Abstract
We determined the nucleotide sequence of the Shiga-like toxin-1 (SLT-1) genes carried by the toxin-converting bacteriophage H-19B. Two open reading frames were identified; these were separated by 12 base pairs and encoded proteins of 315 (A subunit) and 89 (B subunit) amino acids. The predicted protein subunits had N-terminal hydrophobic signal sequences of 22 and 20 amino acids, respectively. The predicted amino acid sequence of the B subunit was identical to that of the B subunit of Shiga toxin. The A chain of ricin was found to be significantly related to the predicted A1 fragment of the SLT-1 A subunit. S1 nuclease protection experiments showed that the two cistrons formed a single transcriptional unit, with the A subunit being proximal to the promoter. A probable promoter was identified by primer extension, and transcription was found to increase dramatically under conditions of iron starvation. A 21-base-pair sequence with dyad symmetry was found in the region of the SLT-1 -10 sequence, which was found to be 68% homologous to a region of dyad symmetry found in the -35 region of the promoter of the iucA gene on plasmid ColV-K30, which specifies the 74,000-dalton ferric-aerobactin receptor protein. Betley et al. (M. Betley, V. Miller, and J. Mekalanos, Annu. Rev. Microbiol. 40:577-605, 1986) have recently summarized evidence suggesting that the slt operon is under the control of the fur regulatory system. The area of dyad symmetry found in both promoters may represent a regulatory site. A rho-independent terminator sequence was found 230 base pairs downstream from the B cistron stop codon.
Full text
PDF





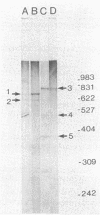
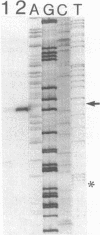
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Betley M. J., Miller V. L., Mekalanos J. J. Genetics of bacterial enterotoxins. Annu Rev Microbiol. 1986;40:577–605. doi: 10.1146/annurev.mi.40.100186.003045. [DOI] [PubMed] [Google Scholar]
- Bindereif A., Neilands J. B. Promoter mapping and transcriptional regulation of the iron assimilation system of plasmid ColV-K30 in Escherichia coli K-12. J Bacteriol. 1985 Jun;162(3):1039–1046. doi: 10.1128/jb.162.3.1039-1046.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock K., Breimer M. E., Brignole A., Hansson G. C., Karlsson K. A., Larson G., Leffler H., Samuelsson B. E., Strömberg N., Edén C. S. Specificity of binding of a strain of uropathogenic Escherichia coli to Gal alpha 1----4Gal-containing glycosphingolipids. J Biol Chem. 1985 Jul 15;260(14):8545–8551. [PubMed] [Google Scholar]
- Braun V., Burkhardt R. Regulation of the ColV plasmid-determined iron (III)-aerobactin transport system in Escherichia coli. J Bacteriol. 1982 Oct;152(1):223–231. doi: 10.1128/jb.152.1.223-231.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brendel V., Trifonov E. N. A computer algorithm for testing potential prokaryotic terminators. Nucleic Acids Res. 1984 May 25;12(10):4411–4427. doi: 10.1093/nar/12.10.4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderwood S. B., Auclair F., Donohue-Rolfe A., Keusch G. T., Mekalanos J. J. Nucleotide sequence of the Shiga-like toxin genes of Escherichia coli. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4364–4368. doi: 10.1073/pnas.84.13.4364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulton J. W., Mason P., Cameron D. R., Carmel G., Jean R., Rode H. N. Protein fusions of beta-galactosidase to the ferrichrome-iron receptor of Escherichia coli K-12. J Bacteriol. 1986 Jan;165(1):181–192. doi: 10.1128/jb.165.1.181-192.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohue-Rolfe A., Keusch G. T., Edson C., Thorley-Lawson D., Jacewicz M. Pathogenesis of Shigella diarrhea. IX. Simplified high yield purification of Shigella toxin and characterization of subunit composition and function by the use of subunit-specific monoclonal and polyclonal antibodies. J Exp Med. 1984 Dec 1;160(6):1767–1781. doi: 10.1084/jem.160.6.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo Y., Mitsui K., Motizuki M., Tsurugi K. The mechanism of action of ricin and related toxic lectins on eukaryotic ribosomes. The site and the characteristics of the modification in 28 S ribosomal RNA caused by the toxins. J Biol Chem. 1987 Apr 25;262(12):5908–5912. [PubMed] [Google Scholar]
- Endo Y., Wool I. G. The site of action of alpha-sarcin on eukaryotic ribosomes. The sequence at the alpha-sarcin cleavage site in 28 S ribosomal ribonucleic acid. J Biol Chem. 1982 Aug 10;257(15):9054–9060. [PubMed] [Google Scholar]
- Fecker L., Braun V. Cloning and expression of the fhu genes involved in iron(III)-hydroxamate uptake by Escherichia coli. J Bacteriol. 1983 Dec;156(3):1301–1314. doi: 10.1128/jb.156.3.1301-1314.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming T. P., Nahlik M. S., McIntosh M. A. Regulation of enterobactin iron transport in Escherichia coli: characterization of ent::Mu d(Apr lac) operon fusions. J Bacteriol. 1983 Dec;156(3):1171–1177. doi: 10.1128/jb.156.3.1171-1177.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouser L. A., Friesen J. D. Mutations in a yeast intron demonstrate the importance of specific conserved nucleotides for the two stages of nuclear mRNA splicing. Cell. 1986 Apr 11;45(1):81–93. doi: 10.1016/0092-8674(86)90540-4. [DOI] [PubMed] [Google Scholar]
- Gray G. L., Smith D. H., Baldridge J. S., Harkins R. N., Vasil M. L., Chen E. Y., Heyneker H. L. Cloning, nucleotide sequence, and expression in Escherichia coli of the exotoxin A structural gene of Pseudomonas aeruginosa. Proc Natl Acad Sci U S A. 1984 May;81(9):2645–2649. doi: 10.1073/pnas.81.9.2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross R., Engelbrecht F., Braun V. Genetic and biochemical characterization of the aerobactin synthesis operon on pColV. Mol Gen Genet. 1984;196(1):74–80. doi: 10.1007/BF00334095. [DOI] [PubMed] [Google Scholar]
- Hantke K. Cloning of the repressor protein gene of iron-regulated systems in Escherichia coli K12. Mol Gen Genet. 1984;197(2):337–341. doi: 10.1007/BF00330982. [DOI] [PubMed] [Google Scholar]
- Hantke K. Regulation of ferric iron transport in Escherichia coli K12: isolation of a constitutive mutant. Mol Gen Genet. 1981;182(2):288–292. doi: 10.1007/BF00269672. [DOI] [PubMed] [Google Scholar]
- Hawley D. K., McClure W. R. Compilation and analysis of Escherichia coli promoter DNA sequences. Nucleic Acids Res. 1983 Apr 25;11(8):2237–2255. doi: 10.1093/nar/11.8.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A., de Grandis S., Friesen J., Karmali M., Petric M., Congi R., Brunton J. L. Cloning and expression of the genes specifying Shiga-like toxin production in Escherichia coli H19. J Bacteriol. 1986 May;166(2):375–379. doi: 10.1128/jb.166.2.375-379.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacewicz M., Clausen H., Nudelman E., Donohue-Rolfe A., Keusch G. T. Pathogenesis of shigella diarrhea. XI. Isolation of a shigella toxin-binding glycolipid from rabbit jejunum and HeLa cells and its identification as globotriaosylceramide. J Exp Med. 1986 Jun 1;163(6):1391–1404. doi: 10.1084/jem.163.6.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson M. P., Newland J. W., Holmes R. K., O'Brien A. D. Nucleotide sequence analysis of the structural genes for Shiga-like toxin I encoded by bacteriophage 933J from Escherichia coli. Microb Pathog. 1987 Feb;2(2):147–153. doi: 10.1016/0882-4010(87)90106-9. [DOI] [PubMed] [Google Scholar]
- Karmali M. A., Petric M., Lim C., Fleming P. C., Arbus G. S., Lior H. The association between idiopathic hemolytic uremic syndrome and infection by verotoxin-producing Escherichia coli. J Infect Dis. 1985 May;151(5):775–782. doi: 10.1093/infdis/151.5.775. [DOI] [PubMed] [Google Scholar]
- Klebba P. E., McIntosh M. A., Neilands J. B. Kinetics of biosynthesis of iron-regulated membrane proteins in Escherichia coli. J Bacteriol. 1982 Mar;149(3):880–888. doi: 10.1128/jb.149.3.880-888.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau P. C., Rowsome R. W., Zuker M., Visentin L. P. Comparative nucleotide sequences encoding the immunity proteins and the carboxyl-terminal peptides of colicins E2 and E3. Nucleic Acids Res. 1984 Nov 26;12(22):8733–8745. doi: 10.1093/nar/12.22.8733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg F., Lund B., Normark S. Gene products specifying adhesion of uropathogenic Escherichia coli are minor components of pili. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1891–1895. doi: 10.1073/pnas.83.6.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenney K., Shimatake H., Court D., Schmeissner U., Brady C., Rosenberg M. A system to study promoter and terminator signals recognized by Escherichia coli RNA polymerase. Gene Amplif Anal. 1981;2:383–415. [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Newland J. W., Strockbine N. A., Miller S. F., O'Brien A. D., Holmes R. K. Cloning of Shiga-like toxin structural genes from a toxin converting phage of Escherichia coli. Science. 1985 Oct 11;230(4722):179–181. doi: 10.1126/science.2994228. [DOI] [PubMed] [Google Scholar]
- O'Brien A. D., Chen M. E., Holmes R. K., Kaper J., Levine M. M. Environmental and human isolates of Vibrio cholerae and Vibrio parahaemolyticus produce a Shigella dysenteriae 1 (Shiga)-like cytotoxin. Lancet. 1984 Jan 14;1(8368):77–78. doi: 10.1016/s0140-6736(84)90006-0. [DOI] [PubMed] [Google Scholar]
- O'Brien A. D., LaVeck G. D. Purification and characterization of a Shigella dysenteriae 1-like toxin produced by Escherichia coli. Infect Immun. 1983 May;40(2):675–683. doi: 10.1128/iai.40.2.675-683.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien A. D., LaVeck G. D., Thompson M. R., Formal S. B. Production of Shigella dysenteriae type 1-like cytotoxin by Escherichia coli. J Infect Dis. 1982 Dec;146(6):763–769. doi: 10.1093/infdis/146.6.763. [DOI] [PubMed] [Google Scholar]
- O'Brien A. D., Laveck G. D. Immunochemical and cytotoxic activities of Shigella dysenteriae 1 (shiga) and shiga-like toxins. Infect Immun. 1982 Mar;35(3):1151–1154. doi: 10.1128/iai.35.3.1151-1154.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien A. O., Lively T. A., Chen M. E., Rothman S. W., Formal S. B. Escherichia coli O157:H7 strains associated with haemorrhagic colitis in the United States produce a Shigella dysenteriae 1 (SHIGA) like cytotoxin. Lancet. 1983 Mar 26;1(8326 Pt 1):702–702. doi: 10.1016/s0140-6736(83)91987-6. [DOI] [PubMed] [Google Scholar]
- Olsnes S., Reisbig R., Eiklid K. Subunit structure of Shigella cytotoxin. J Biol Chem. 1981 Aug 25;256(16):8732–8738. [PubMed] [Google Scholar]
- Reisbig R., Olsnes S., Eiklid K. The cytotoxic activity of Shigella toxin. Evidence for catalytic inactivation of the 60 S ribosomal subunit. J Biol Chem. 1981 Aug 25;256(16):8739–8744. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäffer S., Hantke K., Braun V. Nucleotide sequence of the iron regulatory gene fur. Mol Gen Genet. 1985;200(1):110–113. doi: 10.1007/BF00383321. [DOI] [PubMed] [Google Scholar]
- Seidah N. G., Donohue-Rolfe A., Lazure C., Auclair F., Keusch G. T., Chrétien M. Complete amino acid sequence of Shigella toxin B-chain. A novel polypeptide containing 69 amino acids and one disulfide bridge. J Biol Chem. 1986 Oct 25;261(30):13928–13931. [PubMed] [Google Scholar]
- Shine J., Dalgarno L. Determinant of cistron specificity in bacterial ribosomes. Nature. 1975 Mar 6;254(5495):34–38. doi: 10.1038/254034a0. [DOI] [PubMed] [Google Scholar]
- Smith H. W., Green P., Parsell Z. Vero cell toxins in Escherichia coli and related bacteria: transfer by phage and conjugation and toxic action in laboratory animals, chickens and pigs. J Gen Microbiol. 1983 Oct;129(10):3121–3137. doi: 10.1099/00221287-129-10-3121. [DOI] [PubMed] [Google Scholar]
- Strockbine N. A., Marques L. R., Newland J. W., Smith H. W., Holmes R. K., O'Brien A. D. Two toxin-converting phages from Escherichia coli O157:H7 strain 933 encode antigenically distinct toxins with similar biologic activities. Infect Immun. 1986 Jul;53(1):135–140. doi: 10.1128/iai.53.1.135-140.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN HEYNINGEN W. E., GLADSTONE G. P. The neurotoxin of Shigella shigae. III. The effect of iron on production of the toxin. Br J Exp Pathol. 1953 Apr;34(2):221–229. [PMC free article] [PubMed] [Google Scholar]
- Zimmermann L., Hantke K., Braun V. Exogenous induction of the iron dicitrate transport system of Escherichia coli K-12. J Bacteriol. 1984 Jul;159(1):271–277. doi: 10.1128/jb.159.1.271-277.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Elzen P. J., Walters H. H., Veltkamp E., Nijkamp H. J. Molecular structure and function of the bacteriocin gene and bacteriocin protein of plasmid Clo DF13. Nucleic Acids Res. 1983 Apr 25;11(8):2465–2477. doi: 10.1093/nar/11.8.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Gabain A., Belasco J. G., Schottel J. L., Chang A. C., Cohen S. N. Decay of mRNA in Escherichia coli: investigation of the fate of specific segments of transcripts. Proc Natl Acad Sci U S A. 1983 Feb;80(3):653–657. doi: 10.1073/pnas.80.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. How signal sequences maintain cleavage specificity. J Mol Biol. 1984 Feb 25;173(2):243–251. doi: 10.1016/0022-2836(84)90192-x. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Signal sequences. The limits of variation. J Mol Biol. 1985 Jul 5;184(1):99–105. doi: 10.1016/0022-2836(85)90046-4. [DOI] [PubMed] [Google Scholar]