Abstract
Light chain-associated amyloidosis is characterized by the deposition as fibrils of monoclonal light chain-related components consisting predominately of the variable domain (VL) or the VL plus up to ≈60 residues of the constant domain (CL). Here, we describe a patient (designated BIF) with light chain-associated amyloidosis and κ Bence Jones proteinuria in whom, notably, >80% of the amyloid deposits were comprised of CL-related material. The extracted amyloid protein consisted of 99 aa residues identical in sequence to the main portion of the Cκ region (positions 109–207) of the precursor Bence Jones protein. Remarkably, the CLs from both molecules contained a Ser→Asn substitution at position 177. This heretofore undescribed Cκ alteration did not result from somatic mutation but rather was germline encoded. When tested in our in vitro fibrillogenic kinetic assay, Bence Jones protein BIF was highly amyloidogenic. Notably, endopeptidase treatment of amyloid fibrils prepared from the native light chain revealed the VL to be markedly susceptible to enzymatic digestion, whereas the CL was protease-resistant. Our findings provide evidence that the fragmented light chains typically present in this disease result from proteolytic degradation and suggest that, in this case, conformational differences in VL/CL packing within the fibrils may account for the unusual composition of the amyloid deposits. Additionally, we posit that the previously unrecognized Asn177 substitution represents yet another Cκ allotype, provisionally designated Km4.
Keywords: Ig genes/Bence Jones protein/fibrillogenesis
Light chain-associated (AL) amyloidosis is characterized by birefringent Congophilic fibrillar deposits of monoclonal light chains or, more commonly, light chain-related fragments consisting of the variable domain (VL) or the VL and a contiguous portion of the constant domain (CL) (1, 2). Whether the fragmentary nature of light chains extracted from amyloid deposits results from proteolytic degradation of the native molecule pre or postdeposition [or, alternatively, from aberrant synthesis (3)] has not been established conclusively. In this respect, further information on the composition of AL amyloid components has both pathogenic and therapeutic import.
We report the first case of AL amyloidosis in a patient (designated BIF) with κ Bence Jones proteinuria and widespread disease in whom the fibrillar deposits were not comprised of VL-related material but, rather, consisted almost entirely of CL. Also remarkable was the finding that the Cκ portion of the Bence Jones protein (BJP) contained a Ser→Asn amino acid substitution at position 177. The identical alteration also was present in the Cκ-associated amyloid protein. This heretofore unrecognized difference in Cκ sequence—Asn177—was not caused by somatic mutation, as evidenced from genomic DNA analyses, and presumably represents a genetic marker distinct from that associated with the Km1, Km1,2, and Km3 Cκ-related allotypes (4).
MATERIALS AND METHODS
Clinicopathologic Features.
Patient BIF was a 69-year-old Caucasian male of Italian ancestry in whom a diagnosis of AL amyloidosis was made from examination of material obtained via endoscopic biopsy of a gastric polypoid mass. The Congophilic, green birefringent deposits noted in the lamina propria and within blood vessel walls reacted with an anti-κ light chain antibody. Approximately 20–40% of nucleated cells present in a bone marrow aspirate consisted of a monoclonal (κ+) plasma cell population as evidenced through immunophenotyping studies. Immunofixation electrophoreses of serum and urine revealed a pronounced hypogammaglobulinemia and κ BJP, respectively. Despite treatment with monthly courses of melphalan and prednisone and other supportive care, his condition progressively worsened and he died 4 months later. Postmortem examination revealed extensive amyloid deposition throughout the entire gastrointestinal tract, heart, spleen, and bladder; additionally, lesser deposits were present in the lungs, liver, and kidneys.
Protein Isolation and Characterization.
A 24-h urine specimen containing ≈7 gm of BJP was dialyzed extensively against distilled water and lyophilized. The monoclonal Ig urinary component was isolated from a water-reconstituted urine sample by zone electrophoresis on blocks of polyvinyl chloride/polyvinyl acetate copolymer (Pevikon-870, Hydro Plast A3, Stenungsund, Sweden) and further purified by gel filtration on an acrylamide/agarose column (AcA 54 Ultragel, Pharmacia) as described (5). Amyloid proteins were extracted according to the method of Pras et al. (6) and purified by HPLC (7) from 10-gm portions of heart and spleen that were obtained at autopsy and maintained in a −80°C freezer.
The purity and Mrs of the monoclonal urinary Ig and the amyloid protein were determined by SDS/PAGE in the presence or absence of 2-mercaptoethanol by using a discontinuous buffer system, 12.5% homogeneous polyacrylamide gels, and reference standard proteins. After SDS/PAGE, the proteins were blotted onto poly(vinylidene difluoride) membranes, and the N-terminal sequence was determined.
Sequence Analyses.
Samples (10–20 mg) of completely reduced and pyridylethylated BJP and amyloid protein were cleaved enzymatically with trypsin (Worthington Biochemical, Freehold, NJ) at an enzyme to substrate ratio of 1:100 (wt/wt) as described. (7) The proteins also were digested with an aspartyl residue-specific endopeptidase prepared from a Pseudomonas fragi mutant (Endoproteinase N, Boehringer Mannheim) under aqueous conditions at an enzyme to substrate ratio of 1:100 (wt/wt) for 6 h at 37°C; extension of the digestion time to 24 h also cleaved peptide bonds N-terminally at glutamyl residues. The resultant peptides were separated by HPLC (7), and the amino acid sequences were determined by using an ABI 477A gas-phase protein sequencer connected on-line to an ABI 120A phenylthiohydantoin analyzer (Applied Biosystems).
Preparation of Germ-Line DNA.
Germ-line DNA was prepared from the patient’s cardiac tissue and family members’ buccal cells by using the Puregene DNA Isolation Kit (Gentra, Minneapolis, MN). One-micromolar amounts of 08–018 Vκ1- and Cκ-specific primers (8) were used respectively to amplify the Vκ and Cκ regions from 1 μg of genomic DNA under conditions described (9). PCR products were isolated and purified and then sequenced by using an ABI Prism Model 2.1.1 data collection automated DNA sequencer.
Fibrillogenic Kinetic Assay.
Lyophilized, purified samples of BJP were dissolved in phosphate buffered saline (PBS), pH 7.0 at a final concentration of 1 mg per ml. After filtration through a 0.2 μm filter, 1 ml-aliquots were pipetted into 13 × 100 mm borasilicate test tubes that were placed in an orbital shaker and maintained at 37°C at an angle of 45° to the axis of rotation. The tubes were agitated at 225 rpm and analyzed daily spectroscopically at 320 nm for development of turbidity. Precipitates were harvested by centrifugation at 17,000 × g for 10 min and washed three times with PBS. Aliquots of the washed precipitates were resuspended in PBS, air-dried on microscopic slides, Congo red stained, and examined under polarized light. For electron microscopy, precipitates were applied to Formvar carbon-coated copper grids, air-dried, stained with 1% phosphotungstic acid, and viewed with a Hitachi H-600 electron microscope. Protein aggregation was measured by light scattering at 400 and 600 mm, and the thioflavin T (ThT) fluorometric assay was performed by the method of LeVine (10).
RESULTS
BJP BIF.
By SDS/PAGE, the isolated and purified protein BIF consisted of approximately equimolar mixtures of covalent dimer and monomer with molecular masses of ≈43 and 22 kDa, respectively. Under reducing conditions, the dimer was converted to the monomeric form. When tested by ELISA against a battery of anti-Vκ subgroup specific mAbs (11), protein BIF was typed serologically as a κ1 light chain. This classification was confirmed chemically: The complete amino acid sequence of protein BIF was established from analyses of tryptic- and Asp-N-derived peptides ordered on the basis of published data (12). The portion of the VL domain encoded by the Vκ gene consisted of 95 residues and was most homologous in sequence (identity, 95%) to the deduced product of the Vκ1 germ-line gene 08–018 (8) that was found to be identical to the patient’s germ-line gene counterpart (data not shown). Among the interchanges were the replacement in BJP BIF of Val, Phe, Thr, Val, and Phe for the germline encoded Leu, Leu, Pro, Ile, and Tyr at positions 33, 46, 59, 83, and 87, respectively. The 13 remaining VL residues were joining-(J)-gene-derived and were related most closely in sequence to the predicted product of the Jκ2-germ-line gene (13) with one substitution in protein BIF of Arg for Lys at position 107.
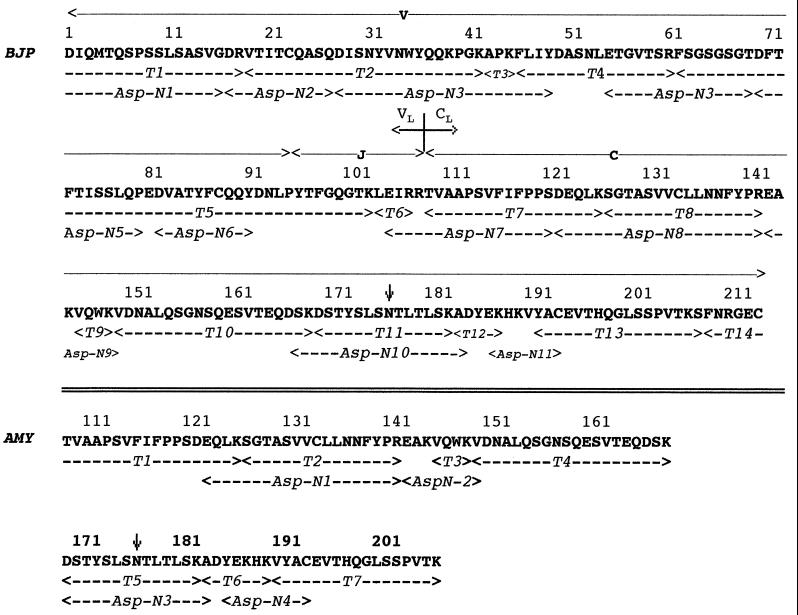
The CL portion of BJP BIF consisted of 107 residues contained within tryptic peptides T7 through T14. With the exception of one amino acid, the sequence was the same as a prototypic Cκ domain containing the Km3 allotypic-associated Ala and Val residues at positions 153 and 191, respectively (4, 12). The main difference was the substitution at position 177 in tryptic peptide T11 of Asn for Ser. This interchange was confirmed through analysis of the overlapping Asp-N 10 peptide. The complete amino acid sequence of BJP BIF is provided in Fig. 1.
Figure 1.
Primary structure of light chain BIF (BJP) and amyloid protein BIF (AMY). The residues marked by dashed arrows were determined from sequencing peptides generated by trypsin (T) cleavage and from enzymatic digestion with the aspartic acid-specific (Asp-N) endoproteinase. The numbering system used is as given in ref. 12 (amino acids are indicated by the single-letter code). The junction between VL and CL domains and the segments encoded by the V, J, and C genes in BJP BIF are as designated. The arrow at position 177 indicates the Asn for Ser substitution found in both proteins.
Amyloid Protein BIF.
The molecular masses of the amyloid extracted from the heart and spleen were determined by SDS/PAGE. Under reducing conditions, the predominant (>80%) component had a molecular mass of ≈11 kDa. That this material was light chain-related was established through N-terminal sequence analyses of the unfractionated amyloid protein extracted from a PVDF membrane and peptides derived from enzymatic cleavage of the completely reduced and pyridylethylated protein purified by HPLC (Fig. 1). The majority of amyloid BIF consisted of 99 residues that were identical in sequence to that of the Cκ region (positions 109–207) of the BJP BIF. The Ser→Asn177 substitution, present in the intact light chain, was found also in the T5 and Asp-N 3 peptides. The seven C-terminal residues (positions 208–214) contained in the native molecule were not detected in the amyloid component. Minor populations of intact light chain identical in N-terminal sequence to BJP BIF, as well as Cκ-related fragments lacking one, three, and 19 N-terminal residues, were noted. There were no demonstrable differences in the composition of heart- and spleen-derived AL components.
In addition to the Cκ-related peptides present in the tryptic digest of the HPLC-purified amyloid protein, a 30-residue component corresponding to amino acids 65–94 of the human neutrophil defensin HNP 1 peptide also was found (14). On a molar basis, this component represented ≈5–10% of the material contained in the digest.
In Vitro Amyloidogenic Studies.
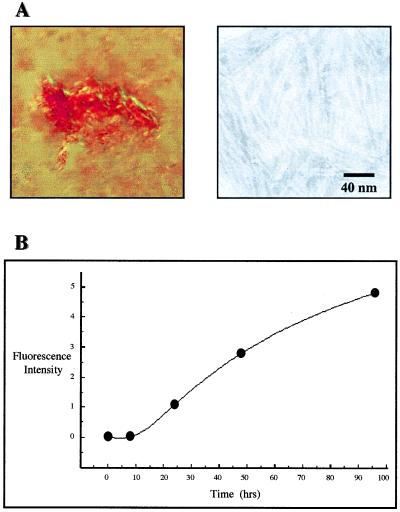
To determine the amyloidogenic potential of native BJPs or VL fragments derived enzymatically or by recombinant technology (15, 16), we have developed an in vitro fibrillogenic kinetic assay in which, under defined conditions, certain soluble proteins form precipitates having the characteristic tinctorial and ultrastructural features of amyloid. Using this assay, the amyloidogenic propensity of BJP BIF was evidenced when solutions of the light chain became turbid within 24 h. By 14 days, >75% of the BJP had precipitated and exhibited Congo red-positive green birefringence when viewed under polarized light and was fibrillar by electron microscopy (Fig. 2A). In other analyses, the in vitro formation of amyloid by BJP BIF also was evidenced when ThT-positive aggregates were formed readily, as demonstrated in fluorescent (Fig. 2B) and light scattering assays. When the synthetic amyloid was digested with trypsin (enzyme to substrate ratio, 1:100) and examined by SDS/PAGE, an ≈11 kDa component comparable to that found in the patient’s amyloid extract was identified. N-terminal sequence analysis revealed that >90% of this material represented the Cκ portion of light chain BIF; the remainder consisted of VL-related material. In similar experiments involving synthetic fibrils comprised of a different amyloid-associated 08–018 κ1 light chain that lacked the Ser→Asn177 alteration, an ≈11 kDa VL component also was generated. However, in contrast to amyloid BIF, this Cκ had undergone proteolysis, and the residual material represented the VL portion of the molecule.
Figure 2.
Synthetic Congo red-positive green birefringent fibrils prepared from BJP BIF. (A, Left) Polarization photomicrograph: Congo red staining, ×400; (Right) Electron photomicrograph: negative staining. (B) ThT assay: fluorescence intensity of the ThT–fibril complex. Excitation and emission wavelengths, 450 and 490 mm, respectively.
DISCUSSION
The results of our studies on the protein extracted from amyloid deposits present in the heart and spleen of a patient (BIF) with widespread AL amyloidosis and κ Bence Jones proteinuria revealed that >80% of this material consisted of virtually the entire Cκ portion of the precursor monoclonal κ light chain. The finding of CL-associated amyloid deposits in this individual has been undescribed heretofore in AL amyloidosis, in which fibrils typically are comprised of either VL, VL plus an indeterminate amount of CL, or, less commonly, the intact light chain (1, 2, 17).
From amino acid sequence analyses, it was determined that the amyloid deposits found in patient BIF were derived from the native BJP. This molecule was deemed particularly amyloidogenic based on the results of our fibrillogenic kinetic assay in which the soluble light chain was converted rapidly into Congophilic birefringent fibrils without enzymatic digestion (18) or chemical modification (19). Noteworthy was that BJP BIF was encoded by the Vκ1-related 08–018 germ-line gene, the products of which preferentially are associated with AL amyloid formation but do not typically contain the Asn177 alteration present in the BIF component (20).
Through comparative sequence analyses of amyloid vs. nonamyloid κ chains, particular residues at certain positions within the VL have been identified that apparently enhance light chain fibrillogenesis (20). These would include the substitutions found in BJP BIF of Val, Thr, and Val for the germline-encoded Leu, Pro, and Ile at positions 33, 59, and 83, respectively. That the destabilizing effects of such mutations lead to partially unfolded intermediates and fibril formation has been shown experimentally (21, 22); however, the molecular event(s) responsible for generation of AL amyloid fibrils has not been elucidated. The demonstration that such material can consist partially or entirely of intact light chains (17) indicates that fragmentation of the precursor protein is not a prerequisite for fibrillogenesis. Based on experiments in which human amyloid-associated BJPs were injected into mice (23), it is probable that AL amyloid is formed in situ as a result of the deposition in tissue of soluble light chains and conversion of this material into fibrils. Subsequent proteolysis of the amyloid protein results in generation of light chain fragments. Several types of endopeptidases including the lysosomal protease cathepsin D have been implicated as being among those enzymes responsible for amyloid digestion (24). The presence of neutrophil-derived defensin components in AA amyloid extracts (25), as also found in material from patient BIF, suggests a role of these molecules in amyloid degradation.
The fact that the CL represented the predominant component contained in the amyloid protein BIF indicates that the VL domain of the precursor light chain was unusually susceptible to proteolysis. Indeed, trypsin digestion of synthetic amyloid fibrils formed from BJP BIF yielded a Cκ fragment having an N-terminal sequence identical to that of the major constituent of the AL deposits found in vivo. By contrast, in similar experiments involving synthetic fibrils formed from a different 08–018-derived κ1 BJP, digestion with trypsin resulted in proteolysis of the CL and yielded only a Vκ fragment. Although there are no x-ray crystallographic data on AL amyloid that would elucidate tertiary structural features of this material, these findings suggest that differences in domain packing within the fibrils may account for the variation that is found in the light chain composition of amyloid deposits. Most commonly, the CL portion of the molecule is exposed to solvent, whereas the VL is buried and protected from enzymatic digestion. In the case of the amyloid formed by light chain BIF, we postulate that the reverse occurred and that the VL portion of the fibrils was sterically accessible. Whether the Ser→Asn substitution at position 177 in the Cκ region affected domain packing and was responsible for this phenomenon is unknown.
The Ig κ-chain locus contains a single Cκ gene (26) that encodes a protein product invariant in sequence except for alterations at positions 153 and 191 (Table 1) that are associated with the expression of the three serologically detected Km allotypes, Km1, Km1,2, and Km3 (4). Remarkably, BJP (and amyloid protein) BIF had a previously unrecognized substitution of Asn for Ser at position 177 in the Cκ region. That this difference did not result from somatic mutation was evidenced from analyses of germline-derived DNA isolated from the tissue of patient BIF. The Asn for Ser difference resulted from a one-base change in codon 177 whereby AGC was altered to AAC. Furthermore, the codon specifying Asn177 was found in germline DNA prepared from buccal cells obtained from the patient’s mother and two of three siblings. In all of the Asn177-positive cases, analyses of several germ-line DNA-derived clones showed the presence of nucleotides encoding the Ser177 as well as the Asn177 residues, thus indicating the heterozygosity of this genetic alteration.
Table 1.
Allotypic-associated Cκ residues
| Allotype | Residue
|
||
|---|---|---|---|
| 153 | 177 | 191 | |
| Km1 | Val | Ser | Leu |
| Km1,2 | Ala | Ser | Leu |
| Km3 | Ala | Ser | Val |
| Km4* | Ala | Asn | Val |
Provisional designation for the Cκ sequence alteration found in light chain BIF.
We presume that a Cκ gene encoding for Asn177 rarely is expressed based on amino acid sequence data on 22 monoclonal κ chains (GenBank) and analysis of DNA derived from 347 individuals (representative of 10 human populations) of which 50 were subjected to direct sequencing of PCR products (27, 28). Presently, there are no known serologic reagents that differentiate between light chains with Asn177 vs. Ser177. However, the use of an allele-specific oligonucleotide in Cκ dot blots of amplified-Cκ DNA (27) will help establish the exact frequency and biologic import of this Cκ allotype that we provisionally designate Km4.
Acknowledgments
We thank Dr. Kristie Bobolis and members of the BIF family for furnishing research specimens. The helpful discussion with Drs. Fred J. Stevens and Ronald Wetzel, technical assistance of Teresa K. Williams, and manuscript preparation by Carolyn Weaver and Valerie Brestel are acknowledged gratefully. This work was supported in part by U.S. Public Health Research Grant CA 10056 from the National Cancer Institute. Alan Solomon is an American Cancer Society Clinical Research Professor.
ABBREVIATIONS
- AL
light chain-associated amyloidosis
- BJP
Bence Jones protein
- VL and CL
variable and constant domains of Ig light chains
- J
joining segment
- ThT
thioflavin T
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. A58703).
References
- 1.Glenner G G. N Engl J Med. 1980;302:1283–1292. doi: 10.1056/NEJM198006053022305. [DOI] [PubMed] [Google Scholar]
- 2.Solomon A, Weiss D T. Amyloid: Int J Exp Clin Invest. 1995;2:269–279. [Google Scholar]
- 3.Buxbaum J. J Clin Invest. 1986;78:798–806. doi: 10.1172/JCI112643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steinberg A G, Milstein C P, McLaughlin C L, Solomon A. Immunogenetics. 1974;1:108–117. [Google Scholar]
- 5.Solomon A. Methods Enzymol. 1985;116:101–121. doi: 10.1016/s0076-6879(85)16008-8. [DOI] [PubMed] [Google Scholar]
- 6.Pras M, Shubert M, Zucker-Franklin D, Rimon A, Franklin C C. J Clin Invest. 1968;47:924–933. doi: 10.1172/JCI105784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eulitz M, Ch’ang L-Y, Zirkel C, Schell M, Weiss D T, Solomon A. J Immunol. 1995;154:3256–3265. [PubMed] [Google Scholar]
- 8.Klein R, Jaenichen R, Zacheau H G. Eur J Immunol. 1993;23:3248–3271. doi: 10.1002/eji.1830231231. [DOI] [PubMed] [Google Scholar]
- 9.Ch’ang L-Y, Yen C-P, Besl L, Schell M, Solomon A. Mol Immunol. 1994;31:531–536. doi: 10.1016/0161-5890(94)90040-x. [DOI] [PubMed] [Google Scholar]
- 10.LeVine H., III Protein Sci. 1993;2:404–410. doi: 10.1002/pro.5560020312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abe M, Goto T, Kennel S J, Wolfenbarger D, Macy S D, Weiss D T, Solomon A. Am J Clin Pathol. 1993;100:67–74. doi: 10.1093/ajcp/100.1.67. [DOI] [PubMed] [Google Scholar]
- 12.Kabat E A, Wu T T, Perry H M, Gottesman K S, Foeller C, editors. Sequences of Proteins of Immunologic Interest. Bethesda, MD: National Institutes of Health; 1991. , NIH Publ. No. 91–3242. [Google Scholar]
- 13.Hieter P A, Maizel J V, Jr, Leder P. J Biol Chem. 1982;257:1516–1522. [PubMed] [Google Scholar]
- 14.Edwards S W. Biochemistry and Physiology of the Neutrophil. Cambridge, MA: Cambridge Univ. Press; 1994. p. 69. [Google Scholar]
- 15.Solomon A, McLaughlin C L. J Biol Chem. 1969;244:3393–3404. [PubMed] [Google Scholar]
- 16.Wilkins-Stevens P, Raffen R, Hanson D K, Deng Y-L, Berrios-Hammond M, Westholm F A, Murphy C, Eulitz M, Wetzel R, Solomon A, et al. Protein Sci. 1995;4:421–432. doi: 10.1002/pro.5560040309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klafki H-W, Kratzin H-D, Pick A I, Eckart K, Karas M, Hilschmann N. Biochemistry. 1992;31:3265–3272. doi: 10.1021/bi00127a031. [DOI] [PubMed] [Google Scholar]
- 18.Glenner G G, Ein D, Eaves E D, Bladen H A, Terry W, Page D L. Science. 1971;174:712–714. doi: 10.1126/science.174.4010.712. [DOI] [PubMed] [Google Scholar]
- 19.Klafki H-W, Peck A I, Pardowitz I, Cole T, Awni L A, Barniksol H-U, Mayer F, Kratzin H D, Hilschmann N. Biol Chem Hoppe-Seyler. 1993;374:1117–1122. doi: 10.1515/bchm3.1993.374.7-12.1117. [DOI] [PubMed] [Google Scholar]
- 20.Stevens F J, Myatt E A, Chang C-H, Westholm F A, Eulitz M, Weiss D T, Murphy C, Solomon A, Schiffer M. Biochemistry. 1995;34:10697–10702. doi: 10.1021/bi00034a001. [DOI] [PubMed] [Google Scholar]
- 21.Hurle M R, Helms L R, Li L, Chan W, Wetzel R. Proc Natl Acad Sci USA. 1994;91:5446–5450. doi: 10.1073/pnas.91.12.5446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wetzel R. Adv Protein Chem. 1997;50:183–242. doi: 10.1016/s0065-3233(08)60322-8. [DOI] [PubMed] [Google Scholar]
- 23.Solomon A, Weiss D T, Pepys M B. Am J Pathol. 1992;140:629–637. [PMC free article] [PubMed] [Google Scholar]
- 24.Epstein W V, Tan M, Wood I S. J Lab Clin Med. 1974;84:107–110. [PubMed] [Google Scholar]
- 25.Liepnieks J J, Kluve-Beckerman B, Benson M D. Biochem Biophys Acta. 1995;1270:81–86. doi: 10.1016/0925-4439(94)00076-3. [DOI] [PubMed] [Google Scholar]
- 26.Hieter P A, Max E E, Seidman J C, Maizel J V, Jr, Leder P. Cell. 1990;22:197–207. doi: 10.1016/0092-8674(80)90168-3. [DOI] [PubMed] [Google Scholar]
- 27.Kurth J H, Bowcock A M, Erlich H A, Nevo S, Cavalli-Sforza L L. Am J Hum Genet. 1991;48:613–620. [PMC free article] [PubMed] [Google Scholar]
- 28.Kurth J H, Cavalli-Sforza L L. Am J Hum Genet. 1994;54:1037–1041. [PMC free article] [PubMed] [Google Scholar]