Abstract
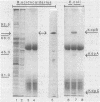
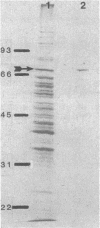
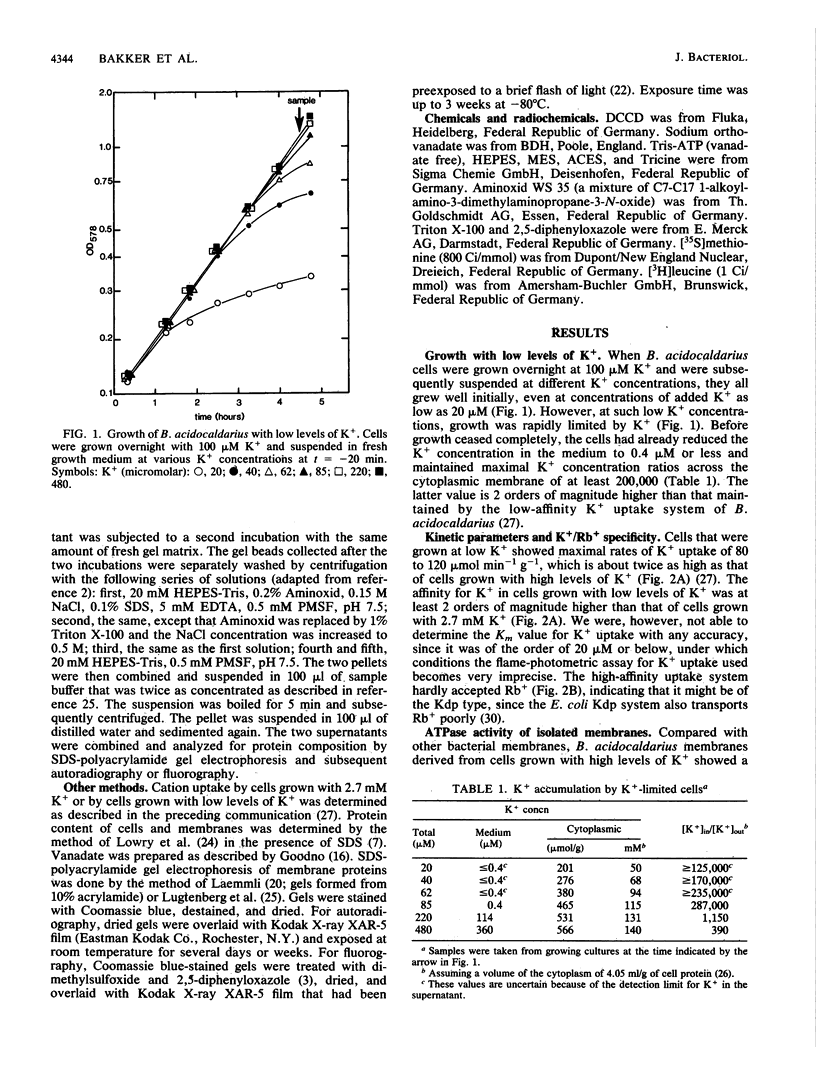
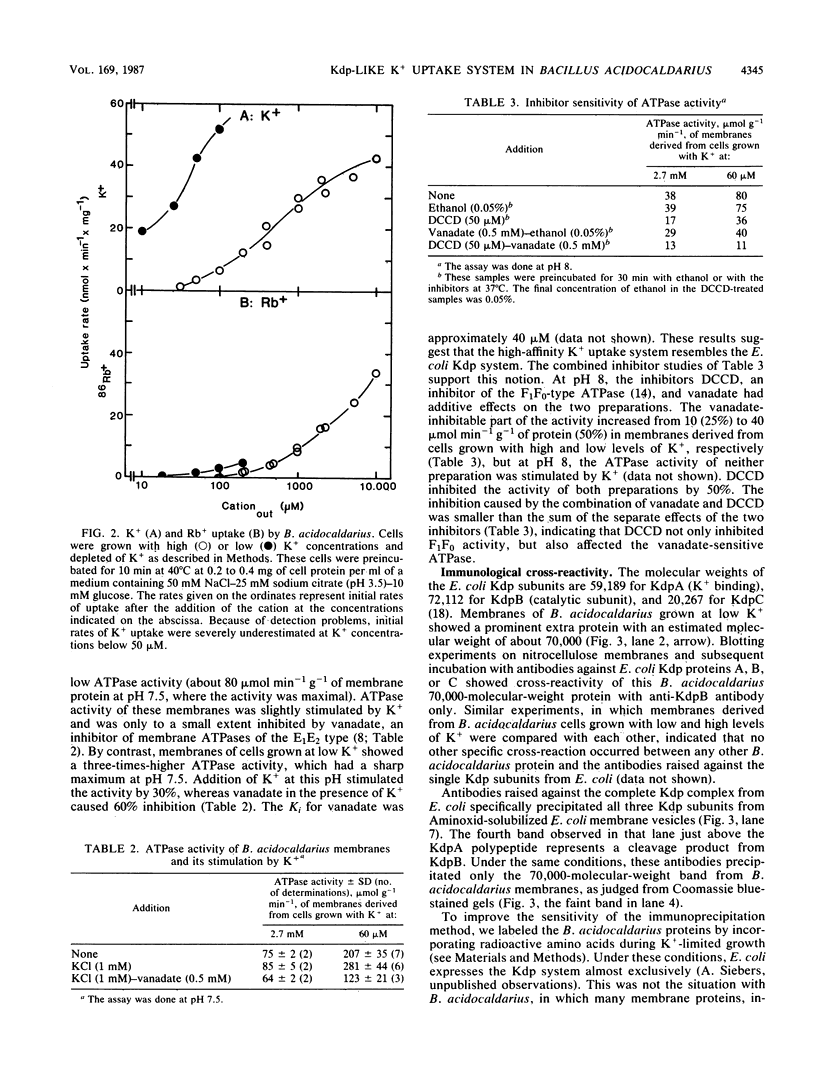
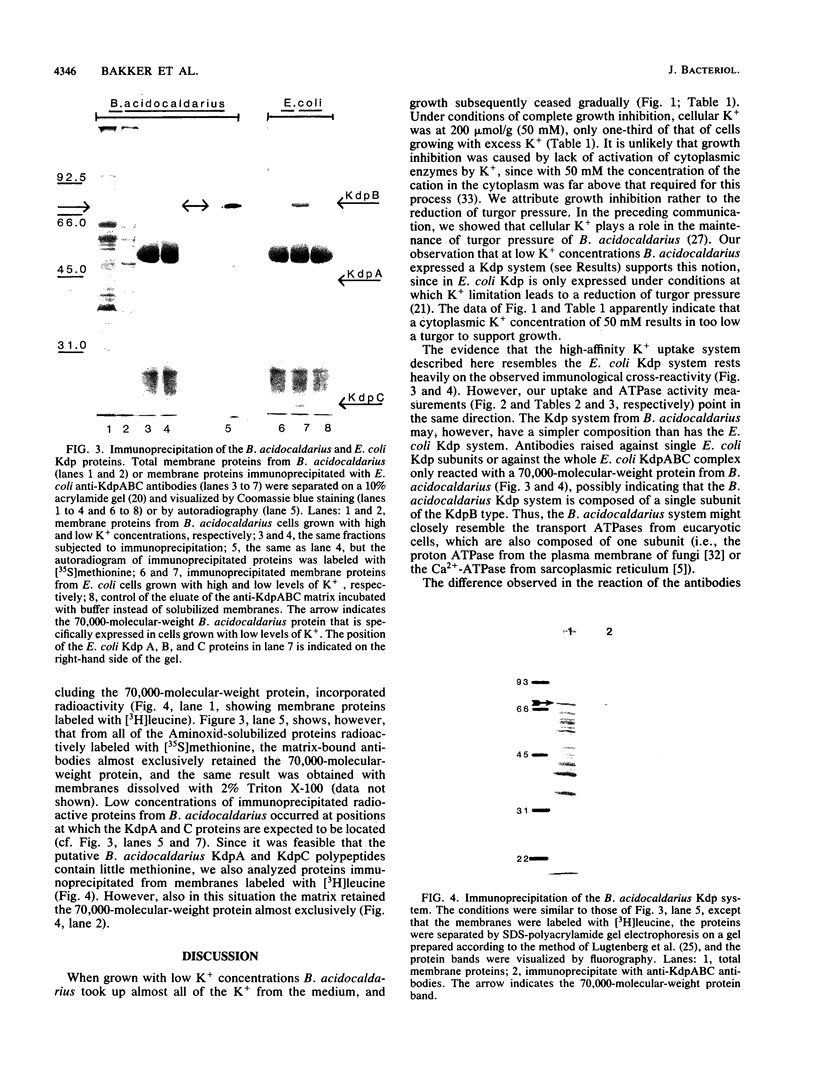
During growth with low levels of K+, Bacillus acidocaldarius expressed a high-affinity K+ uptake system. The following observations indicate that this system strongly resembles the Kdp-ATPase of Escherichia coli: (i) its high affinity for K+ (Km of 20 microM or below); (ii) its poor transport of Rb+; (iii) the enhanced ATPase activity of membranes derived from cells grown with low levels of K+ (this activity was stimulated by K+ and inhibited by vanadate); (iv) the expression of an extra protein with a molecular weight of 70,000 in cells grown with low levels of K+; and (v) the immunological cross-reactivity of this 70,000-molecular-weight protein with antibodies against the catalytic subunit B of the E. coli Kdp system. Antibodies against the complete E. coli Kdp system, which immunoprecipitated the whole E. coli KdpABC complex, almost exclusively precipitated the 70,000-molecular-weight protein from detergent-solubilized B. acidocaldarius membranes. The possibility that the B. acidocaldarius Kdp system consists of a single, KdpB-type subunit is discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold A., Wolf H. U., Ackermann B. P., Bader H. An automated continuous assay of membrane-bound and solube ATPases and related enzymes. Anal Biochem. 1976 Mar;71(1):209–213. doi: 10.1016/0003-2697(76)90029-4. [DOI] [PubMed] [Google Scholar]
- Bakker E. P., Randall L. L. The requirement for energy during export of beta-lactamase in Escherichia coli is fulfilled by the total protonmotive force. EMBO J. 1984 Apr;3(4):895–900. doi: 10.1002/j.1460-2075.1984.tb01902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Booth I. R. Regulation of cytoplasmic pH in bacteria. Microbiol Rev. 1985 Dec;49(4):359–378. doi: 10.1128/mr.49.4.359-378.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandl C. J., Green N. M., Korczak B., MacLennan D. H. Two Ca2+ ATPase genes: homologies and mechanistic implications of deduced amino acid sequences. Cell. 1986 Feb 28;44(4):597–607. doi: 10.1016/0092-8674(86)90269-2. [DOI] [PubMed] [Google Scholar]
- Dulley J. R., Grieve P. A. A simple technique for eliminating interference by detergents in the Lowry method of protein determination. Anal Biochem. 1975 Mar;64(1):136–141. doi: 10.1016/0003-2697(75)90415-7. [DOI] [PubMed] [Google Scholar]
- Epstein W., Kim B. S. Potassium transport loci in Escherichia coli K-12. J Bacteriol. 1971 Nov;108(2):639–644. doi: 10.1128/jb.108.2.639-644.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein W., Whitelaw V., Hesse J. A K+ transport ATPase in Escherichia coli. J Biol Chem. 1978 Oct 10;253(19):6666–6668. [PubMed] [Google Scholar]
- Erecińska M., Deutsch C. J., Davis J. S. Energy coupling to K+ transport in Paracoccus denitrificans. J Biol Chem. 1981 Jan 10;256(1):278–284. [PubMed] [Google Scholar]
- Fillingame R. H. The proton-translocating pumps of oxidative phosphorylation. Annu Rev Biochem. 1980;49:1079–1113. doi: 10.1146/annurev.bi.49.070180.005243. [DOI] [PubMed] [Google Scholar]
- Fürst P., Solioz M. The vanadate-sensitive ATPase of Streptococcus faecalis pumps potassium in a reconstituted system. J Biol Chem. 1986 Mar 25;261(9):4302–4308. [PubMed] [Google Scholar]
- Goodno C. C. Inhibition of myosin ATPase by vanadate ion. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2620–2624. doi: 10.1073/pnas.76.6.2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesse J. E., Wieczorek L., Altendorf K., Reicin A. S., Dorus E., Epstein W. Sequence homology between two membrane transport ATPases, the Kdp-ATPase of Escherichia coli and the Ca2+-ATPase of sarcoplasmic reticulum. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4746–4750. doi: 10.1073/pnas.81.15.4746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugentobler G., Heid I., Solioz M. Purification of a putative K+-ATPase from Streptococcus faecalis. J Biol Chem. 1983 Jun 25;258(12):7611–7617. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laimins L. A., Rhoads D. B., Epstein W. Osmotic control of kdp operon expression in Escherichia coli. Proc Natl Acad Sci U S A. 1981 Jan;78(1):464–468. doi: 10.1073/pnas.78.1.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Lugtenberg B., Meijers J., Peters R., van der Hoek P., van Alphen L. Electrophoretic resolution of the "major outer membrane protein" of Escherichia coli K12 into four bands. FEBS Lett. 1975 Oct 15;58(1):254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- Michels M., Bakker E. P. Generation of a large, protonophore-sensitive proton motive force and pH difference in the acidophilic bacteria Thermoplasma acidophilum and Bacillus acidocaldarius. J Bacteriol. 1985 Jan;161(1):231–237. doi: 10.1128/jb.161.1.231-237.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michels M., Bakker E. P. Low-affinity potassium uptake system in Bacillus acidocaldarius. J Bacteriol. 1987 Sep;169(9):4335–4341. doi: 10.1128/jb.169.9.4335-4341.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plack R. H., Jr, Rosen B. P. Cation/proton antiport systems in Escherichia coli. Absence of potassium/proton antiporter activity in a pH-sensitive mutant. J Biol Chem. 1980 May 10;255(9):3824–3825. [PubMed] [Google Scholar]
- Rhoads D. B., Woo A., Epstein W. Discrimination between Rb+ and K+ by Escherichia coli. Biochim Biophys Acta. 1977 Aug 15;469(1):45–51. doi: 10.1016/0005-2736(77)90324-8. [DOI] [PubMed] [Google Scholar]
- Serrano R., Kielland-Brandt M. C., Fink G. R. Yeast plasma membrane ATPase is essential for growth and has homology with (Na+ + K+), K+- and Ca2+-ATPases. Nature. 1986 Feb 20;319(6055):689–693. doi: 10.1038/319689a0. [DOI] [PubMed] [Google Scholar]
- Suelter C. H. Enzymes activated by monovalent cations. Science. 1970 May 15;168(3933):789–795. doi: 10.1126/science.168.3933.789. [DOI] [PubMed] [Google Scholar]
- Wieczorek L., Altendorf K. Potassium transport in Escherichia coli. Evidence for a K+-transport adenosine-5'-triphosphatase. FEBS Lett. 1979 Feb 15;98(2):233–236. doi: 10.1016/0014-5793(79)80189-1. [DOI] [PubMed] [Google Scholar]
- Wright J. K., Overath P. Purification of the lactose:H+ carrier of Escherichia coli and characterization of galactoside binding and transport. Eur J Biochem. 1984 Feb 1;138(3):497–508. doi: 10.1111/j.1432-1033.1984.tb07944.x. [DOI] [PubMed] [Google Scholar]