Abstract
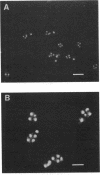
The ascopore wall of Saccharomyces cerevisiae was found to contain more protein, polymeric glucosamine, and beta-glucan than the vegetative cell wall, which was enriched in mannoprotein relative to ascospore walls. Tunicamycin inhibited sporulation, as judged by the absence of refractile ascospores visible by phase-contrast microscopy, but cells completed meiosis, as demonstrated by the presence of multinucleate asci. Such spores lacked the dense outer layer characteristic of normal spores. Thus, the tunicamycin effect was similar to that of glucosamine auxotrophy (W. L. Whelan and C. E. Ballou, J. Bacteriol. 124:1545-1557, 1975).
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold E., Tanner W. An obligatory role of protein glycosylation in the life cycle of yeast cells. FEBS Lett. 1982 Nov 1;148(1):49–53. doi: 10.1016/0014-5793(82)81240-4. [DOI] [PubMed] [Google Scholar]
- Ballou C. E., Maitra S. K., Walker J. W., Whelan W. L. Developmental defects associated with glucosamine auxotrophy in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4351–4355. doi: 10.1073/pnas.74.10.4351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briley M. S., Illingworth R. F., Rose A. H., Fisher D. J. Evidence for a surface protein layer on the Saccharomyces cerevisiae ascospore. J Bacteriol. 1970 Oct;104(1):588–589. doi: 10.1128/jb.104.1.588-589.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briza P., Winkler G., Kalchhauser H., Breitenbach M. Dityrosine is a prominent component of the yeast ascospore wall. A proof of its structure. J Biol Chem. 1986 Mar 25;261(9):4288–4294. [PubMed] [Google Scholar]
- Duksin D., Mahoney W. C. Relationship of the structure and biological activity of the natural homologues of tunicamycin. J Biol Chem. 1982 Mar 25;257(6):3105–3109. [PubMed] [Google Scholar]
- Esposito M. S., Esposito R. E., Arnaud M., Halvorson H. O. Acetate utilization and macromolecular synthesis during sporulation of yeast. J Bacteriol. 1969 Oct;100(1):180–186. doi: 10.1128/jb.100.1.180-186.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GHOSH S., BLUMENTHAL H. J., DAVIDSON E., ROSEMAN S. Glucosamine metabolism. V. Enzymatic synthesis of glucosamine 6-phosphate. J Biol Chem. 1960 May;235:1265–1273. [PubMed] [Google Scholar]
- Kane S. M., Roth R. Carbohydrate metabolism during ascospore development in yeast. J Bacteriol. 1974 Apr;118(1):8–14. doi: 10.1128/jb.118.1.8-14.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz S., Lindquist S. Changing patterns of gene expression during sporulation in yeast. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7323–7327. doi: 10.1073/pnas.81.23.7323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz S., Lindquist S. Subcellular differentiation in sporulating yeast cells. Cell. 1986 Jun 6;45(5):771–779. doi: 10.1016/0092-8674(86)90791-9. [DOI] [PubMed] [Google Scholar]
- Lehle L., Tanner W. The specific site of tunicamycin inhibition in the formation of dolichol-bound N-acetylglucosamine derivatives. FEBS Lett. 1976 Nov 15;72(1):167–170. doi: 10.1016/0014-5793(76)80922-2. [DOI] [PubMed] [Google Scholar]
- Lynn R. R., Magee P. T. Development of the spore wall during ascospore formation in Saccharomyces cerevisiae. J Cell Biol. 1970 Mar;44(3):688–692. doi: 10.1083/jcb.44.3.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILLER G. R., MCCLARY D. O., BOWERS W. D., Jr ULTRAVIOLET AND PHASE MICROSCOPY OF SPORULATING SACCHAROMYCES. J Bacteriol. 1963 Apr;85:725–731. doi: 10.1128/jb.85.4.725-731.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCusker J. H., Haber J. E. Efficient sporulation of yeast in media buffered near pH6. J Bacteriol. 1977 Oct;132(1):180–185. doi: 10.1128/jb.132.1.180-185.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Schroeder R., Breitenbach M. Metabolism of myo-inositol during sporulation of myo-inositol-requiring Saccharomyces cerevisiae. J Bacteriol. 1981 May;146(2):775–783. doi: 10.1128/jb.146.2.775-783.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater M. L. Rapid nuclear staining method for Saccharomyces cerevisiae. J Bacteriol. 1976 Jun;126(3):1339–1341. doi: 10.1128/jb.126.3.1339-1341.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan W. L., Ballou C. E. Sporulation in D-glucosamine auxotrophs of Saccharomyces cerevisiae: meiosis with defective ascospore wall formation. J Bacteriol. 1975 Dec;124(3):1545–1557. doi: 10.1128/jb.124.3.1545-1557.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]