Abstract
Tyrosinemia type 1, caused by mutations in the fumarylacetoacetate hydrolase gene (Fah), is characterized by severe liver injury. We earlier developed a tyrosinemic mouse model with two genetic defects, Fah and 4-hydroxyphenylpyruvate dioxygenase (Hpd) deficiencies. Apoptosis of hepatocytes was induced and an acute onset of liver failure occurred after administration of homogentisic acid (HGA), the intermediate metabolite between the enzymes HPD and FAH. Cytochrome c was released from mitochondria prior to liver failure in the Fah−/− Hpd−/− double-mutant mice after the administration of HGA. In a cell-free system, the addition of fumarylacetoacetate induced the release of cytochrome c from the mitochondria. We also found that caspase inhibitors were highly effective in preventing the liver failure induced by HGA in the double-mutant mice. Therefore, fumarylacetoacetate apparently induces the release of cytochrome c, which in turn triggers activation of the caspase cascade in hepatocytes of subjects with hereditary tyrosinemia type 1.
Hereditary tyrosinemia type 1 (HT1) is caused by mutations in the fumarylacetoacetate hydrolase gene, Fah, which encodes the last enzyme in the tyrosine catabolic pathway (Fig. 1a) (1, 2). HT1 patients show various phenotypes characterized by progressive liver failure during infancy and early development of hepatocellular carcinoma (2, 3). In HT1 hepatocytes, various cellular events have been documented, including inhibition of enzyme activity, such as 4-hydroxyphenylpyruvate dioxygenase (HPD) and δ-aminolevulinate dehydratase (4), aberrant gene expression (5–8), mutagenesis (9), and carcinogenesis (2, 9, 10). These events seem to be related to an aberrant metabolism as a result of a metabolic block at FAH; however, much has remained to be elucidated. FAA and/or related chemicals are obvious candidates.
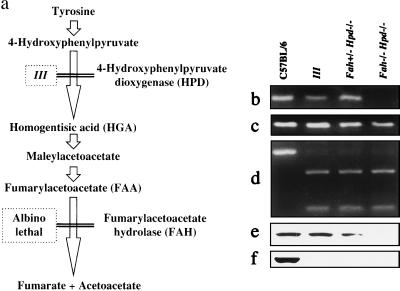
Figure 1.
Characterization of Fah−/− Hpd−/− double-mutant mice. (a) Scheme of tyrosine catabolism and the model mouse for tyrosinemia. The Fah−/−Hpd−/− mice were generated by intercrossing III and albino lethal mice. (b–d) Genotype analysis for Fah−/−Hpd−/− mice by PCR. Deletion in the c14cos mice disrupted the Fah gene, resulting in the absence of exon 1 and 2 sequences (7, 11, 14). The Fah−/− mice were identified by the absence of exon 2 sequences (111 bp) and the presence of exon 8 sequences (132 bp) of the Fah gene after PCR amplification of regions containing each exon, respectively (11). The results of PCR amplification of regions containing exon 2 (b) and exon 8 (c) of the Fah gene are shown. (d) Results of PCR amplification and restriction enzyme digestion of a region containing exon 7 of the Hpd gene. The left lane contains an undigested fragment with normal sequences (386 bp) after treatment with the restriction enzyme HindIII. Lanes 2–4 show products digested by HindIII because of the mutated sequences of the Hpd− allele (272 and 114 bp). DNA fragments were analyzed by electrophoresis on 4% NuSieve agarose gel (FMC BioProducts) and stained with ethidium bromide. (e and f) Immunoblot analysis of FAH (e) and HPD (f) protein in the liver. FAH and HPD proteins were absent from the liver from the Fah−/−Hpd−/− mouse.
We recently developed a tyrosinemic mouse model with Fah and Hpd deficiencies (11). Of the two FAH-deficient mice known, one is albino lethal c14CoS, first described by Gluecksohn-Waelsch in 1979 (12). These mice have a large deletion on chromosome 7, including the albino locus and the Fah gene (13, 14). The homozygous mice are characterized by impairment of expression of hepatocyte-specific genes in the liver during perinatal periods (7, 8, 14). The other FAH-deficient mice were generated by targeted disruption of the Fah gene (10, 15). These mice show essentially the same phenotype and are neonatally lethal (10, 14, 15); however, the double-mutant Fah−/− Hpd−/− mice grow normally (11). In humans, FAH deficiency causes liver disease (1, 2); however, Fah−/− Hpd−/− mice showed no evidence of liver disease and their phenotype was similar to that of Hpd−/− mice (III mice) (11, 16–18). In earlier work, we expressed human HPD in the liver of Fah−/− Hpd−/− mice, by using a recombinant adenovirus (11), the result being a reopening of the tyrosine catabolic pathway in the liver at the step of oxidation of 4-hydroxyphenylpyruvate (Fig. 1a). This manipulation led to rapid and massive apoptosis of hepatocytes and death followed. This model system paved the way toward investigation of processes involved in the cellular injury seen in cases of HT1.
Much attention has been directed to investigations on the role of the mitochondria in apoptosis (19–26). The Bcl-2 family of proteins, predominantly localized in outer mitochondrial membranes, can inhibit permeability transition (19, 20) and the release of apoptogenic proteins—e.g., cytochrome c—from mitochondria (21–25). Cytochrome c is localized in the intermembrane space and on the surface of the inner mitochondrial membrane. Cytochrome c released from the mitochondria interacts with Apaf-1, caspase-9, and pro-caspase-3 to activate caspase-3 and the caspase cascade, leading to fragmentation of the nucleus (24–26). Release of cytochrome c from the mitochondria may be an early event leading to apoptotic cell death.
We now report that the release of cytochrome c from mitochondria precedes liver failure in the Fah−/− Hpd−/− mice. The addition of FAA induces the release of cytochrome c in a cell-free system. We propose that at least one of the signals for cell death in hepatocytes of HT1 is FAA. The liver failure noted in the Fah−/− Hpd−/− was prevented by previous administration of caspase inhibitors.
MATERIALS AND METHODS
Animals and Recombinant Viruses.
The HPD-deficient mice (III mice) (16–18) and Fah−/− Hpd−/− mice (11) have been described. The blood tyrosine ranges between 1100 and 1656 μM (mean 1275 μM, n = 21) in III mice, between 1186 and 2008 μM (mean 1597 μM, n = 13) in Fah−/− Hpd−/− mice, and under 300 μM in BALB/c control mice (n = 10). Immunoblotting for FAH and HPD from liver samples was carried out with rabbit antiserum directed to recombinant human FAH and HPD (11, 18).
Replication-defective recombinant adenoviruses, human adenovirus type 5 (Ad5) lacking the E1A, E1B, and E3 regions and bearing human FAH, HPD, or ornithine transcarbamoylase (OTC) were prepared, as described (18, 27, 28). Expression of HPD is driven by the CAG promoter (28, 29). The adenovirus was purified and titrated as described (30).
In Vitro Experiments.
Primary cultures of hepatocytes obtained from III and Fah−/− Hpd−/− mice were prepared as described by Ueno et al. (31). Cells were counted and placed at a density of 2 × 104 cells per well (2 cm2) of the 24-well plate in William’s medium E (WE) supplemented with 10% fetal calf serum, 2.5 μM dexamethasone (Sigma), 1.0 μM insulin (Sigma), and 5 ng/ml epidermal growth factor (Wakunaga, Hiroshima, Japan). After plating, HGA was added (n = 10) to the culture medium, or cells were transfected with AdHPD (n = 8) for 1 h. Cells were incubated at 37°C for 24 h and harvested by trypsinization, and cell viability was assessed by the trypan blue exclusion method. For the DNA ladder apoptosis assay, chromosomal DNA was prepared from 1 × 106 cells and analyzed as described (32).
To determine whether retrieval of FAH function by transfection with AdFAH would contribute to HGA- or AdHPD-induced apoptosis, we transfected newly isolated hepatocytes with AdFAH (or AdOTC as a control) in a sterile plastic tube for 1 h at a multiplicity of infection (moi) of 5, then the cells were placed at a density of 2 × 104 cells per well (2 cm2) of the 24-well plate in the culture medium described above. After plating, HGA was added (n = 4) to the culture medium, or transfection with AdHPD (n = 4) was done for 1 h. After 24-h incubation, the viability was assessed. We next examined the effect of pretreated AdFAH at various moi values (0, 0.01, 0.1, 1, 10, and 100) in the presence or absence of treatment with 1 mM HGA or with AdHPD at moi 10.
To investigate the in vitro protective effects of apoptosis inhibitors, newly isolated hepatocytes were incubated for 1 h with Ac-Tyr-Val-Ala-Asp-CHO (YVAD) (n = 4) or Ac-Asp-Glu-Val-Asp-CHO (DEVD) (n = 4) (TaKaRa, Tokyo, Japan) at various concentrations (0, 0.1, 1, 10, 100, or 1000 μM), then were treated with 1 mM HGA or with AdHPD at moi 10.
Evaluation of Cytochrome c Release from Mitochondria.
Mice were killed, the livers were quickly removed, and mitochondria and S-100 cytosolic fraction (final volume, 7 ml) were prepared, as described by Schnaitman and Greenawalt (33). A part of the S-100 fraction was filtered through a regenerated cellulose membrane with a mesh size of 5000 (Centrex, Iwaki Glass, Funabashi, Japan) to remove macromolecules. Immunoblots for cytochrome c were carried out with a mAb (7H8.2C12; PharMingen), and mouse IgG was detected with horseradish peroxidase (HRP)-conjugated anti-mouse IgG by using enhanced chemiluminescence (ECL). For analysis of cytochrome c release from mitochondria in a cell-free system, 1 mg of control mitochondria was incubated with 20 μl of filtered S-100 fraction from the mice at 37°C for 2 h. After centrifugation at 7,000 × g at 4°C for 10 min, the supernatant was carefully separated (the first 10 μl was used and the remainder was discarded), then the mitochondrial pellet was suspended in 100 μl of buffer A (0.25 M sucrose/20 mM Hepes, pH 7.4). The supernatant (5 μl) and mitochondrial solution (10 μg of protein) were subjected to SDS/15% PAGE and immunoblot analysis for cytochrome c. To investigate the effect of FAA and HGA on mitochondrial cytochrome c, 1 mg of control mitochondria in 20 μl of buffer A was incubated with various concentrations of FAA and HGA at 37°C for 2 h. The supernatant (5 μl) and mitochondrial suspension (10 μg of protein) were carefully separated by centrifugation at 7,000 × g at 4°C for 10 min, then subjected to SDS/15% PAGE and immunoblot analysis for cytochrome c.
Animal Experiments.
Fah−/− Hpd−/− mice die from acute liver failure with massive apoptosis of hepatocytes within 24 h after intraperitoneal treatment with HGA (LD50 = 300 mg/kg of body weight). At a dose of 800 mg/kg of body weight, the mice died within several hours after the injection of HGA. To evaluate the effect of the caspase inhibitors YVAD or DEVD, mice were given YVAD intraperitoneally (10 μmol in 500 μl of PBS), and 2 h later these mice were given HGA intraperitoneally (800 mg/kg in 100 μl of PBS). The mice were killed 6 h after the HGA injection and tissues were examined histologically.
Biochemical Analysis.
Serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were quantified by using a standard clinical automatic analyzer. Urine succinylacetone was quantified in a stable isotope dilution gas chromatography/mass spectrometry assay (34).
Histological Examinations.
Livers were excised and immediately immersed into 10% neutralized formalin for 1 week prior to embedding, sectioning, and staining with hematoxylin and eosin. The TUNEL [terminal deoxynucleotidyltransferase (TdT)-mediated dUTP-digoxigenin nicked end labeling] procedure kit (ApopTag; Oncor, Gaithersburg, MD) was used to evaluate the ratio of apoptotic hepatocytes.
RESULTS
Apoptosis of Hepatocytes of Fah−/− Hpd−/− Mice.
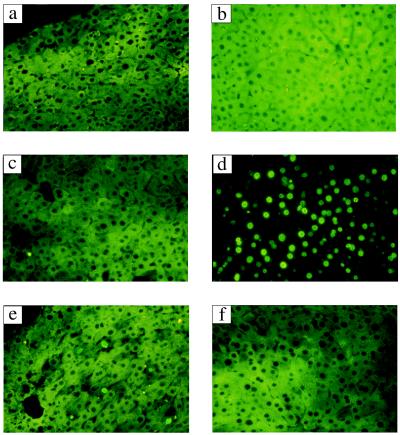
The Fah−/− Hpd−/− mice carried an albino deletion (7, 8, 12–14) on chromosome 7 that disrupted the Fah gene, and a nucleotide substitution in the Hpd gene (16–18) on chromosome 5 that resulted in premature termination of translation and skipping of the constitutive exon (Fig. 1 b–d). As a result, both FAH and HPD proteins in the liver were absent (Fig. 1 e and f). In these mice, the lethal phenotype of FAH deficiency completely disappeared with the introduction of HPD deficiency (Fig. 1a), and their phenotypes were similar to those of Hpd−/− mice (III mice). Acute liver failure in the Fah−/− Hpd−/− mice was achieved either by exogenous in vivo expression of HPD protein in the liver (18) or by administration of HGA (11). In liver sections, massive numbers of hepatocytes were nonviable with fragmentation of nuclei, and approximately 30–80% gave positive signals by in situ detection of DNA fragmentation (see Table 1 and Fig. 6d). Thus, Fah−/− Hpd−/− mice, which are phenotypically normal, can serve as a model of the visceral injuries of HT1 (11).
Table 1.
Effect of HGA and caspase inhibitors on biochemical data of serum and urine, and apoptosis of III and double-mutant mice
| Mice | n | AST, IU/liter | ALT, IU/liter | BUN, mg/dl | SA, nmol/mmol creatinine | Apoptosis of hepatocytes, % |
|---|---|---|---|---|---|---|
| HGA-untreated | ||||||
| III | 6 | 107.0 ± 40.5 | 43.7 ± 23.5 | 26.3 ± 3.7 | 65 | <1 |
| DM | 5 | 89.2 ± 11.5 | 28.8 ± 9.5 | 25.6 ± 2.2 | 150 | <1 |
| DM + YVAD | 1 | 142 | 48 | 39 | ND | <1 |
| HGA-treated | ||||||
| III | 7 | 115.9 ± 33.4 | 26.4 ± 4.2 | 20.4 ± 5.1 | 150 | <1 |
| DM | 7 | 2,509.9 ± 3,116.1 | 1,487.3 ± 2,691.0 | 75.86 ± 71.0 | 205,000 | 30–80 |
| DM + YVAD | 6 | 793.4 ± 467.4 | 505.6 ± 397.1 | 27.2 ± 10.5 | 14,300 | 2–4 |
| DM + DEVD | 2 | 539 | 126 | 23 | ND | <1 |
AST, aspartate aminotransferase; ALT, alanine aminotransferase; BUN, blood urea nitrogen; SA, succinylacetone in pooled urine; DM, double mutant; ND, not done.
Figure 6.
HGA-induced apoptosis of hepatocytes in the Fah−/− Hpd−/− mice and in vivo protective effect of caspase inhibitors. The liver sections were obtained from variously treated mice and investigated as described in the text. The controls were untreated III (a), III treated with HGA (b), and untreated Fah−/− Hpd−/− (c). The positive signals of in situ detection of DNA fragmentation were seen in 30–80% of hepatocytes in the sections from Fah−/− Hpd−/− mice treated with HGA (800 mg/kg body weight) (d), 2–4% of YVAD-pretreated Fah−/− Hpd−/− treated with HGA (e), and below 1% in DEVD-pretreated Fah−/− Hpd−/− treated with HGA (f), compared with below 1% of the controls (a, III without HGA; b, III with HGA; c, Fah−/− Hpd−/− without HGA.). (Original magnification, ×400.)
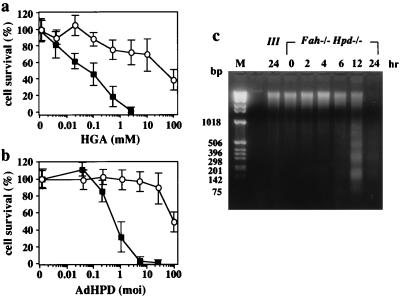
We asked whether primary cultured hepatocytes from III and Fah−/− Hpd−/− mice differed in their sensitivity to HGA (Fig. 2a). There were significant differences in the sensitivity to HGA of cultured hepatocytes from Fah−/− Hpd−/− and III mice. Nearly 90% of Fah−/− Hpd−/− hepatocytes were killed after 24 h incubation in the presence of as low as 1 mM HGA, whereas 70% of the III cells survived in more than 10 mM HGA. Time-dependent progress of apoptotic death of FAH-deficient hepatocytes was demonstrated in cultured cells from the Fah−/− Hpd−/− mice (Fig. 2c). Fragmentation of genomic DNA from the cultured hepatocytes of Fah−/− Hpd−/− mice was evident 6 h after the administration of HGA. A TUNEL assay showed that most of the dead cells treated with HGA were positive, indicating apoptosis (data not shown). We next investigated the sensitivity to recombinant adenovirus expressing human HPD (AdHPD) of primary cultured hepatocytes from III and Fah−/− Hpd−/− mice (Fig. 2b). In this experiment, cultured hepatocytes were infected 24 h after plating with AdHPD at moi 0 to 100. In the control experiments, 90% of the III cells survived, even when the moi exceeded 10. However, the Fah−/− Hpd−/− hepatocytes did not survive after infection with recombinant adenovirus when the moi was more than 10. The numbers of dead cells increased depending on the amounts of recombinant virus used in the experiments. These investigations indicated that reopening of the tyrosine catabolic pathway at the step of HPD in the cultured hepatocytes from Fah−/− Hpd−/− mice resulted in rapid apoptosis because of an intrinsic process of cell death.
Figure 2.
Effect of HGA or exogenous expression of human HPD on cell viability in primary cultured hepatocytes derived from III and Fah−/− Hpd−/− mice. (a) The primary cultured hepatocytes were incubated in medium containing various concentrations of neutralized HGA. The viability of cells was calculated 24 h after exposure to HGA. ○, Means of survival for hepatocytes from III mice (n = 4); ■, those from the Fah−/− Hpd−/− mice (n = 6). The bars represents SD. Fah−/− Hpd−/− hepatocytes are significantly vulnerable to HGA, in a dose-dependent manner, compared with control III hepatocytes. (b) The primary cultured hepatocytes were transfected with recombinant adenovirus bearing human Hpd cDNA (AdHPD) to express HPD exogenously. The primary cultured hepatocytes were treated with various amounts of AdHPD, and the viable cells were calculated 24 h after infection by the recombinant virus. ○, means of survival for hepatocytes from III mice (n = 4); ■, those from the Fah−/− Hpd−/− mice (n = 6). The Fah−/− Hpd−/− hepatocytes are significantly and dose-dependently vulnerable to the infection by AdHPD, compared with control III hepatocytes. (c) Time-dependent progress of apoptotic death of primary cultured hepatocytes from Fah−/− Hpd−/− mice in the presence of 1 mM HGA. DNA samples were prepared from the cells after appropriate intervals and analyzed as described in the text. Lane M, DNA size markers. Fragmentation of genomic DNA from the hepatocytes of Fah−/− Hpd−/− mice was evident 6 h after the administration of HGA.
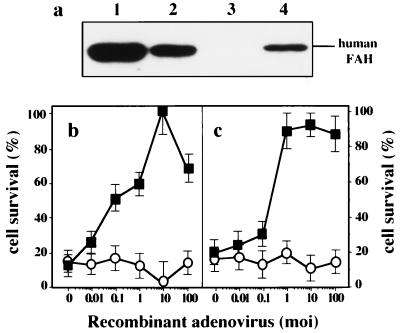
We then investigated whether exogenous expression of FAH in Fah−/− Hpd−/− hepatocytes would prevent HGA- or AdHPD-induced apoptosis (Fig. 3). The expression of human FAH in primary cultured cells was obtained by recombinant adenovirus expressing human FAH (AdFAH) (Fig. 3a). The Fah−/− Hpd−/− hepatocytes that express human FAH were resistant to the cell death induced by HGA (Fig. 3b) or AdHPD (Fig. 3c), compared with results obtained by using recombinant adenovirus expressing human ornithine transcarbamoylase (AdOTC) (see legend for Fig. 3). These results confirmed that cell death seen in the liver of Fah−/− Hpd−/− mice or cultured cells was due to an intrinsic process triggered by substance(s) related to FAH deficiency.
Figure 3.
Prevention of cell death by exogenous expression of FAH. (a) Exogenous expression of FAH by recombinant adenovirus bearing human FAH cDNA (AdFAH) in primary cultured hepatocytes. Lane 1, III cell; lane 2, Fah+/− Hpd−/− cell; Lane 3, Fah−/− Hpd−/− cell; and lane 4, Fah−/− Hpd−/− cells infected with AdFAH at moi 10. Twenty-four hours after infection, cells were harvested, sonicated, and subjected to SDS/PAGE. (b) Effect of AdFAH on HGA-induced apoptosis of primary cultured hepatocytes derived from Fah−/− Hpd−/− mice. The primary cultured hepatocytes were preinfected with AdFAH (■, n = 4) or AdOTC (○, n = 4), in various amounts. The viabilities of cultured hepatocytes, from either III or Fah−/− Hpd−/− mice, after treatment with various moi of AdFAH or AdOTC were similar (data not shown). Twenty-four hours after the infection of recombinant adenovirus, cell death was induced by 1 mM HGA. The viability of the cell was evaluated 24 h after the induction of cell death (adenovirus-infected but not HGA-treated control = 100%). The pretreatment of AdFAH protects Fah−/− Hpd−/− hepatocytes from HGA-induced apoptosis, in a dose-dependent manner. (c) Effect of AdFAH on AdHPD-induced apoptosis of primary cultured hepatocytes derived from Fah−/− Hpd−/− mice. The primary cultured hepatocytes were preinfected with AdFAH (■, n = 4) or AdOTC (○, n = 4), in various amounts. Twenty-four hours after treatment with the recombinant adenovirus, cell death was induced by infection with AdHPD at moi 10. Viability of cells was evaluated 24 h after the induction of cell death (AdFAH or AdOTC-infected but not HGA-treated control = 100%). Pretreatment with AdFAH protected Fah−/− Hpd−/− hepatocytes from AdHPD-induced apoptosis, in a dose-dependent manner.
Release of Cytochrome c from Mitochondria Precedes Liver Failure in Fah−/− Hpd−/− Mice.
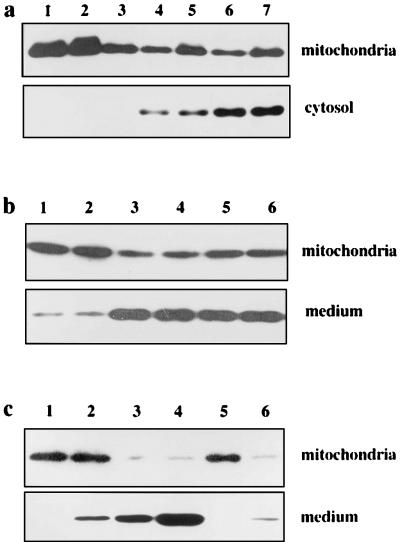
To investigate the status of cytochrome c in hepatocytes that undergo apoptosis, we injected HGA into the mice and examined the livers. One hour after the injection of lethal doses of HGA (800 mg/kg of body weight), there was a considerable release of cytochrome c into the cytosol of HGA-treated Fah−/− Hpd−/− mice (Fig. 4a) but no significant release from HGA-treated III mice or HGA-untreated Fah−/− Hpd−/− mice. Thus, the release of cytochrome c occurred as early as 1 h after the administration of HGA, and it apparently preceded the onset of apoptotic hepatocyte death and liver failure. These results suggest that the release of cytochrome c is a trigger leading to death of Fah−/− Hpd−/− hepatocytes after the administration of HGA.
Figure 4.
Cytochrome c release from mitochondria in vivo and in vitro. (a) Immunoblot analysis of cytochrome c in the liver of Fah−/− Hpd−/− mice. Livers from variously treated mice were resected, and immediately mitochondrial and S-100 cytosolic fractions were prepared and subjected to SDS/PAGE and immunoblotting. Lane 1, untreated III mouse treated with HGA; lane 2, III mouse treated with HGA; lane 3, untreated Fah−/− Hpd−/− mouse; lane 4, Fah−/− Hpd−/− mouse 1 h after treatment with HGA; lane 5, Fah−/− Hpd−/− mouse 6 h after treatment with HGA; lane 6, DEVD-pretreated HGA-treated Fah−/− Hpd−/− mouse; lane 7, YVAD-pretreated HGA-treated Fah−/− Hpd−/− mouse. While a small amount of cytochrome c was detected in the cytosolic fraction (lanes 1–3), significant amounts of cytochrome c were detected in each lane in the case of cytosolic fractions of livers from Fah−/− Hpd−/− mice treated with HGA (lanes 4–7). The amounts of cytosolic cytochrome c remained unchanged by pretreatment with caspase inhibitors DEVD or YVAD (lanes 6 and 7). (b) Cytochrome c is released from control mitochondria in a cell-free system by the addition of cytosolic fraction from the HGA-treated Fah−/− Hpd−/− mouse. Mitochondria (1 mg) from control mice were incubated with S-100 fractions prefiltered to remove cytochrome c and other proteins, as described in the text. After incubation, the mitochondria and the supernatants were separated by centrifugation and analyzed for cytochrome c, using immunoblotting. Lane 1, III mouse treated with HGA; lane 2, untreated Fah−/− Hpd−/− mouse; lane 3, Fah−/− Hpd−/− mouse 1 h after treatment with HGA; lane 4, Fah−/− Hpd−/− mouse 6 h after treatment with HGA; lane 5, DEVD-pretreated HGA-treated Fah−/− Hpd−/− mouse; lane 6, YVAD-pretreated HGA-treated Fah−/− Hpd−/− mouse. A significant release of cytochrome c from mitochondria into the medium was evident in the fractions from HGA-treated Fah−/− Hpd−/− mice, whether pretreated with caspase inhibitors or not (lanes 3, 4, 5, and 6). (c) Cytochrome c is released from mitochondria by purified FAA. Mitochondria (1 mg) from the control mouse were incubated in the presence of purified FAA. After the incubation, the supernatant was analyzed for cytochrome c. Lane 1, buffer A only; lane 2, 1 μM FAA; lane 3, 10 μM FAA; lane 4, 100 μM FAA; lane 5, 10 μM HGA; lane 6, 100 μM HGA. Incubation of the purified FAA with control mitochondria resulted in release of cytochrome c into the medium.
Cytochrome c Release from Mitochondria in a Cell-Free System.
Mitochondria from control mice were incubated with S-100 fractions from HGA-treated Fah−/− Hpd−/− mice or other control mice. The S-100 fractions were filtered to remove cytochrome c and other proteins prior to incubation. As shown in Fig. 4b, when the filtered S-100 fraction from HGA-treated Fah−/− Hpd−/− mice was incubated with control mitochondria, there was a significant release of cytochrome c into the medium. The filtered S-100 fractions from the HGA-treated control mice or untreated Fah−/− Hpd−/− mice showed no such effects (Fig. 4b). These investigations indicate that there is a low molecular weight substance(s) in the cytosol of the liver of HGA-treated Fah−/− Hpd−/− mice that induces the release of cytochrome c from mitochondria. HPLC analysis showed that FAA was present predominantly in the S-100 fraction. We next asked whether purified FAA would react with isolated mitochondria and cause a release of cytochrome c in a cell-free system. As shown in Fig. 4c, incubation of the purified FAA with control mitochondria resulted in a considerable release of cytochrome c into the medium. As FAA is a reagent that causes the release of cytochrome c from mitochondria, it is a candidate for the chemical that triggers apoptotic process in FAH-deficient hepatocytes.
Caspase Inhibitors Prevent Apoptotic Cell Death of Cultured Fah−/− Hpd−/− Hepatocytes.
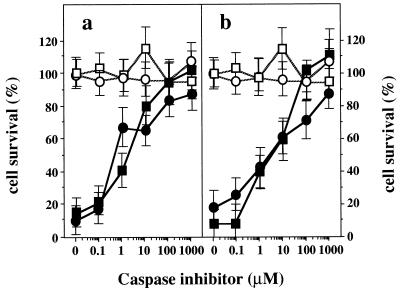
We also investigated the effects of caspase inhibitors on death of HGA- or AdHPD-treated hepatocytes. We used YVAD, which is a potent, selective, cell-permeating inhibitor of caspase-1, and DEVD, which specifically inhibits caspase-1 and caspase-3 (35, 36). In the absence of YVAD and DEVD, 80–90% of Fah−/− Hpd−/− hepatocytes underwent apoptosis 24 h after incubation with 1 mM HGA (Fig. 5a) or after infection of AdHPD at moi 10 (Fig. 5b). However, addition of YVAD or DEVD to the medium markedly decreased the HGA- or AdHPD-induced apoptosis in Fah−/− Hpd−/− hepatocytes. These effects were concentration dependent, and nearly 90% survived in the case of 1000 μM YVAD in the medium, and nearly 100% with 100 μM DEVD. Thus, these inhibitors block the cell death pathway triggered by the cytochrome c released from mitochondria into the cytosol by FAA. However, we did not examine durations of effects of these inhibitors exceeding 1 h. These data suggest that inhibition of this apoptotic process at the level of caspase can prevent the inevitable death of the Fah−/− Hpd−/− cells induced by FAA. Both YVAD and DEVD showed equivalent effects in preventing cell death in the culture system. Because use of either inhibitor led to nearly complete prevention of cell death, these reagents seem to function at the same step of the apoptotic process, or they act on the path of the same cascade in Fah−/− Hpd−/− cells.
Figure 5.
Prevention of cell death by treatment with caspase inhibitors. (a) Effect of caspase inhibitors on HGA-induced apoptosis of primary cultured hepatocytes derived from Fah−/− Hpd−/− mice. Two hours after the treatment with the inhibitors, cell death was induced by 1 mM HGA. Pretreatment with caspase inhibitors, YVAD (•, n = 4) or DEVD (■, n = 4), protected Fah−/− Hpd−/− hepatocytes from HGA-induced apoptosis in a dose-dependent manner. Open symbols indicate means of survival rate for hepatocytes treated with YVAD (○, n = 3) or DEVD (□, n = 3) without HGA. (b) Effect of caspase inhibitors on AdHPD-induced apoptosis of primary cultured hepatocytes derived from Fah−/− Hpd−/− mice. Two hours after treatment with the inhibitors, cell death was induced by infection with AdHPD at moi 10. Pretreatment with caspase inhibitors YVAD (•, n = 4) or DEVD (■, n = 4), protects Fah−/− Hpd−/− hepatocytes from AdHPD-induced apoptosis in a dose-dependent manner. Open symbols indicate means of survival for hepatocytes that were treated by YVAD (○, n = 3) or DEVD (□, n = 3) without AdHPD.
In Vivo Administration of Caspase Inhibitors Is Highly Effective in Reducing Liver Failure in Fah−/− Hpd−/− Mice.
We expected that in vivo administration of caspase inhibitors YVAD or DEVD would protect the mice from acute liver failure. To evaluate the in vivo effect of these inhibitors on prevention of liver failure, Fah−/− Hpd−/− mice were given 10 μmol of YVAD (n = 6) or DEVD (n = 2) intraperitoneally 2 h before injection of 800 mg/kg of body weight of HGA. All the treated animals were killed 6 h after the administration of HGA and examined for liver function (Table 1) and liver histology. The HGA-treated Fah−/− Hpd−/− mice showed extensive abnormalities in serum transaminases and blood urea nitrogen (BUN), whereas the untreated mice had normal values. However, preadministration of the inhibitor significantly suppressed elevation of the aspartate aminotransferase, alanine aminotransferase, and BUN in the Fah−/− Hpd−/− mice (Table 1). Nevertheless, urinary excretion of succinylacetone, which is derived from FAA and is a diagnostic marker of tyrosinemia type 1 (2, 3), markedly increased after injection of HGA. The levels of urinary succinylacetone in HGA-treated mice pretreated with YVAD were also high (not determined in DEVD-treated mice). Liver histology showed that previous administration of the inhibitor significantly reduced the numbers of apoptotic hepatocytes (Fig. 6). Although the long-term effects of a single administration of inhibitors remains to be determined, these in vivo observations do suggest that pretreatment with caspase inhibitors can prevent the liver injury induced by HGA in the Fah−/− Hpd−/− mice. Thus, inhibition of cell death processes is equivalent to the prevention of liver failure in the Fah−/− Hpd−/− mice.
DISCUSSION
Our previous observations that liver injury in FAH deficiency is associated with apoptotic death of hepatocytes suggested that an intrinsic reagent(s) derived from HGA triggers the process of apoptosis and that the apoptotic hepatocyte death is the central feature of liver disease in HT1 (11). In the present study, we reproduced apoptosis in primary cultured hepatocytes from Fah−/− Hpd−/− mice by retrieval of the tyrosine catabolic pathway at the step of HPD. The apoptosis of Fah−/− Hpd−/− cells was apparently due to an intrinsic process in hepatocytes. Cytochrome c is an essential macromolecule that initiates activation of the caspase cascade, leading to fragmentation of the nucleus (21–26). Release of cytochrome c from the mitochondria into the cytosol seems to be an essential step in initiating the process of apoptosis. This protein was released in the intrinsic process of apoptosis of Fah−/− Hpd−/− cells. In addition, the release of cytochrome c was caused by FAA, a primary metabolite associated with FAH deficiency and a candidate for causing visceral injury in HT1 (11). Effective treatment of an HT1 patient with 2-(2-nitro-4-trifluoromethylbenzoyl)-1,3-cyclohexanedione (NTBC) (2, 3, 37), which inhibits HPD activity, and complete rescue of Fah−/− mice by null mutation of HPD (11) support this concept. We found here that FAA directly interacted with the mitochondria and induced the release of cytochrome c. On the basis of these observations, we propose that FAA is at least one agent responsible for apoptotic hepatocyte death in FAH deficiency.
If the attack against mitochondria by FAA and the subsequent activation of the caspase cascade are essential events in the cellular injury in FAH deficiency, inhibition of the caspase cascade should modify the liver phenotype of Fah−/−. Indeed, caspase inhibitors effectively prevented death of Fah−/− Hpd−/− cells. In addition, the HGA-induced liver failure was prevented by the caspase inhibitor YVAD. Thus, activation of the caspase cascade plays an essential role in liver injury in FAH deficiency. In other words, the challenge directed to the mitochondria by FAA can be separated from the subsequent cell death process. While FAA with its alkylating potential (2, 7) seems to attack membranes of various cellular components, our observations suggest that a critical part of liver injury in FAH deficiency is activation of the cell death process. FAA seems to be a major metabolite responsible for the cell death signal via mitochondria.
HT1 is a complex disease (2, 3), in which many cellular events are affected, including inhibition of enzyme activity (4), aberrant gene expression (5–8), mutagenesis (9), and carcinogenesis (2, 9, 10), in addition to cell death. Among these events, inhibition of δ-aminolevulinate dehydratase is caused by direct interaction of the enzyme protein with succinylacetone (38), a derivative of FAA. Mutagenesis and carcinogenesis may be related to possible DNA damage, perhaps caused by FAA (7). Apoptosis followed by severe DNA damage has been observed in many cell types subjected to various insults (39, 40). Cell death processes induced by the accumulation of FAA seem to be rapid. Because it is unlikely that DNA damage by FAA induces the apoptosis of hepatocytes in FAH deficiency, we speculate that FAA might directly attack the mitochondria, leading to apoptosis (short-term effect) and to DNA damage (long-term effect).
The clinical features of HT1 are not unique. There are several metabolic liver diseases that are difficult to distinguish from HT1 without specific tests. These similarities include the liver pathology. Diseases such as galactosemia type I and hereditary fructose intolerance are examples. In addition, other diseases related to neonatal liver failure syndrome show similar clinical and pathological findings (41). These disorders are often characterized by progressive loss of hepatocytes without elevation of transaminases and impaired synthesis of blood proteins. In this context, modifications of cell death processes of metabolic liver diseases by caspase inhibitors, as shown in the present study, may have clinical significance.
Acknowledgments
We are grateful to R. J. Desnick (Department of Human Genetics, Mount Sinai School of Medicine) for critical review of this manuscript, R. Tasaki (Chemo-Sero-Therapeutic Research Institute, Kumamoto, Japan) for amino acid analysis, T. Kubo (Kumamoto University) for technical assistance with the histological tissue preparations, and M. Ohara for reading the manuscript. This work was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, Sports and Culture of Japan and by a grant from the Ministry of Health and Welfare (Japan).
ABBREVIATIONS
- HT1
hereditary tyrosinemia type 1
- HPD
4-hydroxyphenylpyruvate dioxygenase
- HGA
homogentisic acid
- FAA
fumarylacetoacetate
- FAH
fumarylacetoacetate hydrolase
- moi
multiplicity of infection
- YVAD
Ac-Tyr-Val-Ala-Asp-CHO
- DEVD
Ac-Asp-Glu-Val-Asp-CHO
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Phaneuf D, Lambert M, Laframboise R, Mitchell G, Lettre F, Tanguay R M. J Clin Invest. 1992;90:1185–1192. doi: 10.1172/JCI115979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mitchell G A, Lambert M, Tanguay R M. In: The Metabolic and Molecular Basis of Inherited Disease. 7th Ed. Scriver C R, Beaudet A L, Sly W S, Valle D, editors. New York: McGraw-Hill; 1995. pp. 1077–1106. [Google Scholar]
- 3.Holme E, Lindstedt S. Curr Opin Pediatr. 1995;7:726–732. doi: 10.1097/00008480-199512000-00017. [DOI] [PubMed] [Google Scholar]
- 4.Lindblad B, Lindstedt S, Steen G. Proc Natl Acad Sci USA. 1977;74:4641–4645. doi: 10.1073/pnas.74.10.4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Awata H, Endo F, Matsuda I. Genomics. 1994;23:534–539. doi: 10.1006/geno.1994.1540. [DOI] [PubMed] [Google Scholar]
- 6.Haber B A, Chuang E, Lee W, Taub R. Hepatology. 1996;24:65–71. doi: 10.1002/hep.510240113. [DOI] [PubMed] [Google Scholar]
- 7.Ruppert S, Kelsey G, Schedl A, Schmid E, Thies E, Schütz G. Genes Dev. 1992;6:1430–1443. doi: 10.1101/gad.6.8.1430. [DOI] [PubMed] [Google Scholar]
- 8.Tönjes R R, Xanthopoulos K G, Darnell J E, Jr, Paul D. EMBO J. 1992;11:127–133. doi: 10.1002/j.1460-2075.1992.tb05035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kvittingen E A, Rootwelt H, Berger R, Brandtzaeg P. J Clin Invest. 1994;94:1657–1661. doi: 10.1172/JCI117509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grompe M, Lindstedt S, al-Dhalimy M, Kennaway N G, Papaconstantinou J, Torres-Ramos C A, Ou C N, Finegold M. Nat Genet. 1995;10:453–460. doi: 10.1038/ng0895-453. [DOI] [PubMed] [Google Scholar]
- 11.Endo F, Kubo S, Awata H, Kiwaki K, Katoh H, Kanegae Y, Saito I, Miyazaki J, Yamamoto T, Jakobs C, Hattori S, Matsuda I. J Biol Chem. 1997;272:24426–24432. doi: 10.1074/jbc.272.39.24426. [DOI] [PubMed] [Google Scholar]
- 12.Gluecksohn-Waelsch S. Cell. 1979;16:225–237. doi: 10.1016/0092-8674(79)90001-1. [DOI] [PubMed] [Google Scholar]
- 13.Klebig M L, Russell L B, Rinchik E M. Proc Natl Acad Sci USA. 1992;89:1363–1367. doi: 10.1073/pnas.89.4.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kelsey G, Ruppert S, Beermann F, Grund C, Tanguay R M, Schütz G. Genes Dev. 1993;7:2285–2297. doi: 10.1101/gad.7.12a.2285. [DOI] [PubMed] [Google Scholar]
- 15.Grompe M, al-Dhalimy M, Finegold M, Ou C N, Burlingame T, Kennaway N G, Soriano P. Genes Dev. 1993;7:2298–2307. doi: 10.1101/gad.7.12a.2298. [DOI] [PubMed] [Google Scholar]
- 16.Katoh H, Endo F, Suzuki K, Matsuda I. Mouse Genome. 1991;89:572. [Google Scholar]
- 17.Endo F, Katoh H, Yamamoto S, Matsuda I. Am J Hum Genet. 1991;48:704–709. [PMC free article] [PubMed] [Google Scholar]
- 18.Kubo S, Kiwaki K, Awata H, Katoh H, Kanegae Y, Saito I, Yamamoto T, Miyazaki J, Matsuda I, Endo F. Human Gene Ther. 1997;8:65–71. doi: 10.1089/hum.1997.8.1-65. [DOI] [PubMed] [Google Scholar]
- 19.Kroemer G. Nat Med. 1997;3:614–620. doi: 10.1038/nm0697-614. [DOI] [PubMed] [Google Scholar]
- 20.Reed J C. Nature (London) 1997;387:773–776. doi: 10.1038/42867. [DOI] [PubMed] [Google Scholar]
- 21.Yang J, Liu X, Bhalla K, Kim C N, Ibrado A M, Cai J, Peng T I, Jones D P, Wang X. Science. 1997;275:1129–1132. doi: 10.1126/science.275.5303.1129. [DOI] [PubMed] [Google Scholar]
- 22.Kluck R M, Wetzel E B, Green D R, Newmeyer D D. Science. 1997;275:1132–1136. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- 23.Liu X, Kim C N, Yang J, Jemmerson R, Wang X. Cell. 1996;86:147–157. doi: 10.1016/s0092-8674(00)80085-9. [DOI] [PubMed] [Google Scholar]
- 24.Zou H, Henzel W J, Liu X, Lutschg A, Wang X. Cell. 1997;90:405–413. doi: 10.1016/s0092-8674(00)80501-2. [DOI] [PubMed] [Google Scholar]
- 25.Li P, Nijhawan D, Budihardjo I, Srinivasula S M, Ahmad M, Alnemri E S, Wang X. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 26.Liu X, Zou H, Slaughter C, Wang X. Cell. 1997;89:175–184. doi: 10.1016/s0092-8674(00)80197-x. [DOI] [PubMed] [Google Scholar]
- 27.Kiwaki K, Kanegae Y, Saito I, Komaki S, Nakamura K, Miyazaki J, Endo F, Matsuda I. Human Gene Ther. 1996;7:821–830. doi: 10.1089/hum.1996.7.7-821. [DOI] [PubMed] [Google Scholar]
- 28.Miyazaki J, Takai S, Araki K, Tashiro F, Tominaga A, Takatsu K, Yamamura K. Gene. 1989;79:269–277. doi: 10.1016/0378-1119(89)90209-6. [DOI] [PubMed] [Google Scholar]
- 29.Niwa H, Yamamura K, Miyazaki J. Gene. 1991;108:193–199. doi: 10.1016/0378-1119(91)90434-d. [DOI] [PubMed] [Google Scholar]
- 30.Kanegae Y, Makimura M, Saito I. Jpn J Med Sci Biol. 1994;47:157–166. doi: 10.7883/yoken1952.47.157. [DOI] [PubMed] [Google Scholar]
- 31.Ueno T, Miyamura T, Saito I, Mizuno K. Hum Cell. 1993;6:126–135. [PubMed] [Google Scholar]
- 32.Ni R, Tomita Y, Matsuda K, Ichihara A, Ishimura K, Ogasawara J, Nagata S. Exp Cell Res. 1994;215:332–337. doi: 10.1006/excr.1994.1349. [DOI] [PubMed] [Google Scholar]
- 33.Schnaitman C, Greenawalt J W. J Cell Biol. 1968;38:158–175. doi: 10.1083/jcb.38.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jakobs C, Dorland L, Wikkerink B, Kok R M, de Jong A P, Wadman S K. Clin Chim Acta. 1988;171:223–231. doi: 10.1016/0009-8981(88)90147-7. [DOI] [PubMed] [Google Scholar]
- 35.Thornberry N A, Bull H G, Calaycay J R, Chapman K T, Howard A D, Kostura M J, Miller D K, Molineaux S M, Weidner J R, Aunins J, et al. Nature (London) 1992;356:768–774. doi: 10.1038/356768a0. [DOI] [PubMed] [Google Scholar]
- 36.Nicholson D W, Ali A, Thornberry N A, Vaillancourt J P, Ding C K, Gallant M, Gareau Y, Griffin P R, Labelle M, Lazebnik Y A, et al. Nature (London) 1995;376:37–43. doi: 10.1038/376037a0. [DOI] [PubMed] [Google Scholar]
- 37.Lindstedt S, Holme E, Lock E A, Hjalmarson O, Strandvik B. Lancet. 1992;340:813–817. doi: 10.1016/0140-6736(92)92685-9. [DOI] [PubMed] [Google Scholar]
- 38.Sassa S, Kappas A. J Clin Invest. 1983;71:625–634. doi: 10.1172/JCI110809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maltzman W, Czyzyk L. Mol Cell Biol. 1984;4:1689–1694. doi: 10.1128/mcb.4.9.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kastan M B, Onyekwere O, Sidransky D, Vogelstein B, Craig R W. Cancer Res. 1991;51:6304–6311. [PubMed] [Google Scholar]
- 41.Shneider B L. Curr Opin Pediatr. 1996;8:495–501. doi: 10.1097/00008480-199610000-00013. [DOI] [PubMed] [Google Scholar]