Abstract
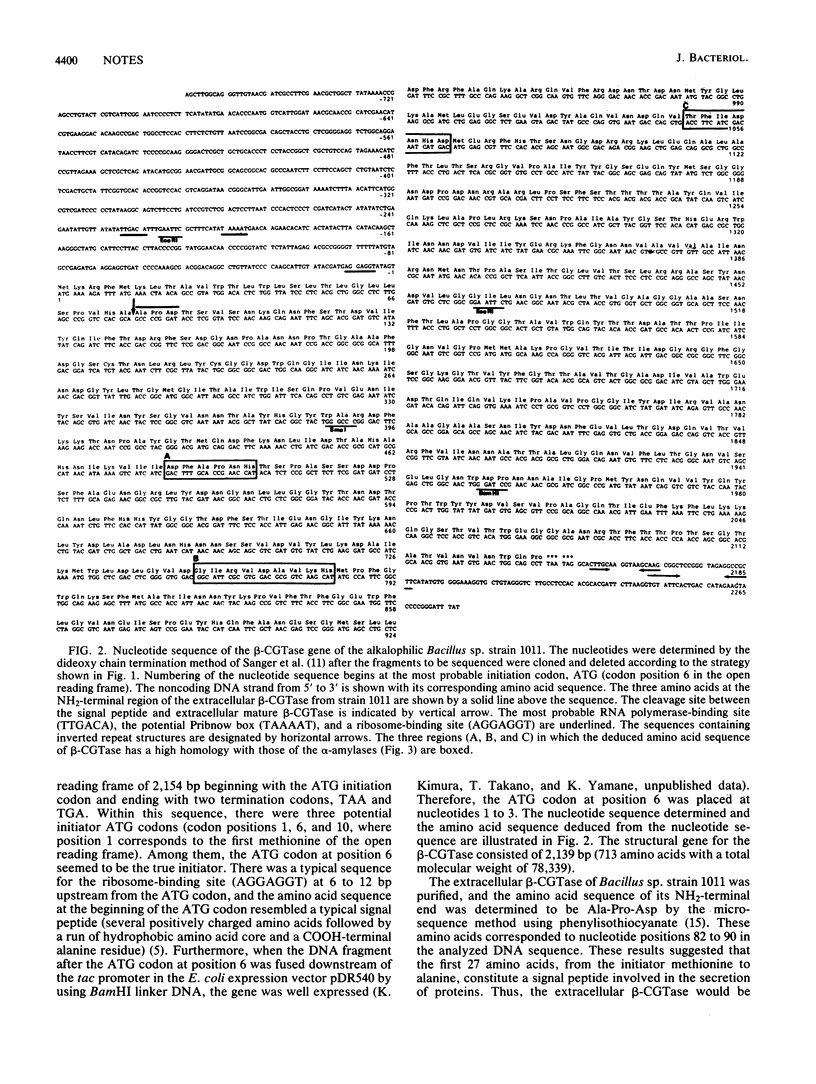
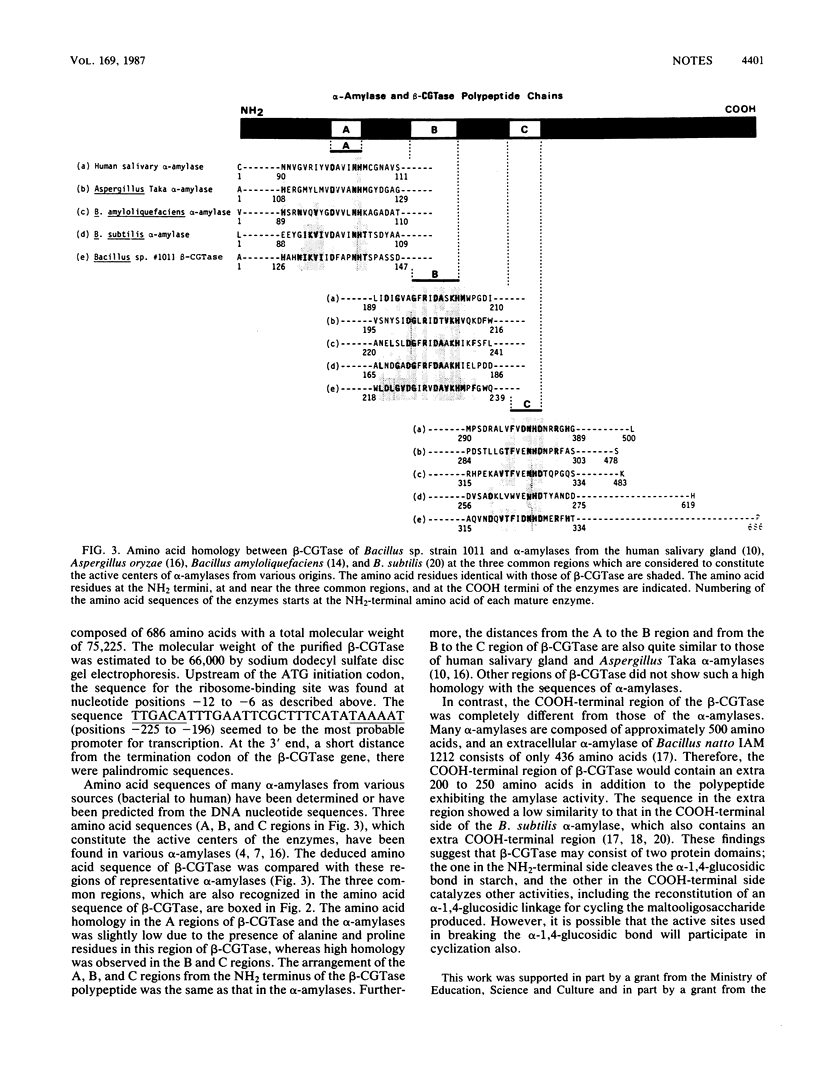
The nucleotide sequence of the gene for cyclodextrin glucanotransferase of alkalophilic Bacillus sp. strain 1011 was determined. The deduced amino acid sequence at the NH2-terminal side of the enzyme showed a high homology with the sequences of alpha-amylase in the three regions which constitutes the active centers of alpha-amylases.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRENCH D. The Schardinger dextrins. Adv Carbohydr Chem. 1957;12:189–260. doi: 10.1016/s0096-5332(08)60209-x. [DOI] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Ihara H., Sasaki T., Tsuboi A., Yamagata H., Tsukagoshi N., Udaka S. Complete nucleotide sequence of a thermophilic alpha-amylase gene: homology between prokaryotic and eukaryotic alpha-amylases at the active sites. J Biochem. 1985 Jul;98(1):95–103. doi: 10.1093/oxfordjournals.jbchem.a135279. [DOI] [PubMed] [Google Scholar]
- Inouye M., Halegoua S. Secretion and membrane localization of proteins in Escherichia coli. CRC Crit Rev Biochem. 1980;7(4):339–371. doi: 10.3109/10409238009105465. [DOI] [PubMed] [Google Scholar]
- Kobayashi S., Kainuma K., Suzuki S. Purification and some properties of Bacillus macerans cycloamylose (cyclodextrin) glucanotransferase. Carbohydr Res. 1978 Mar;61:229–238. doi: 10.1016/s0008-6215(00)84484-5. [DOI] [PubMed] [Google Scholar]
- Matsuura Y., Kusunoki M., Harada W., Kakudo M. Structure and possible catalytic residues of Taka-amylase A. J Biochem. 1984 Mar;95(3):697–702. doi: 10.1093/oxfordjournals.jbchem.a134659. [DOI] [PubMed] [Google Scholar]
- Matsuzaki H., Yamane K., Yamaguchi K., Nagata Y., Maruo B. Hybrid alpha-amylases produced by transformants of Bacillus subtilis. I. Purification and characterization of extracellular alpha-amylases produced by the parental strains and transformants. Biochim Biophys Acta. 1974 Sep 13;365(1):235–247. doi: 10.1016/0005-2795(74)90268-2. [DOI] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Nishide T., Emi M., Nakamura Y., Matsubara K. Corrected sequences of cDNAs for human salivary and pancreatic alpha-amylases [corrected]. Gene. 1984 May;28(2):263–270. doi: 10.1016/0378-1119(84)90265-8. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabak H. F., Flavell R. A. A method for the recovery of DNA from agarose gels. Nucleic Acids Res. 1978 Jul;5(7):2321–2332. doi: 10.1093/nar/5.7.2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano T., Fukuda M., Monma M., Kobayashi S., Kainuma K., Yamane K. Molecular cloning, DNA nucleotide sequencing, and expression in Bacillus subtilis cells of the Bacillus macerans cyclodextrin glucanotransferase gene. J Bacteriol. 1986 Jun;166(3):1118–1122. doi: 10.1128/jb.166.3.1118-1122.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takkinen K., Pettersson R. F., Kalkkinen N., Palva I., Söderlund H., Käriäinen L. Amino acid sequence of alpha-amylase from Bacillus amyloliquefaciens deduced from the nucleotide sequence of the cloned gene. J Biol Chem. 1983 Jan 25;258(2):1007–1013. [PubMed] [Google Scholar]
- Tarr G. E. Improved manual sequencing methods. Methods Enzymol. 1977;47:335–357. doi: 10.1016/0076-6879(77)47036-8. [DOI] [PubMed] [Google Scholar]
- Yamane K., Hirata Y., Furusato T., Yamazaki H., Nakayama A. Changes in the properties and molecular weights of Bacillus subtilis M-type and N-type alpha-amylases resulting from a spontaneous deletion. J Biochem. 1984 Dec;96(6):1849–1858. doi: 10.1093/oxfordjournals.jbchem.a135019. [DOI] [PubMed] [Google Scholar]
- Yang M., Galizzi A., Henner D. Nucleotide sequence of the amylase gene from Bacillus subtilis. Nucleic Acids Res. 1983 Jan 25;11(2):237–249. doi: 10.1093/nar/11.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]



