Abstract
Infection by the opportunistic bacterial pathogen Shigella flexneri stimulates tyrosine phosphorylation of host cell proteins, but the kinases involved and their effects on the regulation of cell signaling pathways during bacterial entry remain largely undefined. Here, we demonstrate a requirement for the Abl family of tyrosine kinases during Shigella internalization. Family members Abl and Arg are catalytically activated upon Shigella infection, accumulate at the site of bacterial entry, and are required for efficient bacterial uptake, as internalization is blocked upon targeted deletion of these kinases or treatment with a specific pharmacological inhibitor. We identify the adapter protein Crk as a target for Abl kinases during Shigella uptake, and show that a phosphorylation-deficient Crk mutant significantly inhibits bacterial uptake. Moreover, we define a novel signaling pathway activated during Shigella entry that links Abl kinase phosphorylation of Crk to activation of the Rho family GTPases Rac and Cdc42. Together, these findings reveal a new role for the Abl kinases, and suggest a novel approach to treatment of Shigella infections through inhibition of host cell signaling pathways.
Keywords: Abl tyrosine kinases/Cdc42/Crk/Rac/Shigella flexneri
Introduction
Multiple bacterial pathogens have evolved mechanisms that engage intracellular signaling pathways in the host cell to achieve successful infection (Zaharik et al., 2002). Among these pathogens is the Gram-negative bacterium Shigella flexneri, the etiologic agent for the diarrheal disease shigellosis (Sansonetti, 2001). A key step in the pathogenesis of shigellosis is the ability of the bacteria to enter the normally non-phagocytic cells of the colonic mucosa. At the site of Shigella entry, the host actin cytoskeleton undergoes dramatic changes, including the formation of filopodia and lamellipodia, which are subsequently organized into long, actin-rich extensions that engulf the invading bacterium (Adam et al., 1995). The changes in the actin cytoskeleton observed during Shigella infection are mediated by virulence plasmid-expressed bacterial effectors that are part of a Type III Secretion System (TTSS) that is activated following contact between the bacterium and the host cells. The TTSS inserts a pore complex, comprised of Shigella proteins IpaB and IpaC, into the host cell plasma membrane that allows for delivery of other bacterial effector proteins into the host cell (Tran Van Nhieu et al., 1997; Blocker et al., 1999; Niebuhr et al., 2000; Zaharik et al., 2002). A key event during the initial phase of infection is the induction of actin polymerization at the site of Shigella contact with the host cell membrane, inducing massive cytoskeletal rearrangements, and the formation of actin foci at the sites of the invading bacteria (Adam et al., 1995). The insertion of Shigella IpaC into the membrane results in changes in the actin cytoskeleton, characteristic of the activation of the Rho family GTPases Cdc42 and Rac (Tran Van Nhieu et al., 1999). Both Cdc42 and Rac localize to the site of bacterial entry, and their activation has been shown to be required for efficient uptake of Shigella (Mounier et al., 1999; Shibata et al., 2002). The tyrosine kinase Src is also translocated to the site of the invading bacterium, and is thought to act as both a positive and negative regulator of the entry process. Src exerts its positive role by promoting the formation of actin foci, but it also acts negatively to down-regulate Rho (Dumenil et al., 1998, 2000). However, the role of tyrosine phosphorylation in the uptake of S.flexneri has not been fully explored, and the link between tyrosine kinases and Rho GTPase-dependent actin polymerization during this process has yet to be defined.
The Abl tyrosine kinase has been shown to regulate Rac-dependent cytoskeletal dynamics in mammalian cells (Plattner et al., 1999, 2003), suggesting that Abl kinases may play a role in bacterial uptake. The mammalian Abl family of tyrosine kinases is comprised of Abl and Arg (Abl2), and has been implicated in the regulation of cell proliferation, survival, adhesion and migration (Pendergast, 2002). While the functions of the constitutively active chimeric oncoprotein Bcr-Abl have been well described, the cellular functions of Abl and Arg have remained elusive. Genetic studies have implicated Abl and Arg in the regulation of cytoskeletal dynamics. Drosophila melanogaster that lack Abl exhibit defects in growth cone motility, axon guidance and epithelial cell polarity (Pendergast, 2002). The defective growth cone phenotype is similar to that of Drosophila lacking profilin, a protein known to be involved in cytoskeletal dynamics (Wills et al., 1999). A similar phenotype is observed in flies expressing dominant negative Cdc42, or mutants of Trio, a guanine nucleotide exchange factor for Rac and Rho (Wills et al., 1999; Bateman et al., 2000; Liebl et al., 2000). Since Rho family GTPases have been shown to regulate the formation of F-actin structures such as filopodia and lamellipodia, these observations suggest that Drosophila Abl may regulate cytoskeletal reorganization and cell motility. Mice lacking Abl and Arg also exhibit cytoskeletal defects, resulting in delayed closure of the neural tube, and death before embryonic day 11 (Koleske et al., 1998). Normal neuroepithelium display an ordered pattern of actin filaments at their apical surface, where Abl and Arg are normally located. In the Abl/Arg null mice, this apical actin latticework pattern is absent, and unorganized bundles of actin filaments are found at the basolateral surface of the cell (Koleske et al., 1998). Moreover, we have shown that Abl is required for formation of Rac-dependent lamellipodia and chemotaxis in response to PDGF (Plattner et al., 1999, 2003). These properties of the normal Abl family tyrosine kinases are consistent with the observed changes in the actin cytoskeleton of Bcr-Abl-expressing cells. Expression of Bcr-Abl induces the formation of filopodia and lamellipodia, and extension of pseudopods onto a fibronectin matrix (Salgia et al., 1997). These cytoskeletal effects are the result of the increased tyrosine kinase activity of Bcr-Abl, and are reversed in the presence of Abl kinase inhibitors (Gaston et al., 2000). Furthermore, Abl and Arg are unique among all known tyrosine kinases in that they contain a C-terminal actin binding domain, and have been shown to have actin bundling activity (Wang et al., 2001; Pendergast, 2002). Altogether, the Abl kinases are uniquely suited to link extracellular stimuli, such as infection by bacterial pathogens, to reorganization of the actin cytoskeleton. In this study, we demonstrate a requirement for Abl and Arg in S.flexneri infection, and link the requirement for Abl kinase activity to the activation of the Rho family GTPases Cdc42 and Rac during bacterial uptake.
Results
Abl family kinases are required for uptake of S.flexneri
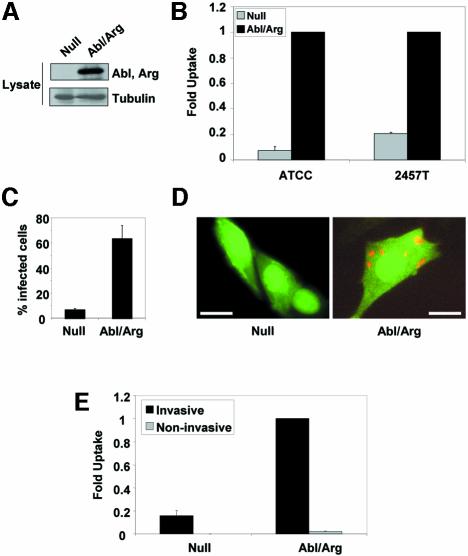
To determine whether the Abl kinases are involved in the uptake of S.flexneri, we employed mouse embryo fibroblasts (MEFs) that lack both Abl and Arg. These cells were reconstituted with either vector alone (Null) or with Abl and Arg (Abl/Arg), to levels similar to those of endogenous Abl and Arg proteins (Plattner et al., 2003) (Figure 1A; Supplementary figure 1 available at The EMBO Journal Online). We compared the ability of these cell lines to internalize two different strains of S.flexneri by the gentamicin protection assay (Figure 1B). A 93% decrease in the number of intracellular bacteria (S.flexneri strain ATCC® serotype 2a) was observed in cells lacking Abl and Arg (P = 0.001). The Null cells also exhibited a 79% decrease in uptake using the more invasive S.flexneri strain 2457T (P = 0.0002). The reconstituted Abl/Arg MEFs internalized S.flexneri 2457T to the same level as wild-type MEFs (Supplementary figure 1). We also examined bacterial uptake in these cell lines by immunofluorescence microscopy, and quantitated the percentage of cells containing intracellular Shigella. The Null cells exhibited a 90% decrease in the number of infected cells, compared with the reconstituted Abl/Arg cells (P < 0.0001) (Figure 1C). Notably, while most of the Abl/Arg cells engulfed several bacteria, the large majority of Null cells remained uninfected (Figure 1D). To demonstrate that the bacterial uptake observed in these MEF cell lines was dependent upon the TTSS, we compared the level of uptake of an invasive strain of Shigella 2457T with a non-invasive strain that has lost the virulence plasmid (Supplementary figure 2). While the invasive strain readily infected the Abl/Arg-expressing MEFs, the level of uptake of the non-invasive strain was negligible in these cells (Figure 1E). Additionally, there was no significant difference in the low level of uptake between the Null and Abl/Arg cells using the non-invasive strain (Figure 1E). These observations demonstrate that Abl and Arg are specifically required for TTSS-mediated internalization of Shigella.
Fig. 1. Abl and Arg are required for Shigella internalization. (A) MEFs from mice lacking both Abl and Arg were reconstituted with either vector alone (Null) or with Abl and Arg expression constructs (Abl/Arg). The expression of Abl and Arg was confirmed by western blotting with anti-Abl 8E9, which recognizes the catalytic domain of both Abl and Arg. Anti-β-tubulin immunoblotting was used to demonstrate equal protein loading. (B) Null (gray bars) or Abl/Arg cells (black bars) were infected with S.flexneri strains ATCC® serotype 2a (ATCC) or 2457T, and bacterial uptake was measured by the gentamicin protection assay. Results shown correspond to three independent experiments, each performed in triplicate. (C) Null or Abl/Arg cells plated on coverslips were infected with S.flexneri 2457T, and incubated with gentamicin to eliminate extracellular bacteria. The percentage of infected cells was quantitated by immunofluorescence microscopy. (D) Null or Abl/Arg cells (both GFP-positive, due to stable expression of MIGR1 plasmids) plated on coverslips were infected with Shigella 2457T and incubated with gentamicin to eliminate extracellular bacteria. Cells were immunostained with anti-GFP (green) and anti-Shigella (red) antibodies, and visualized by immunofluorescence microscopy. Calibration bars = 50 µm. (E) Null or Abl/Arg cells were infected with either invasive (black bars) or plasmid-cured, non-invasive (gray bars) variants of S.flexneri 2457T, and bacterial uptake was measured by the gentamicin protection assay.
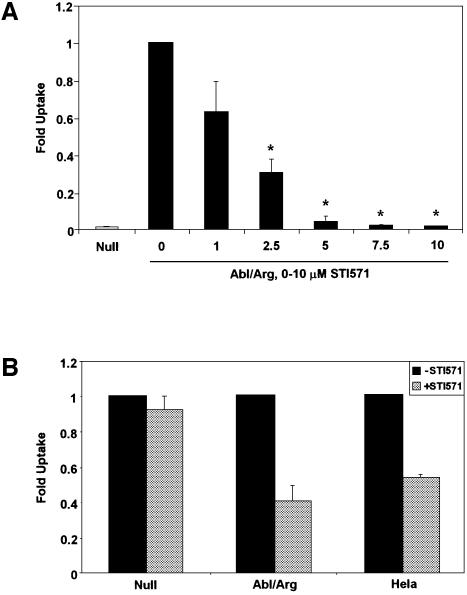
To determine whether the catalytic activities of Abl and Arg are required for bacterial uptake, we used a specific inhibitor of the Abl family kinases, STI571, also known as Gleevec™ (Druker et al., 2001). This compound does not inhibit other non-receptor tyrosine kinases, including Src (Buchdunger et al., 2002; Nagar et al., 2003). Abl/Arg-expressing cells were infected with S.flexneri ATCC® serotype 2a in the presence of increasing concentrations of STI571. We observed a dose-dependent decrease in the ability of Abl/Arg cells to be infected with Shigella (Figure 2A). At the 5 μM concentration of STI571, the level of Shigella uptake was reduced to that of the Null MEFs, and the 10 µM concentration inhibited internalization by 98%, compared with untreated cells (P < 0.0001). We also examined the ability of STI571 to inhibit cellular uptake by the more invasive S.flexneri strain 2457T. Addition of 10 μM STI571 resulted in a 60% reduction in uptake of S.flexneri 2457T into Abl/Arg cells (P = 0.0243), and a 47% reduction in HeLa cells (P = 0.0018), demonstrating that the observed effect is not cell-type specific (Figure 2B). Additionally, STI571 treatment had no significant effect on bacterial uptake in the Null cells (Figure 2B), suggesting that the specific targets of this inhibitor are Abl and Arg. Taken together, these findings show that efficient S.flexneri uptake requires functional Abl family tyrosine kinases.
Fig. 2. Abl and Arg kinase activities are required for Shigella uptake. (A) MEFs lacking (Null, gray bar) or expressing Abl and Arg (Abl/Arg, black bars) were infected with S.flexneri ATCC® serotype 2a in the presence of 0–10 µM STI571, and bacterial uptake was measured by the gentamicin protection assay. The asterisks represent concentrations of STI571 at which the decrease in uptake is statistically significant (P < 0.05). (B) MEFs either lacking (Null) or re-expressing Abl and Arg (Abl/Arg) and HeLa cells were infected with S.flexneri 2457T in the absence (black bars) or presence (gray bars) of 10 µM STI571, and bacterial uptake was measured by the gentamicin protection assay. Results shown correspond to three independent experiments, each performed in triplicate, and are normalized with respect to the 0 µM STI571 treatment.
Abl family kinases are activated during Shigella uptake
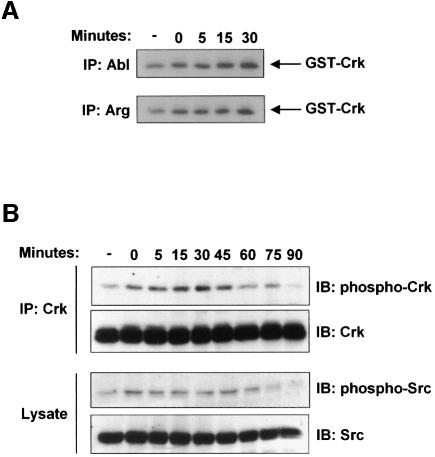
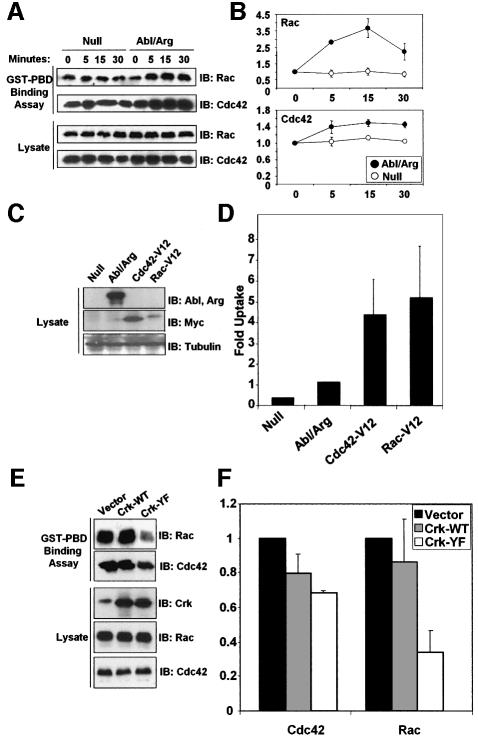
The ability of STI571 to prevent uptake of S.flexneri suggested that the Abl family tyrosine kinases might become catalytically activated during infection. To test this hypothesis, we analyzed Abl and Arg kinase activity in cells infected with S.flexneri from 0 to 30 min, the length of time required for bacterial entry into the cell (Dehio et al., 1995). Both Abl and Arg were catalytically activated during infection by S.flexneri, with their activities increasing >2-fold over uninfected cells at the 30 min timepoint (Figure 3A). Another approach to examine the activation of the Abl family kinases is through analysis of the phosphorylation state of the adapter protein Crk at tyrosine 221. Abl is known to specifically phosphorylate this site on Crk, resulting in a conformational change, thereby altering the ability of Crk to interact with other signaling effectors (Feller et al., 1994). Crk was immunoprecipitated from lysates of cells at various stages of infection by S.flexneri, and the immunoprecipitates were analyzed with an antibody that specifically recognizes phosphorylation of Crk at tyrosine 221 (Figure 3B, upper panels). We observed an increase in tyrosine phosphorylation of Crk at tyrosine 221, with maximal phosphorylation occurring at the 30 min timepoint. The Src tyrosine kinase has been previously demonstrated to have a role in Shigella uptake (Dumenil et al., 1998), and has been functionally linked to Abl activation in response to growth factors (Plattner et al., 1999). Thus, we examined the activation of endogenous Src during Shigella infection by immunoblotting with an antibody that recognizes the activated form of Src (Figure 3B, lower panels). The activation of endogenous Src followed an equivalent time course as the activation of endogenous Abl and Arg, and Src and the Abl kinases were catalytically activated to a similar extent.
Fig. 3. Abl and Arg are catalytically activated during Shigella infection. (A) Abl and Arg were immunoprecipitated from lysates of NIH-3T3 cells that were either uninfected (-) or infected with S.flexneri 2457T for 0–30 min. The immunoprecipitates were used in an in vitro kinase assay, using GST–Crk as a substrate. (B) NIH-3T3 cells were infected with S.flexneri 2457T for 0–90 min. Anti-Crk immunoprecipitates were examined by immunoblotting with anti-phospho-Crk-Y221 or anti-Crk (upper panels). Total lysates were examined by immunoblotting with anti-phospho-Src-Y418 or anti-Src (lower panels).
Tyrosine phosphorylation of Crk is required for Shigella uptake
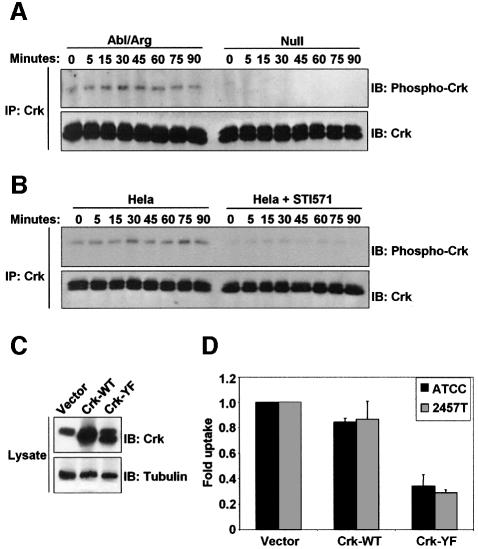
Phosphorylation of Crk at tyrosine 221 has been observed following stimulation by growth factors and integrins, and has been shown to regulate the activity of Rac (Abassi and Vuori, 2002). In these systems, Abl and Arg have been shown to specifically phosphorylate tyrosine 221 on Crk (Kain and Klemke, 2001). Our observation that Crk becomes tyrosine phosphorylated during Shigella infection suggests that Crk may play a role in the signaling pathways mediating bacterial uptake downstream of Abl family kinases. We sought to determine whether phosphorylation of Crk was reduced in Shigella-infected cells lacking Abl kinase activity, and whether tyrosine phosphorylation of Crk during infection was required for the cellular uptake of Shigella. To this end, we examined the tyrosine phosphorylation levels of the adapter protein Crk during Shigella infection in cells either lacking or re-expressing both Abl and Arg. Crk was immunoprecipitated from Null and Abl/Arg cells at various time points during Shigella infection, and its tyrosine phosphorylation state was examined by immunoblotting. The phosphorylation of Crk at tyrosine 221 was completely ablated in cells lacking Abl and Arg (Figure 4A). A decrease in tyrosine phosphorylation of Crk was observed during infection of HeLa cells that were pre-treated with STI571 (Figure 4B). These data demonstrate that Abl and Arg are required for phosphorylation of Crk tyrosine 221 during S.flexneri infection, and suggest that Crk phosphorylation plays a role during bacterial uptake. To determine whether phosphorylation of Crk at tyrosine 221 is required during Shigella infection, we expressed either wild-type chicken Crk (Crk-WT) or a Crk mutant containing a tyrosine to phenylalanine substitution at position 222 (Crk-YF), which corresponds to tyrosine 221 in the human and murine forms of Crk (Figure 4C). We examined the effect of expression of these Crk constructs on bacterial internalization using the gentamicin protection assay. Expression of wild-type Crk did not significantly reduce bacterial uptake, compared with the vector control using either strain of Shigella. However, expression of Crk-YF resulted in a 65–70% inhibition of bacterial uptake of both Shigella strains (Figure 4D). These data demonstrate that phosphorylation of Crk by the Abl family kinases is an essential step for efficient Shigella internalization.
Fig. 4. Phosphorylation of Crk by the Abl kinases is required for Shigella internalization. (A) Cells lacking (Null) or re-expressing Abl and Arg (Abl/Arg) were infected with S.flexneri 2457T for noted times. Anti-Crk immunoprecipitates were examined by immunoblotting with anti-phospho-Crk-Y221 (upper panel) and anti-Crk (lower panel). (B) HeLa cells were serum-starved for 3 h in the presence or absence of STI571, and infected for the indicated times with S.flexneri 2457T. Anti-Crk immunoprecipitates were examined by immunoblotting with anti-phospho-Crk-Y221 (upper panel) and anti-Crk (lower panel). (C) MIGR1 vector, Crk-WT, and Crk-Y222F were introduced into NIH-3T3 cells. Crk expression was analyzed by immunoblotting with anti-Crk (upper panel). Anti-β-tubulin immunoblotting was used to assess equal protein loading (lower panel). (D) NIH-3T3 cells expressing MIGR1 vector, Crk-WT, or Crk-Y222F were infected with S.flexneri strains ATCC® serotype 2a (black bars) or 2457T (gray bars), and bacterial uptake was measured by the gentamicin protection assay. Results shown correspond to three independent experiments, each performed in triplicate.
Cdc42 and Rac activation is regulated by Abl kinases during Shigella infection
Abl has been previously linked to the Rho family GTPases, through genetic studies in Drosophila, and loss-of-function studies in mammalian fibroblasts (Plattner et al., 1999, 2003; Pendergast, 2002). Additionally, phosphorylation of the Abl substrate Crk at tyrosine 221 modulates the ability of Crk to interact with other signaling effectors, and has been shown to regulate the localization of Rac, and Rac-dependent signaling (Abassi and Vuori, 2002). Our observations that Abl-mediated tyrosine phosphorylation of Crk is required for bacterial uptake led us to hypothesize that Abl and Arg might be functionally linked to the activation of the Rho family GTPases during Shigella infection. To test this hypothesis, we analyzed the levels of activated Rac and Cdc42 during Shigella infection of cells either lacking or re-expressing Abl and Arg. In Abl/Arg-expressing cells, Shigella infection increased Rac and Cdc42 activities, peaking at an average of 3.7- and 1.5-fold, respectively, over uninfected cells. In contrast, this increase in Rac and Cdc42 activity was not observed in cells lacking Abl and Arg (Figure 5A and B). These data suggest that the Abl family kinases mediate the activation of the Rho family GTPases during Shigella infection. A prediction from this finding is that expression of activated forms of Cdc42 and Rac in cells lacking Abl and Arg would rescue the ability of the Null cells to engulf bacteria. Indeed, we observed a dramatic increase in the ability of the Null cells to internalize Shigella in the presence of activated Cdc42 and Rac (Figure 5C and D). These data demonstrate that activation of the Rho family GTPases can compensate for the loss of Abl and Arg during Shigella infection. Since Crk phosphorylation by the Abl family kinases is a major signaling event regulating bacterial uptake (Figure 4D), we examined whether the Crk-Y221F mutant had an effect on the activation of Rac and Cdc42 during Shigella infection. Indeed, Crk-Y221F expression reduced Cdc42 and Rac activation by 32 and 66%, respectively, while expression of Crk-WT had no significant effect (Figure 5E and F). Taken together, these observations define a signaling pathway activated during Shigella infection that connects the Abl family kinases to tyrosine phosphorylation of Crk and to the activation of Rac and Cdc42.
Fig. 5. The Abl kinases act upstream of activation of Cdc42 and Rac during Shigella infection. (A) Cells lacking (Null) or re-expressing Abl and Arg (Abl/Arg) were infected with S.flexneri 2457T for noted times. Lysates were incubated with GST–PBD to precipitate GTP-bound Cdc42 and Rac, and the bound proteins were analyzed by immunoblotting with anti-Rac and anti-Cdc42 (upper panels). Cellular lysates were examined by immunoblotting with anti-Rac and anti-Cdc42 (lower panels). (B) The GST–PBD binding assays to assess Rac (upper panel) or Cdc42 (lower panel) activation in the Null and Abl/Arg cells were quantitated by densitometry. Results correspond to three independent experiments. (C) Activated forms of Cdc42 and Rac (Cdc42-V12 and Rac-V12) were introduced into cells lacking Abl and Arg. Expression of Abl and Arg was analyzed by western blotting with anti-Abl 8E9 (upper panel). Expression of myc-tagged Cdc42-V12 and Rac-V12 was analyzed by immunoblotting with anti-myc (middle panel). Anti-β-tubulin immunoblotting was used to assess equal protein loading (lower panel). (D) Null, Abl/Arg and Null cells expressing Cdc42-V12, and Rac-V12 were infected with S.flexneri, and bacterial uptake was analyzed by the gentamicin protection assay. Results represent three independent experiments, each performed in triplicate. (E) NIH-3T3 cells expressing MIGR1 vector, Crk-WT or Crk-Y222F were infected with S.flexneri 2457T for 30 min, and analyzed for Cdc42 and Rac activities using the GST–PBD binding assay, as in (A). (F) The GST–PBD binding assays to assess Cdc42 or Rac activities in cells expressing Crk-WT or Crk-Y222F were quantitated by densitometry. Results correspond to three independent experiments.
Abl, Arg and Crk localize to the sites of Shigella entry within the host cell
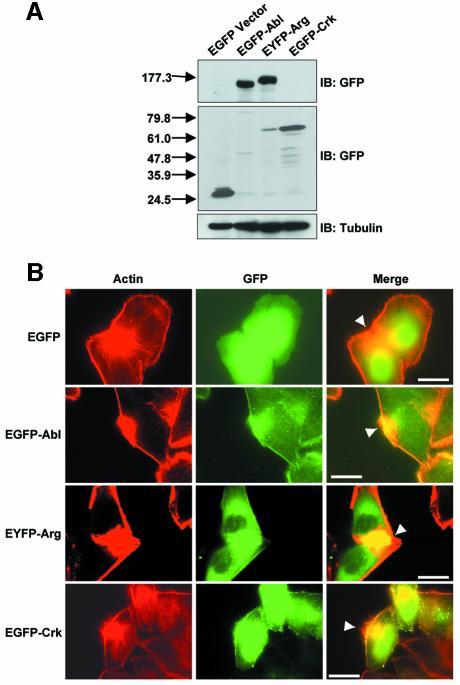
We have identified a novel signaling pathway activated during Shigella infection that involves the Abl family kinases and the adapter protein Crk. To visualize the participation of these signaling molecules in Shigella internalization, we used immunofluorescence microscopy to localize Abl, Arg and Crk to sites of bacterial entry. At the onset of Shigella infection, the activation of host cell signaling pathways results in the formation of actin foci at sites of bacterial entry (Adam et al., 1995). Co-localization with these large clusters of actin has been used to implicate a number of host cell signaling molecules in Shigella uptake, including Src and the Rho family GTPases (Dehio et al., 1995; Adam et al., 1996; Mounier et al., 1999; Dumenil et al., 2000). We used a similar approach to localize Abl, Arg and Crk to sites of bacterial entry, employing fluorescently-tagged versions of these signaling proteins. HeLa cells were transfected with EGFP vector, EGFP–Abl, EYFP–Arg or EGFP–Crk (Figure 6A), and infected with S.flexneri 2457T prior to fixation. The cells were immunostained with DAPI to localize the bacteria (data not shown), rhodamine–phalloidin to label the actin (Figure 6B, left panels) and anti-GFP to label the EGFP/EYFP–tagged proteins (Figure 6B, middle panels). Abl and Arg both localized at the cell periphery, and were concentrated at sites of bacterial entry (Figure 6B, middle and right panels). Crk localized to the cell periphery in a more punctate pattern, and was also enriched at the actin foci (Figure 6B, middle and right panels). These patterns of localization were not observed in cells expressing GFP alone, demonstrating that the localization of Abl, Arg and Crk was specific (Figure 6B, uppermost panels). The findings that the Abl kinases and Crk localize to sites of bacterial entry provide further support for a role of these signaling molecules in Shigella internalization.
Fig. 6. Abl, Arg, and Crk localize to the site of bacterial entry. (A) HeLa cells were transfected with EGFP vector, EGFP–Abl, EYFP–Arg or EGFP–Crk, as noted. Cells were lysed and analyzed for fusion protein expression by immunoblotting with anti-GFP (upper panels). Anti-β-tubulin immunoblotting was used to assess equal protein loading (lower panel). (B) HeLa cells expressing EGFP vector, EGFP–Abl, EYFP–Arg, or EGFP–Crk were infected with S.flexneri for 30 min, and analyzed by immunofluorescence microscopy. Sites of bacterial entry were identified by staining the bacteria with DAPI (data not shown) and staining the actin foci with rhodamine–phalloidin (red, left panels). Abl, Arg and Crk localization was performed by staining with anti-GFP (green, middle panels). These images were merged to show co-localization of Abl, Arg and Crk with the focus of actin at the site of bacterial entry (yellow, right panels). The sites of actin foci formation and co-localization with Abl, Arg and Crk are noted by the arrowheads. Calibration bar (shown in the right panel only) = 50 µm.
Discussion
This study reveals a novel role for the Abl tyrosine kinases in bacterial pathogenesis. Here we demonstrate a requirement for the Abl family of tyrosine kinases in the cellular uptake of S.flexneri. Additionally, the Abl kinases are catalytically activated during the initial stages of Shigella infection, and mediate the tyrosine phosphorylation of the adapter protein Crk, an event that contributes to efficient Shigella uptake. We have also demonstrated that Abl family kinases and Crk accumulate at the site of bacterial entry. Moreover, we define a signaling pathway triggered by bacterial infection, that leads to the catalytic activation of Abl and Arg tyrosine kinases, phosphorylation of Crk, and activation of the Rho family GTPases Cdc42 and Rac.
Previously, the cytoplasmic pool of Abl and Arg has been implicated in signaling pathways downstream of growth factor receptors, such as PDGF (Plattner et al., 1999). The signaling pathways activated during the initial stages of infection by S.flexneri are strikingly similar to those involved in growth factor receptor signaling. Src is activated by both growth factor stimulation and S.flexneri infection, as are the Rho family GTPases (Dumenil et al., 1998; Mounier et al., 1999). In this study, we demonstrate that the Abl kinase, another component of the PDGF receptor signaling pathway, is also activated during Shigella infection. Following stimulation of the PDGF receptor, Abl activity increases 3-fold (Plattner et al., 1999), which is similar to the level of activation of both Abl and Arg during Shigella infection (Figure 3A). This increase in Abl kinase activity is likely to reflect the localized activation of Abl kinases at specific subcellular compartments, such as the site of bacterial entry, or at the membrane in growth factor-stimulated cells. Cells lacking Abl exhibit a dramatic reduction in membrane ruffling in response to PDGF, indicating that small changes in overall Abl kinase activity are sufficient to mediate the cellular response to extracellular stimuli (Plattner et al., 1999). Here, we have shown that the induction of Abl and Arg kinase activity is essential for efficient Shigella infection, since disruption of these kinases either by targeted deletion or pharmacological inhibition interferes with bacterial uptake. A recent manuscript has provided further support for the requirement of Abl kinase activity during Shigella invasion, by reporting that inhibition of PLCγ, a downstream target of the PDGF receptor, with the PLCγ inhibitor U73122 blocks signaling pathways induced during Shigella invasion (Tran Van Nhieu et al., 2003). Our laboratory recently identified a link between PLCγ and Abl kinase activation, and demonstrated that the U73122 inhibitor blocks the catalytic activation of Abl following stimulation of the PDGF receptor (Plattner et al., 2003). These observations further support a link between the Abl family kinases and signaling events that occur during Shigella infection.
We have identified the adapter protein Crk as a downstream target of Abl and Arg kinase activity during Shigella infection. Phosphorylation of Crk at tyrosine 221 by Abl during cell spreading and migration has been well documented (Escalante et al., 2000; Kain and Klemke, 2001). Prior to our findings, Crk had not been identified as a target of tyrosine kinases during Shigella infection. The fact that this site remains unphosphorylated in cells lacking the Abl family kinases demonstrates that Crk is a major target of Abl and Arg during Shigella infection (Figure 4A). Additionally, we have shown that phosphorylation of Crk by Abl kinases promotes Shigella internalization, since expression of a Crk mutant that can no longer be phosphorylated by Abl causes a reduction in bacterial uptake. Previous studies have demonstrated that Crk mediates the activation of Rac downstream of growth factors and integrins, and that expression of dominant negative mutants of Crk inhibits Rac-dependent cell processes such as cell migration and lamellipodia formation (Abassi and Vuori, 2002). Here, we provide a new link between Crk phosphorylation and activation of the Rho GTPases, by showing that the Crk-Y221F mutant inhibits Rac and Cdc42 activation during Shigella infection (Figure 5). Our data suggest that Crk is a component of the host cell signaling pathway that mediates bacterial uptake, linking upstream signals from the Abl kinases to GTPases during Shigella infection.
Previous studies using genetics and cell biology have suggested links between the Abl kinases and cellular processes regulated by the Rho family GTPases Rac and Cdc42 (Plattner et al., 1999, 2003; Pendergast, 2002). However, these studies did not show a requirement for the Abl kinases in the activation of Rac and Cdc42 in response to extracellular stimuli. Here, we demonstrate that the Abl family kinases are required for the activation of Rac and Cdc42 during cellular infection by S.flexneri. Cells lacking Abl and Arg are unable to activate endogenous Rac and Cdc42 in response to Shigella infection. In contrast, cells expressing Abl and Arg exhibit a 3.7- and 1.5-fold activation of Rac and Cdc42, respectively. These increases are consistent with the 1.7-fold activation of Rac observed following stimulation of the PDGF receptor (Hawkins et al., 1995). Previous studies have demonstrated a requirement for Rac and Cdc42 during Shigella uptake using expression dominant negative mutants, but activation of the endogenous GTPases was not determined (Mounier et al., 1999; Dumenil et al., 2000). Here, we measure the activation of endogenous Rac and Cdc42 during Shigella infection, and demonstrate that the Abl kinases mediate this response. Furthermore, we show that expression of activated Rac and Cdc42 can rescue the ability of cells lacking Abl and Arg to engulf S.flexneri. In contrast, expression of these mutants in wild-type cells has no effect on Shigella uptake (data not shown), consistent with previous findings (Mounier et al., 1999). Expression of the activated GTPases in the Null cells may compensate for the lack of inducible Rac and Cdc42 activity exhibited by cells lacking Abl and Arg (Figure 5). Together, these observations indicate that Abl and Arg are upstream components in the signaling pathway regulating the activation of Cdc42 and Rac. Indeed, the requirement for Abl and Arg during Shigella entry is similar to that of Rac and Cdc42. Expression of dominant negative forms of Rac and Cdc42 in HeLa cells reduced the levels of Shigella internalization by 68–74% (Mounier et al., 1999). Similarly, MEFs derived from Cdc42 knockout mice exhibit a 68–85% reduction in Shigella entry, compared with wild-type fibroblasts (Shibata et al., 2002). Abl/Arg-null fibroblasts exhibit a 79–93% decrease in Shigella infection, depending on the strain employed. While the activities of both Rac and Cdc42 have been shown to be required for the uptake of S.flexneri, the mechanism of their activation has not been fully explored (Mounier et al., 1999). The data presented here support a model whereby activation of the Rho family GTPases during Shigella internalization is preceded by the activation of the Abl family kinases, and the tyrosine phosphorylation of Crk.
The requirement for Abl and Arg during S.flexneri infection suggests that these kinases could be potential targets for antimicrobial therapy. Antibiotic resistance to shigellae is widespread, and the development of novel strategies to treat shigellosis is imperative (Sack et al., 1997). Our findings suggest that inhibition of Abl and Arg with STI571 may be used as novel strategy to treat Shigella infections. STI571, also known as Gleevec™, was approved by the Federal Drug Administration in 2001, and has been successful in the treatment of Bcr-Abl-positive chronic myelogenous leukemia patients with minimal side effects (Druker et al., 2001). Inhibition of the Abl kinases represents a unique approach to antimicrobial therapy, as it targets host cell proteins, rather than the infectious agent itself. This potential strategy for antimicrobial treatment awaits further inquiry.
Materials and methods
Bacterial strains and infections
The S.flexneri serotype 2a strain was obtained from the American Type Culture Collection. The S.flexneri 2457T strain was a generous gift from M.Goldberg (Harvard University). The non-invasive strain was created by plasmid curing the 2457T strain at 4°C for three months, isolating white colonies on Congo Red agar plates, and analyzing the strain for HeLa cell infectivity and presence of the virulence plasmid (Supplementary figure 2). All strains were grown on tryptic soy broth (TSB) agar plates and liquid cultures. For infection, overnight cultures of S.flexneri were grown in TSB, diluted 1:100, and grown to mid-logarithmic phase (OD600 = 0.3).
Antibodies and chemical reagents
Anti-Arg antiserum was generated by injection of rabbits with a peptide comprised of a sequence unique to the Arg C-terminus (DKDRPRRVKPK). The following antibodies were obtained from commercial sources: anti-Abl 8E9 (BD Pharmingen), anti-Abl K12, anti-GFP, anti-myc 9E10, anti-Src, horseradish peroxidase-linked goat anti-mouse IgG (Santa Cruz Biotechnology), anti-Cdc42, anti-Crk, anti-Rac (BD Transduction Laboratories), anti-Shigella (Maine Biotechnology Services), anti-phospho-Crk Y221 (Cell Signaling Technology), anti-phosphotyrosine (Upstate Biotechnology), anti-phospho-Src Y418 (Biosource), anti-β-tubulin (Sigma). Protein A–Sepharose, Protein G–Sepharose, horseradish peroxidase-linked protein A, and the ECL western blotting reagents were obtained from Amersham Biosciences. Rhodamine–phalloidin, DAPI, and Cy2- and Cy3-conjugated secondary antibodies were obtained from Molecular Probes. STI571 was a generous gift from B. Druker (Oregon Health Sciences University).
DNA constructs
The pCan-Cdc42-V12 and pExv-Rac-V12 constructs were provided by A. Abo (Onyx Pharmaceuticals). The Cdc42 and Rac coding sequences were subcloned into the bicistronic retroviral vector MIGR1 (Pear et al., 1998). The MIGR1-c-Abl construct was previously described (Plattner et al., 1999). The PK1-Arg expression construct was previously described (Plattner et al., 2003). The chicken Crk constructs were a generous gift of R. Tsien (University of California, San Diego). The Crk coding sequences were amplified by PCR and subcloned into MIGR1 and pEGFP (Clontech). The GST–PBD construct was provided by K. Burridge (University of North Carolina). The EGFP–Abl construct was provided by J.V. Small (Austrian Academy of Sciences). The EYFP–Arg construct (Wang et al., 2001) was provided by A. Koleske (Yale University).
Cell culture
MEFs from mice doubly null for Abl and Arg were kindly provided by A. Koleske (Yale University), and were maintained in Dulbecco’s modified eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS) (Koleske et al., 1998). PK1-Arg was introduced into the MEFs by transfection and selection of a puromycin-resistant population. MIGR1 vector and MIGR1-c-Abl were introduced into null or Arg-expressing MEFs by retroviral infection, as described (Plattner et al., 2003). MIGR1-Cdc42-V12 and MIGR1-Rac-V12 were introduced into the MEFs by retroviral infection, and GFP-positive cells were selected, as described (Plattner et al., 1999). HeLa cells were obtained from the Cell Culture Facility at the Duke Comprehensive Cancer Center, and were maintained in DMEM supplemented with 10% FBS. NIH-3T3 cells were provided by C. Der (University of North Carolina), and were maintained in DMEM supplemented by 10% calf serum (Hyclone). MIGR1-Crk constructs were introduced into NIH-3T3 cells by retroviral infection, as described (Plattner et al., 1999). HeLa cells were transfected using Lipofectamine (Invitrogen) according to the manufacturer’s instructions.
Invasion assays
The gentamicin protection assay was performed as described (Elsinghorst, 1994). Briefly, mid-logarithmic phase bacteria were pelleted, and resuspended in DMEM containing 10% FBS and 50 mM HEPES pH 7.3. The bacteria were overlayed onto a cell monolayer at a multiplicity of infection of 50, and the infection was initiated by centrifuging the plates at 700 g for 10 min. The plates were transferred to a 37°C incubator for a 30–120 min invasion incubation. The cell monolayers were washed, and media containing 50 µg/ml gentamicin was added for 2 h. The cells were lysed with 1% Triton X-100, and diluted lysates were plated on TSB agar plates. The results presented are compiled from three independent experiments, each performed in triplicate. Shigella uptake was measured by dividing the number of internalized bacteria by the number of input bacteria (c.f.u./input). The fold uptake is a normalization of this calculation for each cell type or experimental condition. Analysis of invasion by immunofluorescence microscopy was performed as described (Shibata et al., 2002).
Immunoprecipitation and in vitro kinase assays
Mid-logarithmic phase bacteria were resuspended in serum-free DMEM containing 50 mM HEPES pH 7.3 and overlayed onto a cell monolayer. The plates were incubated at room temperature for 10 min, and transferred to a 37°C incubator for noted times. The 0 timepoint represents cells that were incubated with bacteria at room temperature for 10 min, but not transferred to 37°C. The cells were washed with cold PBS, and lysed as for the in vitro kinase assay (Plattner et al., 1999). Lysates were incubated with noted antibodies, and immunoprecipitated with protein A- or protein G–Sepharose. To infect cells for the in vitro kinase assay, bacteria were resuspended as above, overlayed onto a cell monolayer, and centrifuged at 700 g for 10 min. The plates were transferred to a 37°C incubator for the noted times, and processed for use in the in vitro kinase assay, as described (Plattner et al., 1999).
Cdc42 and Rac activation assays
The GST–PBD binding assay to assess endogenous Cdc42 and Rac activation was essentially performed as described (Bagrodia et al., 1998). In brief, the cells were infected with S.flexneri 2457T for 0–30 min as described above, washed with cold HEPES-buffered saline (HBS), and lysed in a modified RIPA buffer (1% NP-40, 500 mM NaCl, 0.5% DOC, 0.1% SDS, 50 mM Tris pH 8.0, 10 mM MgCl2) containing protease and phosphatase inhibitors. Equal amounts of lysate were incubated with 20 µg GST–PBD for 30 min, and the beads were washed 3–5 times with HBS wash buffer (1% NP-40, 120 mM NaCl, 20 mM HEPES, 10 mM MgCl2). The samples were separated by SDS–PAGE, transferred to nitrocellulose and immunoblotted with either anti-Rac or anti-Cdc42. Cell lysates were examined by immunoblotting to demonstrate equal levels of total Rac and Cdc42 for each sample. Rac and Cdc42 activities were analyzed using densitometry and quantitated using ImageQuant software.
Immunofluorescence microscopy
Cells plated on coverslips were infected with S.flexneri 2457T as described, washed with ice-cold PBS, and fixed in 4% paraformaldehyde. Cells were lysed in 0.5% Triton X-100 in 4% paraformaldehyde, washed with PBS, and incubated in block (2% BSA in PBS). Antibodies were diluted in block as follows: anti-GFP (1:100), anti-Shigella (1:20 000), rhodamine–phalloidin (1:1000), DAPI (1:100 000), Cy2-anti-mouse secondary (1:100), Cy3-anti-rabbit secondary (1:2000). Samples were viewed at 63× magnification on a Zeiss Axioskop microscope, and analyzed using Metamorph software (Universal Imaging).
Supplementary data
Supplementary data are available at The EMBO Journal Online.
Acknowledgments
Acknowledgements
We thank Dr A.R.Means, Dr X.F.Wang and Dr P.A.Zipfel for critically reading the manuscript. This work was supported by N.I.H. grants CA70940 and GM62375 (A.M.P). E.A.B. is a Fellow of The Leukemia & Lymphoma Society (5912-01).
References
- Abassi Y.A. and Vuori,K. (2002) Tyrosine 221 in Crk regulates adhesion-dependent membrane localization of Crk and Rac and activation of Rac signaling. EMBO J., 21, 4571–4582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam T., Arpin,M., Prevost,M.C., Gounon,P. and Sansonetti,P.J. (1995) Cytoskeletal rearrangements and the functional role of T-plastin during entry of Shigella flexneri into HeLa cells. J. Cell Biol., 129, 367–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam T., Giry,M., Boquet,P. and Sansonetti,P. (1996) Rho-dependent membrane folding causes Shigella entry into epithelial cells. EMBO J., 15, 3315–3321. [PMC free article] [PubMed] [Google Scholar]
- Bagrodia S., Taylor,S.J., Jordon,K.A., Van Aelst,L. and Cerione,R.A. (1998) A novel regulator of p21-activated kinases. J. Biol. Chem., 273, 23633–23636. [DOI] [PubMed] [Google Scholar]
- Bateman J., Shu,H. and Van Vactor,D. (2000) The guanine nucleotide exchange factor trio mediates axonal development in the Drosophila embryo. Neuron, 26, 93–106. [DOI] [PubMed] [Google Scholar]
- Blocker A., Gounon,P., Larquet,E., Niebuhr,K., Cabiaux,V., Parsot,C. and Sansonetti,P. (1999) The tripartite type III secreton of Shigella flexneri inserts IpaB and IpaC into host membranes. J. Cell Biol., 147, 683–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchdunger E., O’Reilly,T. and Wood,J. (2002) Pharmacology of imatinib (STI571). Eur. J. Cancer, 38, S28–S36. [DOI] [PubMed] [Google Scholar]
- Dehio C., Prevost,M.C. and Sansonetti,P.J. (1995) Invasion of epithelial cells by Shigella flexneri induces tyrosine phosphorylation of cortactin by a pp60c-src-mediated signalling pathway. EMBO J., 14, 2471–2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druker B.J., Sawyers,C.L., Kantarjian,H., Resta,D.J., Reese,S.F., Ford,J.M., Capdeville,R. and Talpaz,M. (2001) Activity of a specific inhibitor of the BCR-ABL tyrosine kinase in the blast crisis of chronic myeloid leukemia and acute lymphoblastic leukemia with the Philadelphia chromosome. N. Engl. J. Med., 344, 1038–1042. [DOI] [PubMed] [Google Scholar]
- Dumenil G., Olivo,J.C., Pellegrini,S., Fellous,M., Sansonetti,P.J. and Nhieu,G.T. (1998) Interferon α inhibits a Src-mediated pathway necessary for Shigella- induced cytoskeletal rearrangements in epithelial cells. J. Cell Biol., 143, 1003–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumenil G., Sansonetti,P. and Tran Van Nhieu,G. (2000) Src tyrosine kinase activity down-regulates Rho-dependent responses during Shigella entry into epithelial cells and stress fibre formation. J. Cell Sci., 113, 71–80. [DOI] [PubMed] [Google Scholar]
- Elsinghorst E.A. (1994) Measurement of invasion by gentamicin resistance. Methods Enzymol., 236, 405–420. [DOI] [PubMed] [Google Scholar]
- Escalante M., Courtney,J., Chin,W.G., Teng,K.K., Kim,J.I., Fajardo,J.E., Mayer,B.J., Hempstead,B.L. and Birge,R.B. (2000) Phosphorylation of c-Crk II on the negative regulatory Tyr222 mediates nerve growth factor-induced cell spreading and morphogenesis. J. Biol. Chem., 275, 24787–24797. [DOI] [PubMed] [Google Scholar]
- Feller S.M., Knudsen,B. and Hanafusa,H. (1994) c-Abl kinase regulates the protein binding activity of c-Crk. EMBO J., 13, 2341–2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaston I., Stenberg,P.E., Bhat,A. and Druker,B.J. (2000) Abl kinase but not PI3-kinase links to the cytoskeletal defects in Bcr- Abl transformed cells. Exp. Hematol., 28, 77–86. [DOI] [PubMed] [Google Scholar]
- Hawkins P.T. et al. (1995) PDGF stimulates an increase in GTP-Rac via activation of phosphoinositide 3-kinase. Curr. Biol., 5, 393–403. [DOI] [PubMed] [Google Scholar]
- Kain K.H. and Klemke,R.L. (2001) Inhibition of cell migration by Abl family tyrosine kinases through uncoupling of Crk-CAS complexes. J. Biol. Chem., 276, 16185–16192. [DOI] [PubMed] [Google Scholar]
- Koleske A.J., Gifford,A.M., Scott,M.L., Nee,M., Bronson,R.T., Miczek,K.A. and Baltimore,D. (1998) Essential roles for the Abl and Arg tyrosine kinases in neurulation. Neuron, 21, 1259–1272. [DOI] [PubMed] [Google Scholar]
- Liebl E.C., Forsthoefel,D.J., Franco,L.S., Sample,S.H., Hess,J.E., Cowger,J.A., Chandler,M.P., Shupert,A.M. and Seeger,M.A. (2000) Dosage-sensitive, reciprocal genetic interactions between the Abl tyrosine kinase and the putative GEF trio reveal trio’s role in axon pathfinding. Neuron, 26, 107–118. [DOI] [PubMed] [Google Scholar]
- Maurelli A.T., Blackmon,B. and Curtiss,R.,3rd (1984) Loss of pigmentation in Shigella flexneri 2a is correlated with loss of virulence and virulence-associated plasmid. Infect. Immun., 43, 397–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menard R. and Sansonetti,P.J. (1994) Shigella flexneri: isolation of noninvasive mutants of gram-negative pathogens. Methods Enzymol., 236, 493–509. [DOI] [PubMed] [Google Scholar]
- Mounier J., Laurent,V., Hall,A., Fort,P., Carlier,M.F., Sansonetti,P.J. and Egile,C. (1999) Rho family GTPases control entry of Shigella flexneri into epithelial cells but not intracellular motility. J. Cell Sci., 112, 2069–2080. [DOI] [PubMed] [Google Scholar]
- Nagar B., Hantschel,O., Young,M.A., Scheffzek,K., Veach,D., Bornmann,W., Clarkson,B., Superti-Furga,G. and Kuriyan,J. (2003) Structural basis for the autoinhibition of c-Abl tyrosine kinase. Cell, 112, 859–871. [DOI] [PubMed] [Google Scholar]
- Niebuhr K., Jouihri,N., Allaoui,A., Gounon,P., Sansonetti,P.J. and Parsot,C. (2000) IpgD, a protein secreted by the type III secretion machinery of Shigella flexneri, is chaperoned by IpgE and implicated in entry focus formation. Mol. Microbiol., 38, 8–19. [DOI] [PubMed] [Google Scholar]
- Pear W.S. et al. (1998) Efficient and rapid induction of a chronic myelogenous leukemia-like myeloproliferative disease in mice receiving P210 bcr/abl-transduced bone marrow. Blood, 92, 3780–3792. [PubMed] [Google Scholar]
- Pendergast A.M. (2002) The Abl family kinases: mechanisms of regulation and signaling. Adv. Cancer Res., 85, 51–100. [DOI] [PubMed] [Google Scholar]
- Picking W.L., Mertz,J.A., Marquart,M.E. and Picking,W.D. (1996) Cloning, expression and affinity purification of recombinant Shigella flexneri invasion plasmid antigens IpaB and IpaC. Protein Expr. Purif., 8, 401–408. [DOI] [PubMed] [Google Scholar]
- Plattner R., Kadlec,L., DeMali,K.A., Kazlauskas,A. and Pendergast,A.M. (1999) c-Abl is activated by growth factors and Src family kinases and has a role in the cellular response to PDGF. Genes Dev., 13, 2400–2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plattner R., Irvin,B.J., Guo,S., Blackburn,K., Kazlauskas,A., Abraham,R.T., York,J.D. and Pendergast,A.M. (2003) A new link between the c-Abl tyrosine kinase and phosphoinositide signalling through PLC-γ1. Nat. Cell Biol., 5, 309–319. [DOI] [PubMed] [Google Scholar]
- Sack R.B., Rahman,M., Yunus,M. and Khan,E.H. (1997) Antimicrobial resistance in organisms causing diarrheal disease. Clin. Infect. Dis., 24, S102–S105. [DOI] [PubMed] [Google Scholar]
- Salgia R. et al. (1997) BCR/ABL induces multiple abnormalities of cytoskeletal function. J. Clin. Invest., 100, 46–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansonetti P.J. (2001) Microbes and microbial toxins: paradigms for microbial-mucosal interactions III. Shigellosis: from symptoms to molecular pathogenesis. Am. J. Physiol. Gastrointest. Liver. Physiol., 280, G319–G323. [DOI] [PubMed] [Google Scholar]
- Shibata T., Takeshima,F., Chen,F., Alt,F.W. and Snapper,S.B. (2002) Cdc42 facilitates invasion but not the actin-based motility of Shigella. Curr. Biol., 12, 341–345. [DOI] [PubMed] [Google Scholar]
- Tran Van Nhieu G., Ben-Ze’ev,A. and Sansonetti,P.J. (1997) Modulation of bacterial entry into epithelial cells by association between vinculin and the Shigella IpaA invasin. EMBO J., 16, 2717–2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran Van Nhieu G., Caron,E., Hall,A. and Sansonetti,P.J. (1999) IpaC induces actin polymerization and filopodia formation during Shigella entry into epithelial cells. EMBO J., 18, 3249–3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran Van Nhieu G., Clair,C., Bruzzone,R., Mesnil,M., Sansonetti,P. and Combettes,L. (2003) Connexin-dependent inter-cellular com munication increases invasion and dissemination of Shigella in epithelial cells. Nat. Cell Biol., 5, 720–726. [DOI] [PubMed] [Google Scholar]
- Wang Y., Miller,A.L., Mooseker,M.S. and Koleske,A.J. (2001) The Abl-related gene (Arg) nonreceptor tyrosine kinase uses two F-actin-binding domains to bundle F-actin. Proc. Natl Acad. Sci. USA, 98, 14865–14870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J. et al. (2003) Complete genome sequence and comparative genomics of Shigella flexneri serotype 2a strain 2457T [erratum appears in Infect. Immun., 2003, 71, 4223]. Infect. Immun., 71, 2775–2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills Z., Marr,L., Zinn,K., Goodman,C.S. and Van Vactor,D. (1999) Profilin and the Abl tyrosine kinase are required for motor axon outgrowth in the Drosophila embryo. Neuron, 22, 291–299. [DOI] [PubMed] [Google Scholar]
- Zaharik M.L., Gruenheid,S., Perrin,A.J. and Finlay,B.B. (2002) Delivery of dangerous goods: type III secretion in enteric pathogens. Int. J. Med. Microbiol., 291, 593–603. [DOI] [PubMed] [Google Scholar]