Abstract
Obesity causes its complications through functional and morphologic damage to remotely situated tissues via undetermined mechanisms. In one rodent model of obesity, the Zucker diabetic fatty fa/fa rat, overaccumulation of triglycerides in the pancreatic islets may be responsible for a gradual depletion of β cells, leading to the most common complication of obesity, non-insulin-dependent diabetes mellitus. At the onset of non-insulin-dependent diabetes mellitus, the islets from fa/fa rats contain up to 100 times the fat content of islets from normal lean rats. Ultimately, about 75% of the β cells disappear from these fat-laden islets as a consequence of apoptosis induced by long-chain fatty acids (FA). Here we quantify Bcl-2, the anti-apoptosis factor in these islets, and find that Bcl-2 mRNA and protein are, respectively, 85% and 70% below controls. In normal islets cultured in 1 mM FA, Bcl-2 mRNA declined by 68% and completely disappeared in fa/fa islets cultured in FA. In both groups, suppression was completely blocked by the fatty acyl-CoA synthetase inhibitor, triacsin C, evidence of its mediation by fatty acyl-CoA. To determine whether leptin action blocked FA-induced apoptosis, we cultured normal and fa/fa islets in 1 mM FA with or without leptin. Leptin completely blocked FA-induced Bcl-2 suppression in normal islets but had no effect on islets from fa/fa rats, which are unresponsive to leptin because of a mutation in their leptin receptors (OB-R). However, when wild-type OB-R is overexpressed in fa/fa islets, leptin completely prevented FA-induced Bcl-2 suppression and DNA fragmentation.
Earlier studies from our laboratory have indicated that the islets of leptin-unresponsive Zucker diabetic fatty (ZDF) fa/fa rats accumulate excessive amounts of triglycerides early in the disease (1–3) and subsequently lose substantial numbers of β cells through apoptosis (4). These studies further showed that the high lipid content causes an increase in de novo ceramide synthesis, as excess palmitoyl CoA condenses with serine to form ceramide in excessive quantities (4). Ceramide increases the expression of inducible nitric oxide synthase and the resulting overproduction of nitric oxide (NO) triggers the apoptotic program (4, 5). This proposed lipoapoptotic pathway involving excessive de novo ceramide synthesis and NO overproduction is based on the demonstration that C2-ceramide mimics the apoptotic effects of long-chain fatty acids (FA) on cultured islets from ZDF rats, and that apoptosis can be entirely blocked by inhibitors of both ceramide and NO synthesis (4, 5). The fact that the foregoing effects occur in the fat-laden islets of ZDF fa/fa rats, which are completely unresponsive to leptin because of a Gln-269 → Pro mutation in the leptin receptor (OB-R) (6, 7), implies that the lipoapoptosis is somehow linked to lack of leptin action. The present report was designed to test the hypothesis that lack of leptin action in β cells makes them vulnerable to lipoapoptosis.
MATERIALS AND METHODS
Animals.
Lean wild-type (+/+) male ZDF rats and obese homozygous (fa/fa) male ZDF rats were bred in our laboratory from [ZDF/Drt-fa(F10)] rats purchased from R. Peterson (University of Indiana School of Medicine, Indianapolis, IN).
Islet Isolation and Culture.
Pancreatic islets were isolated by the method of Naber et al. (11) with modifications (5). Isolated islets were cultured as described (5, 12). In some experiments, islets were cultured with or without 1 mM FA (2:1 oleate/palmitate, Sigma) in the absence and presence of 20 ng/ml recombinant leptin (kindly provided by Hector Beltrandelrio and Gayle Yamamoto, Zymogenetics, Seattle), and 10 μM triacsin C (BIOMOL Research Laboratories, Plymouth Meeting, PA).
Semiquantitation of Bcl-2 and Bax mRNA by RT-PCR.
Bcl-2 and Bax mRNA expression was analyzed in cultured islets by using reverse transcriptase–PCR (RT-PCR) as described in detail (7). Briefly, total RNA was extracted by using a TRIzol isolation kit (Life Technologies) and treated with RNase-free DNase. First-strand cDNA was obtained by using a first-strand cDNA synthesis kit (CLONTECH). Two microliters of cDNA was amplified by PCR as described (5). Primers used to amplify each gene were as follows: 5′-GCAACCGAACGCCCGCTGTG-3′ (398–417) and 5′-GTGATGCAGGCCCCCACCAG-3′ (842–871) for rat Bcl-2 (GenBank accession no. L14680, 474-bp fragments), 5′-GCGAATTGGAGATGAACTGG-3′(192–211) and 5′-GTGAGCGAGGCGGTGAGGAC-3′)(538–557) for rat Bax (accession no. U49729, 366-bp fragments), and 5′-TTGTAACCAACTGGGACGATATGG-3′ (1552–1575) and 5′-GATCTTGATCTTCATGGTGCTAGG-3′ (2991–2844) for rat β-actin (accession no. J00691, 764-bp fragment). The products were electrophoresed on a 1.2% agarose gel. After being transferred to Hybond-N Nylon membrane (Amersham), DNA samples were hybridized with [32P]ATP-labeled specific probes and analyzed in the Molecular Imager (Bio-Rad). The internal primers were 5′-GAGGAGGCCACAATGCGACCCCAGTTTACC-3′ for Bcl-2, 5′-CAACCACGCGGCCCCAGTTGAAGTTGCCAT-3′ for Bax, and 5′-GGTCAGGATCTTCATGAGGTAGTCTGTCAG-3′ for β-actin. Levels of Bcl-2 and Bax mRNA were expressed as the ratio of signal intensity for those genes relative to that for β-actin.
Immunoblotting of Bcl-2.
Groups of islets were washed with ice-cold PBS and homogenized in buffer (pH 7.5) containing 20 mM Tris⋅HCl, 1 mM EDTA, and 0.1 mM phenylmethane sulfonylfluoride. The extract was centrifuged at 8,000 × g for 5 min at 4°C and then at 100,000 × g for 1 h at 4°C. The infranatant containing soluble proteins was stored at −70°C until used. Protein concentrations in the extract were measured by the Bio-Rad protein assay using BSA as a standard. Extracted protein (40 g) from each pool was subjected to SDS/PAGE and electrophoretically transferred to a nitrocellulose membrane. The blot was probed with a goat polyclonal antibody that recognizes Bcl-2 (Santa Cruz Biotechnology) and then detected with horseradish peroxidase conjugated with enhanced chemiluminescence (Pierce) according to the manufacturer’s protocol. The signals were quantified with a Molecular Imager (Bio-Rad).
DNA Fragmentation Assay.
DNA fragmentation was assayed by a modification of the method of Duke and Sellins (13) Groups of freshly isolated or cultured islets were washed twice with cold PBS and suspended in 100 μl of lysis buffer (10 mM Tris⋅HCl/10 mM EDTA/0.5% Triton X-100, pH 8.0), vortexed, sonicated, and incubated on ice for 20 min. After centrifugation for 20 min at 40°C (14,000 × g), the supernatant containing fragmented (soluble) DNA was transferred to another tube. Lysis buffer (100 μl) was added to the pellet containing insoluble DNA. Both samples were treated with RNase A (0.5 mg/ml) for 1 h at 37°C and then with proteinase-K (Sigma, 0.4 mg/ml) for 1 h at 37°C. After adding 20 μl of 5 M NaCl and 120 μl of isopropanol, the samples were incubated overnight at −20°C, and the DNA concentrations were measured by the method of Hopcroft et al. (14). Fragmented DNA was calculated as 100% × soluble DNA/soluble + insoluble DNA). The soluble fraction of DNA was determined by electrophoresis on a 1.5% agarose gel and has a ladder-like appearance.
RESULTS
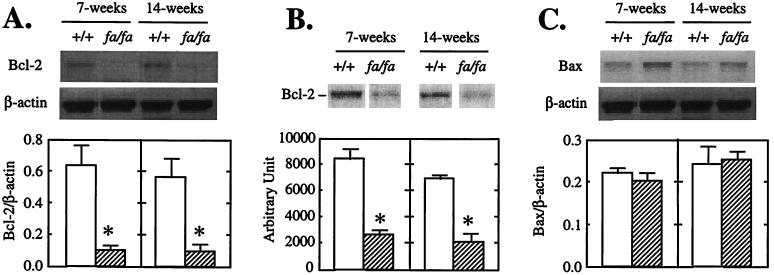
Cell homeostasis is controlled by a family of Bcl-2-related proteins (8–10). The family includes the death antagonists such as Bcl-2, Bcl-xL, Bfl-1, and Bcl-w and death agonists such as Bax, Bak, and Bad (8–10). To determine whether the enhanced tendency to apoptosis observed in islets of fa/fa ZDF with mutated leptin receptors might be the result of abnormalities in expression of Bcl-2 family genes that control cell homeostasis, we semiquantified Bcl-2 and Bax mRNA by RT-PCR in the islets of prediabetic (7-week-old) and diabetic (14-week-old) fa/fa obese ZDF male rats and age-matched +/+ lean ZDF controls. The fa/fa Bcl-2/β-actin mRNA ratio in islets of both prediabetic and diabetic rats was only 15% of the controls (Fig. 1A). Bcl-2 protein was also strikingly reduced in the islets of fa/fa rats at both 7 and 14 weeks of age to 30% and 31% of levels in islets of +/+ rats, respectively (Fig. 1B). There were no differences in Bax mRNA between the two groups at either age (Fig. 1C). Thus, the Bcl-2/Bax ratio was extremely low at both 7 and 14 weeks compared with controls (0.5 and 0.3 vs. 2.8 and 3.6, P < 0.01).
Figure 1.
Bcl-2 and Bax expression in pancreatic islets of lean wild type (+/+) ZDF or obese (fa/fa) ZDF rats. (A) Representative Southern blot of Bcl-2 RT-PCR products. (B) Immunoblot of Bcl-2. (C) Representative Southern blot of Bax RT-PCR products. Bars represent the mean ± SEM of Bcl-2/β-actin (n = 3) or Bax/β-actin ratios (A and C) or arbitrary densitometric unit (B) (n = 3). ∗, P < 0.01 vs. +/+ values.
Effect of FA on Bcl-2 Expression.
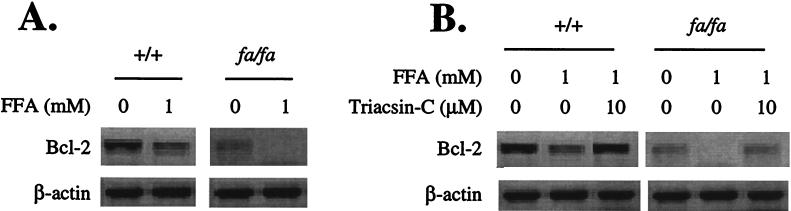
Apoptosis and other abnormalities observed in the islets of fa/fa ZDF rats have been attributed to excess intracellular fatty acyl-CoA derived both from the increased plasma lipids and the high intracellular fat content (1, 2–4). Therefore, we studied the effect of a 1 mM oleate/palmitate mixture on Bcl-2 expression in +/+ and fa/fa islets. In both groups, this mixture resulted in profound lowering of islet Bcl-2 mRNA. In the +/+ islets, the mean baseline Bcl-2 level was reduced by 68%. In the fa/fa islets, the baseline Bcl-2 mRNA level was about 66% below the levels of controls, and it disappeared almost completely in the presence of 1 mM FA (Fig. 2A). To determine whether the Bcl-2-lowering action of fatty acids was the result of their metabolism to fatty acyl-CoA rather than to nonspecific stress injury, we blocked fatty acyl-CoA synthesis by culturing islets in the presence of 10 μM triacsin C. This step completely prevented the FA-induced fall in Bcl-2 (Fig. 2B). We had previously reported that triacsin C completely prevents FA-induced DNA fragmentation (4).
Figure 2.
(A) Effect of FA on Bcl-2 mRNA in pancreatic islets from +/+ and obese homozygous (fa/fa) ZDF rats. (B) The effect of 10 μM triacsin C on the Bcl-2-lowering action of FA in islets of +/+ and fa/fa ZDF rats.
Role of Leptin in Bcl-2 Expression.
The profound underexpression of Bcl-2 observed in the islets of leptin-unresponsive fa/fa rats strongly implies that leptin action is required to prevent this deficit. To test the hypothesis that leptin prevents FA-induced reduction in Bcl-2 expression and protects against apoptosis, two types of experiments were carried out. In the first, we compared Bcl-2 mRNA in islets from normal +/+ rats cultured in 1 mM FA with or without leptin. The presence of 20 ng/ml recombinant leptin in the medium completely abolished the Bcl-2-lowering effect of 1 mM FA (Fig. 3). However, in islets of fa/fa rats with defective OB-R, leptin failed to prevent the FA-induced reduction of Bcl-2 mRNA to undetectable levels.
Figure 3.
Effect of 1 mM FA alone or in the presence of recombinant leptin on Bcl-2 mRNA in islets from 7-week-old +/+ or fa/fa ZDF rats. Bars represent the mean ± SEM of the Bcl-2/β-actin mRNA ratio. ∗, P < 0.01 vs. 0 mM FA; †, P < 0.01 vs. 1 mM FA.
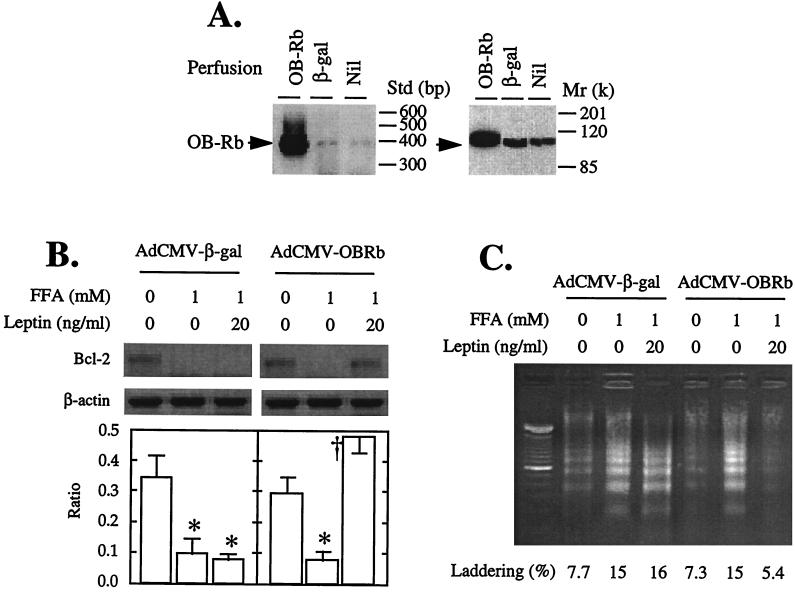
To determine whether restoration of leptin action in fa/fa rats would block the Bcl-2-lowering action of FA and prevent apoptosis, we carried out a second type of experiment. We overexpressed the long isoform of the normal leptin receptor, OB-Rb, in ZDF fa/fa islets. We did this by perfusing the pancreata of 10-week-old diabetic fa/fa rats with recombinant adenovirus containing either the cDNA of the wild-type long leptin receptor isoform (OB-Rb) or, as a control, the gene encoding β-galactosidase (β-gal) as previously described (15). Islets were then isolated and cultured for 2 days, during which time they overexpressed OB-Rb or β-gal (Fig. 4A). They were then exposed to 1 mM FA either with or without 20 ng/ml of leptin for an additional 24 h. In the β-gal-overexpressing control fa/fa islets, FA completely suppressed Bcl-2 mRNA whether leptin was present or not. In the OB-Rb-overexpressing islets, by contrast, leptin completely blocked the Bcl-2-lowering action of FA (Fig. 4B).
Figure 4.
Effects of overexpressing OB-Rb or, as a control, β-gal in pancreatic islets of 10-week-old obese fa/fa ZDF rats. (A) Expression of OB-Rb mRNA by RT-PCR and protein by immunoblotting. (B) Effects of 20 ng/ml recombinant leptin on Bcl-2 mRNA levels in islets cultured for 24 h with 1 mM FA. Bars represent the mean ± SEM of the Bcl-2/β-actin mRNA ratio. ∗, P < 0.01 vs. 0 mM FFA; †, P < 0.01 vs. 1 mM FA. (C) Effect of 20 ng/ml recombinant leptin on DNA fragmentation in pancreatic islets for 24 h with 1 mM FA. DNA laddering as a percentage of total DNA is indicated by the numbers.
Effect of OB-Rb Overexpression in ZDF Islets on Fatty Acid-Induced DNA Fragmentation.
To determine whether the prevention by leptin of FA-mediated lowering of Bcl-2 levels in the fa/fa islets would block FA-induced apoptosis, we quantified DNA fragmentation in OB-Rb-overexpressing and β-gal-overexpressing islets cultured with 0 or 1 mM FA with or without 20 ng/ml leptin. In the β-gal controls, 1 mM FA increased DNA laddering from a baseline of 8% to 15%, and leptin did not prevent this increase (Fig. 4C). In the OB-Rb-overexpressing islets, by contrast, baseline laddering was 7%, and it rose to 15% in the presence of FA; 20 ng/ml leptin completely prevented the FA-induced increase in laddering, suppressing it to 5%, below the baseline (Fig. 4C).
DISCUSSION
These findings disclose a hitherto unsuspected and potentially important leptin action on the homeostasis of the cells of the pancreatic islets. We had shown previously that leptin has profound lipopenic effects on normal islets but has no lipopenic effect on islets of the OB-R-mutated fa/fa ZDF rats, which are fat-laden as a result (11). Overexpression of the wild-type OB-Rb in such islets restores their sensitivity to the lipopenic action of leptin and rescues impaired β cell function (ref. 16 and M.-Y.W., K. Koyama, M.S., D. Mangelsdorf, C.B.N., and R.H.U., unpublished observation). We now show that leptin action completely blocks the apoptogenic effects of FA. Although our data do not indicate if this is a direct effect of leptin or secondary to its lipopenic action, it clearly indicates that FA lower Bcl-2 and that this is prevented by leptin. We suggest that ceramide, which is believed to mediate the TNF-α-induced lowering of Bcl-2 (17–19), also mediates FA-induced lowering of this antiapoptosis factor. We base this on the facts that FA increase ceramide synthesis in islets and that the ceramide synthetase blocker, fumonisin-B1, completely inhibits lipoapotosis (4).
Bcl-2 expression in islets seems to be inversely related to islet fat content, which in turn is controlled by leptin action. Apoptosis is minimal in islets with normal fat content because of higher Bcl-2 expression. Even when islet fat content is increased by culturing normal islets in 1 mM FA, the reduced Bcl-2 level in these islets still exceeds the baseline levels observed in fa/fa islets in the absence of FA. In these fat-laden islets, the addition of 1 mM FA further reduces Bcl-2 to undetectable levels, thereby expediting their demise. Teleologically, the assisted suicide of cells rendered dysfunctional by the surfeit of fat would provide room for new, fully functional replacements. As long as a cell remains sensitive to leptin, it is protected from overaccumulation of fat and underexpression of Bcl-2; however, if it becomes insensitive to leptin, as is the case in most diet-induced obesity (20), the accumulation of fat and the accompanying decline in Bcl-2 might mark it for elimination.
Acknowledgments
We thank Drs. Xiaodong Wang and Daniel Foster for critical review of the paper. We also thank Tagan Ferguson and Kay McCorkle for excellent technical work and Tess Perico for superb secretarial assistance. This work was supported by the National Institutes of Health (Grant DK02700-37), by the National Institutes of Health/Juvenile Diabetes Foundation Diabetes Interdisciplinary Research Program, and by Department of Veterans Affairs Institutional Support (SMI 821-109).
ABBREVIATIONS
- FA
long-chain fatty acids
- ZDF
Zucker diabetic fatty
- RT
reverse transcriptase
- β-gal
β-galactosidase
- OB-R
leptin receptor
References
- 1.Lee Y, Hirose H, Ohneda M, Johnson J H, McGarry J D, Unger R H. Proc Natl Acad Sci USA. 1994;91:10878–10882. doi: 10.1073/pnas.91.23.10878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee Y, Hirose H, Zhou Y-T, Esser V, McGarry J D, Unger R H. Diabetes. 1997;46:408–413. doi: 10.2337/diab.46.3.408. [DOI] [PubMed] [Google Scholar]
- 3.Ohneda M, Inman L R, Unger R H. Diabetologia. 1995;38:173–179. doi: 10.1007/BF00400091. [DOI] [PubMed] [Google Scholar]
- 4.Shimabukuro M, Zhou Y-T, Levi M, Unger R H. Proc Natl Acad Sci USA. 1998;95:2498–2502. doi: 10.1073/pnas.95.5.2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shimabukuro M, Ohneda M, Lee Y, Unger R H. J Clin Invest. 1997;100:290–295. doi: 10.1172/JCI119534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iida M, Murakami T, Ishida K, Mizuno A, Kuwajami M, Shima K. Biochem Biophys Res Commun. 1996;224:597–604. doi: 10.1006/bbrc.1996.1070. [DOI] [PubMed] [Google Scholar]
- 7.Phillips M S, Liu Q, Hammond H, Dugan V, Hey P, Caskey C T, Hess J F. Nat Genet. 1996;13:18–19. doi: 10.1038/ng0596-18. [DOI] [PubMed] [Google Scholar]
- 8.Kroemer G. Nat Med. 1997;3:614–620. doi: 10.1038/nm0697-614. [DOI] [PubMed] [Google Scholar]
- 9.Reed J C. J Cell Biol. 1994;124:1–6. doi: 10.1083/jcb.124.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang J, Liu X, Bhalla K, Kim C N, Ibrado A M, Cai J, Peng T-I, Jones D P, Wang X. Science. 1997;275:1129–1132. doi: 10.1126/science.275.5303.1129. [DOI] [PubMed] [Google Scholar]
- 11.Naber S P, McDonald J M, Jarrett L, McDaniel M L, Ludvigsen C W, Larcy P E. Diabetologia. 1980;19:439–444. doi: 10.1007/BF00281823. [DOI] [PubMed] [Google Scholar]
- 12.Milburn J L, Hirose H, Lee Y H, Nagasawa Y, Ogawa A, Ohneda M, Beltrandelrio H, Newgard C, Johson J H, Unger R H. J Biol Chem. 1995;270:1295–1299. doi: 10.1074/jbc.270.3.1295. [DOI] [PubMed] [Google Scholar]
- 13.Duke R C, Sellins C B. In: Cellular Basis of Immune Modulation. Kaplan J G, editor. New York: Liss; 1989. pp. 311–314. [Google Scholar]
- 14.Hopcroft D W, Mason D R, Scott R S. Horm Metabol Res. 1985;17:559–561. doi: 10.1055/s-2007-1013606. [DOI] [PubMed] [Google Scholar]
- 15.Wang M-Y, Koyama K, Shimabukuro M, Newgard C B, Unger R H. Proc Natl Acad Sci USA. 1998;95:714–718. doi: 10.1073/pnas.95.2.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shimabukuro M, Koyama K, Chen G, Wang M-Y, Trieu F, Lee Y, Newgard C B, Unger R H. Proc Natl Acad Sci USA. 1997;94:4637–4641. doi: 10.1073/pnas.94.9.4637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thompson C B. Science. 1995;267:1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- 18.Wieder T, Geilen C C, Kolter T, Sadeghlar F, Sandhoff K, Brossmer R, Ihrig P, Perry D, Orfanos C E, Hannun Y A. FEBS Lett. 1997;411:260–264. doi: 10.1016/s0014-5793(97)00717-5. [DOI] [PubMed] [Google Scholar]
- 19.Herrmann J L, Bruckheimer E, McDonnell T J. Biochem Soc Trans. 1996;24:1059–1065. doi: 10.1042/bst0241059. [DOI] [PubMed] [Google Scholar]
- 20.Caro J F, Sinha M K, Kolaczynski J W, Zhang P L, Considine R V. Diabetes. 1996;45:1455–1462. doi: 10.2337/diab.45.11.1455. [DOI] [PubMed] [Google Scholar]