Abstract
Mitochondrial function and ambulatory activity were monitored after feeding old rats acetyl-l-carnitine (ALCAR). Young (3–5 mo) and old (22–28 mo) rats were given a 1.5% (wt/vol) solution of ALCAR in their drinking water for 1 mo, were sacrificed, and their liver parenchymal cells were isolated. ALCAR supplementation significantly reverses the age-associated decline of mitochondrial membrane potential, as assessed by rhodamine 123 staining. Cardiolipin, which declines significantly with age, is also restored. ALCAR increases cellular oxygen consumption, which declines with age, to the level of young rats. However, the oxidant production per oxygen consumed, as measured by 2′,7′-dichlorofluorescin fluorescence levels, is ≈30% higher than in untreated old rats. Cellular glutathione and ascorbate levels were nearly 30% and 50% lower, respectively, in cells from ALCAR-supplemented old rats than in untreated old rats, further indicating that ALCAR supplementation might increase oxidative stress. Ambulatory activity in young and old rats was quantified as a general measure of metabolic activity. Ambulatory activity, defined as mean total distance traveled, in old rats is almost 3-fold lower than in young animals. ALCAR supplementation increases ambulatory activity significantly in both young and old rats, with the increase being larger in old rats. Thus, ALCAR supplementation to old rats markedly reverses the age-associated decline in many indices of mitochondrial function and general metabolic activity, but may increase oxidative stress.
Mitochondria are the cellular organelles that provide ATP for metabolism and help to maintain calcium homeostasis within the cell. Damage that compromises these key functions may adversely affect survival of the organism. Mitochondrial decay appears to play a major role in the aging process (1–3). We recently showed that hepatocytes become heterogeneous with respect to mitochondrial function as the rat ages (2). The majority of cells (67% of the total) had mitochondria with significantly lower average mitochondrial membrane potential (2) than cells from young animals. Smaller cell subpopulations had mitochondria that were moderately impaired or retained the same functional characteristics as seen in cells from young rats. We separated these cell subpopulations by centrifugal cell elutriation and characterized some of the underlying events that may have caused the appearance of mitochondrial heterogeneity (2).
Cells containing the most impaired mitochondria were the least metabolically active, had mitochondria that were more uncoupled, and had a higher leakage of oxidants than cells from young rats (2). The other cell subpopulations from old rats also showed varying degrees of the same age-related alterations (2).
There is growing evidence that mitochondria ultimately cause their own decay, although the factors involved in mitochondrial dysfunction and heterogeneity remain to be clarified. Mitochondrial electron transport is not completely efficient, and a small, yet detectable, level of oxidants is constitutively produced. Enhanced mitochondrial susceptibility to oxidative damage is suggested by the decline with age in cellular antioxidant levels (4, 5), coupled with increased oxidant production (6) and increased lipid unsaturation in the inner mitochondrial membrane.
Mitochondrial DNA, proteins, and lipids are oxidatively damaged and metabolically interconnected, and their decay could cause the age-associated decline in mitochondrial function. A constant oxidative damage to mtDNA leads to mtDNA lesions, which could result in decreased transcription (7), an epigenetic change, or mutation. These alterations may result in decreased mitochondrial transcription (7). Elevated levels of oxidized proteins would decrease efficiency of electron transport and would further increase oxidant production. Resultant protein oxidation (8) could result in loss of substrate affinity and Vmax of enzymes that would also lead to loss of electron transport efficiency. Changes to phospholipid ultrastructure because of increased unsaturation may alter membrane fluidity and may change the conformation of transmembrane proteins embedded in the lipid bilayer, causing decreased substrate transport (9). This decrease, in turn, would affect the ability of mitochondria to meet cellular energy demands.
The higher rates of oxidants produced from such inefficiency could decrease levels of key metabolites. Cardiolipin, an important phospholipid that serves as a cofactor for a number of critical mitochondrial transport proteins, declines significantly with age (10). This loss may reflect enhanced oxidative damage and removal of cardiolipin from the membrane but may also be because of decreased de novo synthesis. Finally, carnitine also becomes limiting with age (11, 12), depriving mitochondria of fatty acids for β-oxidation. Supplying mitochondria with metabolites that have become limited with age, by means of dietary supplementation, could improve mitochondrial function.
Carnitine serves to shuttle acetyl moieties derived from fatty acids into the mitochondria for conversion into ATP. Because carnitine levels and carnitine transport decline significantly with age and the beneficial effects of acetyl-l-carnitine (ALCAR) supplementation on mitochondrial function have been described (11–16), we gave rats ALCAR to determine whether this derivative of l-carnitine could reverse the mitochondrial decay that we previously observed in hepatocytes isolated from old rats. We also examined how ALCAR supplementation affected overall ambulatory activity in young and old rats as a general parameter of metabolic activity.
MATERIALS AND METHODS
The following chemicals were used: [ethylene glycol bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid], trypan blue, glutathione (GSH), heparin (sodium salt), and rhodamine 123 (R123) (Sigma); reduced 2′,7′-dichlorofluorescin diacetate (DCFH) (Molecular Probes); collagenase (type D) (Boehringer Mannheim); and l-ascorbic acid and metaphosphoric acid (Fluka). ALCAR (chloride salt) was a gift of Sigma Tau (Pomezia, Italy) and was purchased from Aldrich. All other reagents were reagent grade or better. Double distilled/deionized water was used throughout.
Rats (Fischer 344, virgin male, outbred albino; 3–5 mo) were obtained from Simonsen Laboratories (Gilroy, CA). Old rats (identical strain; 20–28 mo) were from the National Institute on Aging animal colonies. All animals were fed Purina rodent chow and water ad libitum. There was no discernable difference in food consumption in ALCAR vs. untreated rats. All animals were acclimatized at the Northwest Animal Facilities on the University of California, Berkeley, CA, campus for at least 1 wk before experimentation.
ALCAR Supplementation.
Old and young rats were given a 1.5% (wt/vol; pH adjusted to ≈6) solution of ALCAR in their drinking water and allowed to drink ad libitum for 1 mo before sacrifice and hepatocyte isolation. Both young and old rats typically drank ≈25 ml per rat per day (data not shown), which would provide a daily ALCAR dose of approximately 0.5 g/kg of body weight per day for old rats and 0.7 g/kg of body weight per day for young rats. All animal experiments were done with appropriate Animal Use Committee clearances.
Cell Isolation.
Liver tissue was dispersed into single cells by collagenase perfusion (17). Cell number was assessed by using a hemocytometer, and viability was determined by trypan blue exclusion. Viability was usually >90% in both age groups.
Flow Cytometry.
Hepatocytes (2.0 × 106 cells) were incubated with R123 (0.01 mg/ml) for 30 min at 37°C and were then subjected to flow cytometry in an instrument constructed according to Steinkamp et al. (18). Stained cells were passed through a flow chamber at ≈500 cells per sec in a stream that intersected the beam of an argon laser tuned to 488 nm. Emitted light, collected at 90° from the laser beam and cell stream, was collected through a band pass filter centered at 550 nm with spread of 10 nm and a transmittance of 50%. Signals from the photomultiplier were collected in an Oxford multichannel analyzer after analog-to-digital conversion. Nonspecific light scatter was subtracted and all pertinent data were graphed as the number of cells showing a particular fluorescence. Because different numbers of cells were analyzed in separate experiments, all data were normalized to cell number.
Assay of Oxidants with DCFH.
Formation of oxidants (19–21) in cells was determined by fluorescence over time by using DCFH, a reduced, nonfluorescent derivative of fluorescein (22). Quadruplicate samples were routinely analyzed. Fluorescence was monitored by using a Cytofluor 2350 fluorescent measurement system (Millipore) using standard fluorescein filters and Cytocalc software. Because the majority of cells from old rats consume oxygen at lower rates than cells from young animals (2), the rate of oxidant production was normalized to the level of oxygen consumed. Cellular oxygen consumption was measured by using a Yellow Springs Instruments 5300 oxygen electrode and monitor. Cells (4.0 × 106 cells) were added to 3 ml of Krebs–Henseleit balanced salts medium, supplemented with 1 mM glucose and 7 mM glutamate, pH 7.4, that had been equilibrated previously to 20°C, and oxygen consumption was monitored for at least 15 min.
Cardiolipin Measurement.
Cardiolipin was separated from cellular lipid extracts by using a Waters RCM 100 Radial Module and Radial Pak Resolve silica cartridge (5 μm particle size; 0.8 × 10 cm) by using a cyclohexane/2-propanol/water solvent system (45:50:5, vol/vol) at a flow rate of 1 ml/min (23). Cardiolipin was detected at 203 nm by using a Kratos Spectraflow 773 UV detector and was quantified relative to standards.
GSH Analysis.
GSH was measured by HPLC, as described by Reed et al. (24). Briefly, cells were mixed with 5-sulfosalicylic acid [7.5% (wt/vol), final concentration] and the samples were spun for 1 min at 13,000 rpm in a microcentrifuge to remove denatured debris. An aliquot (0.4 ml) of the supernatant was heated with 100 μl of 40 mM fresh aqueous iodoacetic acid (4 μmol). The reaction mixture was brought to pH 7 with NaHCO3. After 60 min in the dark at room temperature, 500 μl of 2,4-dinitrofluorobenzene [1.5% (vol/vol) in absolute ethanol] and 100 μl of K2CO3 were added to bring the final pH to >10. The reaction was allowed to proceed for 4 h in the dark at room temperature. The resultant dinitrophenyl derivatives were separated on 10-μm Ultrasphere-amine (4.6 mm × 25 cm) in a Waters HPLC system, using solvents as described (24).
Ascorbate Analysis.
Total ascorbic acid analysis was performed after reduction with dithiothreitol, as described (25). The samples were placed in a chilled (2°C) auto sampler for analysis. The system used for separation was reversed-phase HPLC (Hewlett–Packard) with coulometric detection (ESA, Bedford, MA). The peak area corresponding to ascorbic acid was integrated by using HP ChemStation software (Hewlett-Packard).
Activity Tests.
Each night, rats were moved from group housing to individual cages (48 cm long × 25 cm wide × 20 cm high) at least 4 h before the quantification of ambulatory parameters. The room was on a 12-h light/dark cycle (lights on 6:00 a.m. to 6:00 p.m.). At 8:00 p.m., a very low light illuminated the test subjects for video tracking. Quantification began at 9:00 p.m. and continued for 4 h. One hour later, the low light turned off and the room remained in total darkness until 6:00 a.m., when the standard light/dark cycle lighting began. A video signal from a camera suspended directly above the individual cages was fed directly into a Videomex-V (Columbus Instruments, Columbus, OH) computer running the Multiple Zone Distance Traveled software. The system quantified ambulatory activity parameters and was calibrated to report distance traveled in cm. In addition to total distance traveled, the time each subject spent in ambulatory (locomotor), stereotypic (grooming), and resting (nonmovement) activity was recorded in hourly segments on an IBM computer using the Multiple Objects Multiple Zones monitoring software (Columbus Instruments). No additional modifications (such as fur dyeing) were needed to accurately and continuously track the subjects. At 9:00 a.m., animals were removed from individual housing and returned to group housing. Results are shown as the mean cm traveled per h ± SEM. Before ALCAR supplementation and for two consecutive nights, the ambulatory activity of each rat was recorded. After ALCAR supplementation and for two consecutive nights, the same spontaneous locomotor parameters were determined. With this design, each rat was its own control.
Statistical Analysis.
Statistical significance was determined by using the paired two-tailed Student’s t test or single-factor ANOVA. Results are expressed as the mean ± SEM, unless otherwise noted.
RESULTS
Effects of ALCAR on Hepatocellular Mitochondrial Function.
To determine the effects of ALCAR supplementation on mitochondrial function, we performed a series of studies in isolated hepatocytes that examined: (i) changes to mitochondrial membrane potential, (ii) changes in cardiolipin (a phospholipid that is essential for mitochondrial substrate transport and cytochrome c oxidase activity), and (iii) rates of oxidant production in isolated hepatocytes and their effects on small molecular weight antioxidant (GSH and ascorbate) status.
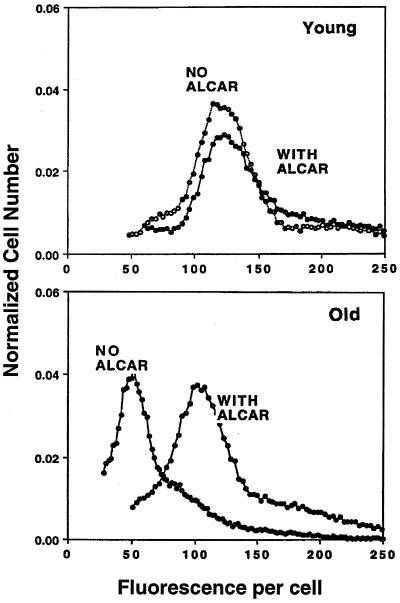
To assess what effect ALCAR supplementation had on average cellular mitochondrial membrane potential, cells were incubated with R123, a fluorescent dye that accumulates in the mitochondria as a function of the inner mitochondrial membrane potential. R123 staining for the majority of hepatocytes from non-ALCAR-supplemented old rats was 59.9% ± 2.6% (n = 6) lower than that for cells isolated from young animals, reflecting a significant age-dependent decline in mitochondrial membrane potential (P < 0.04). In old rats supplemented with ALCAR, hepatocellular mitochondrial membrane potential was significantly higher: only 10.2% ± 4.5% (n = 5) lower than untreated or ALCAR-supplemented young rats, which was not significantly (P > 0.05) different. Previously, we corroborated that R123 fluorescence is a valid measure of mitochondrial membrane potential in cells from old rats (2). ALCAR supplementation virtually abolished the age-related appearance of cell subpopulations that we had previously observed (ref. 2; Fig. 1). ALCAR supplementation did not affect the average membrane potential in cells taken from young rats.
Figure 1.
ALCAR reverses the age-associated decline in R123 fluorescence. Hepatocytes isolated from rats either supplemented with 1.5% (wt/vol) ALCAR for 1 mo or unsupplemented were incubated with R123 30 min before analysis by flow cytometry. Results show that ALCAR supplementation significantly reverses the age-associated decline in mitochondrial membrane potential and abolishes the appearance of mitochondrial heterogeneity with age. Shown is a fluorogram typical of that seen in at least six experiments.
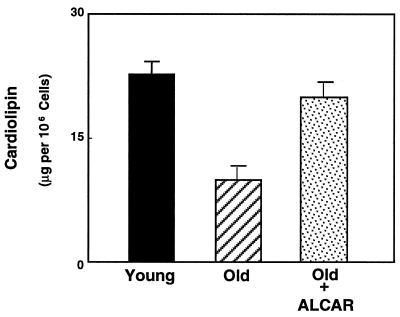
Cardiolipin levels, which decline with age, were also measured after ALCAR feeding. Cardiolipin is an essential cofactor for a number of substrate transport proteins, and its loss with age could severely compromise the ability of mitochondria to generate ATP in quantities sufficient to meet cellular energy demands. As shown in Fig. 2, hepatocytes from old rats exhibit a marked decline in cardiolipin, which dropped from 21.2 ± 2.5 (n = 5) to 10.5 ± 3.6 μg per 106 cells (n = 4) (P < 0.005) in cells from young rats vs. cells from old rats. ALCAR supplementation reversed this loss in cardiolipin concentrations to a level not significantly different from young animals (Fig. 2). Thus, ALCAR supplementation not only facilitates increased average mitochondrial membrane potential, but may effectively reverse any age-associated decline in substrate transport because of loss of cardiolipin.
Figure 2.
Cardiolipin, a key phospholipid necessary for mitochondrial substrate transport, was extracted from hepatocytes and the levels were assessed by HPLC. Results show that mitochondria in cells from old rats have significantly lower cardiolipin when compared with cells isolated from young rats. ALCAR supplementation restores this level to that of young rats.
ALCAR also increased cellular respiration, which had declined with age. Hepatocytes from untreated old rats had an overall oxygen consumption rate of 328 ± 42 μM O2 per min per 107 cells. In contrast, the oxygen consumption characteristics for hepatocytes from ALCAR-treated rats was 450 ± 42 μM O2 per min per 107 cells (n = 6), which was not significantly different from cells from young rats (P = 0.06). These results indicate that ALCAR supplementation significantly increases overall hepatocellular metabolic activity in old animals.
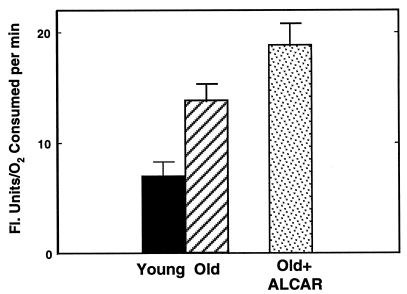
Whereas ALCAR supplementation reversed many of the altered characteristics evident in mitochondrial metabolism with age, analysis of the rate of cellular oxidant flux (as measured by the rate of DCF fluorescence) showed that supplementation increased the rate of oxidant production. As shown in Fig. 3, the rate of oxidant flux in hepatocytes from ALCAR-supplemented rats was 18.2 ± 1.2 fluorescence units/μM O2 per min per 107 cells, approximately a 30% increase over oxidant production in cells from untreated old rats. In contrast, ALCAR supplementation did not alter the rate of oxidant production in cells from young animals (data not shown). These results show that ALCAR cannot mask the age-associated loss of efficiency of electron transport that leads to increased oxidant production in these more rapidly respiring mitochondria.
Figure 3.
DCFH was used as a probe to determine the rate of oxidant production in cells isolated from animals (with or without ALCAR). ALCAR supplementation causes an enhanced rate of oxidant production. These results indicate that ALCAR may improve mitochondrial function but may also increase the amount of oxidants produced as by-products in mitochondrial electron transport.
To explore further whether ALCAR supplementation increased oxidative stress, we measured GSH and ascorbate levels in hepatocytes from ALCAR-treated rats and their age-matched controls. Results show that the levels of both antioxidants decline with age (Table 1). Hepatocellular ascorbate and GSH concentrations declined significantly (P < 0.03). ALCAR supplementation caused a small but nonsignificant decline in both antioxidant levels in cells from young rats; however, both GSH and ascorbate were markedly lower in cells from old supplemented animals (Table 1). GSH levels declined 32% when compared with cells from untreated old rats. Overall, GSH levels were nearly 50% lower in ALCAR-supplemented old rats vs. the levels found in cells from young rats (Table 1). Cellular ascorbate levels were even more affected by ALCAR supplementation, with losses of nearly 50% and 75% when compared with levels in unsupplemented old or young rats, respectively (Table 1). Therefore, whereas ALCAR may reverse many aspects of mitochondrial dysfunction with age, this feeding regimen may concommitantly increase oxidative stress.
Table 1.
Hepatocellular GSH and ascorbate levels with or without ALCAR
| Age, mo | Level, nmol per 106 cells
|
|||
|---|---|---|---|---|
| Untreated
|
ALCAR-treated
|
|||
| GSH | Ascorbate | GSH | Ascorbate | |
| 3–5 | 43.7 ± 2.4 (6) | 7.3 ± 3.0 (15) | 35.5 ± 4.7 (8) | 6.6 ± 4.9 (3) |
| 22–24 | 33.3 ± 2.8 (10)† | 3.4 ± 0.7 (11)† | 22.7 ± 2.4 (8)*† | 1.8 ± 1.2 (3)*† |
Numbers in parentheses denote n value. GSH values are expressed as mean ± SEM: ascorbate values are the mean ± SD. Values with a dagger denote statistical significance between young and old rats (P < 0.03); values with an asterisk denote statistical significance from untreated animals of similar age (P < 0.05).
ALCAR Supplementation Reverses the Age-Associated Decline in General Activity.
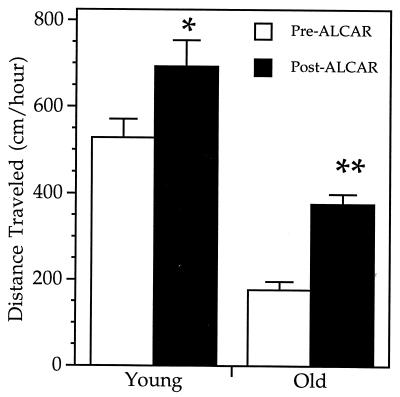
To gauge whether ALCAR treatment resulted in increased levels of metabolism, we monitored ambulatory activity in young and old rats (before and after ALCAR supplementation) as a means to quantify general changes in metabolic rate (see Materials and Methods). Ambulatory activity, defined as total distance traveled per hour (cm/hr) was assessed first in unsupplemented rats. Results, as shown in Fig. 4, showed that untreated old rats were significantly less active than young rats (P = 0.0014). Total distance traveled in old rats was 177 ± 19 cm/hr (n = 5) versus 528 ± 43 cm/hr (n = 5), a 3-fold decline in ambulatory activity with age.
Figure 4.
Distance traveled by young and old rats. Each bar represents the mean distance traveled (cm/h) ± SEM from 8 h of quantification, as described in the text. Distance was determined from the same young (n = 5) and old (n = 5) rats before and after ALCAR treatment. ∗∗, P = 0.0005 when comparing pre-ALCAR old and post-ALCAR old. ∗, P = 0.0011 when comparing pre-ALCAR young and post-ALCAR young. P = 0.0610 when comparing post-ALCAR old and pre-ALCAR young (by using the two-tailed Student’s t test).
After assessment of basal ambulatory activity, animals were supplemented with ALCAR for 1 mo, after which activity was reanalyzed. ALCAR supplementation significantly reversed the age-associated decline in ambulatory activity (P = 0.0005). Activity levels in old rats increased to 376 ± 23 cm/hr (n = 5), a 2-fold increase over the pre-ALCAR supplementation values. The improvement in ambulatory activity was so substantial that the mean distance traveled in ALCAR-supplemented old rats was no longer significantly different (P = 0.061) from unsupplemented young rats. ALCAR supplementation thus reverses the age-associated decline in general ambulatory activity of old rats and may reflect a general underlying improvement of metabolic function in aged animals.
DISCUSSION
The degree of cellular dysfunction because of mitochondrial damage, or how such changes could affect organ function, is not yet known. In part, this may be because of a technical obstacle: a high degree of mitochondrial lysis occurs during purification from aged tissue. Furthermore, mitochondrial isolation from a whole organ cannot determine alterations that take place in specific cells within an organ. Therefore, isolating mitochondria, even if the fraction obtained accurately represented that found in vivo, would not reflect the extent of age-associated alterations at the cellular level (26). We have avoided these problems by assaying for mitochondrial changes in isolated cells from young and old rats (2).
We and others have shown that mitochondrial membrane potential, respiratory control ratios, and cellular oxygen consumption decline with age, and oxidant production increases (2, 3, 6, 11, 14). Both genetic and epigenetic changes to mitochondria and to cells may be involved. Mutations in genes that encode mitochondrial proteins could compromise mitochondria by altering components of the electron transport chain (3), resulting in inefficient electron transport and increased superoxide production (6). The resultant oxidative damage to mitochondria may compromise their ability to meet cellular energy demands. Oxidized proteins accumulate with age (8) and these may also cause mitochondrial inefficiencies, leading to oxidant formation, perhaps due, in part, to deformation of enzyme structure leading a poorer Km for substrate, which could be ameliorated by higher substrate levels. Oxidants may also cause increased damage and use of critical metabolites such as ubiquinone or small molecular weight antioxidants. The significant loss of cardiolipin in aging may be in part because of greater oxidative damage and/or reduced biosynthesis. Loss of cardiolipin, coupled with oxidation of critical thiol groups in key proteins, may adversely affect transport of substrates and cytochrome c oxidase activity (15) necessary for mitochondrial function. These changes could directly impact the ability of mitochondria to maintain their membrane potential and lead to the alterations observed.
The argument that epigenetic factors significantly affect mitochondrial function in aging is supported by our ALCAR supplementation studies. ALCAR effectively abolished the heterogeneity in average mitochondrial membrane potential seen in cells from old rats and increased this key functional parameter to the levels evident in cells from young animals. Moreover, we show that ALCAR reverses the age-associated decline in cardiolipin levels, indicating that ALCAR can not only improve fatty acid transport into mitochondria, but also has a remarkable ability to maintain the structure and function of the mitochondrial inner membrane. ALCAR treatment could, however, induce a greater flux of electrons through an inefficient transport chain, accounting for the enhanced oxidant production.
Our results are consistent with the work of Paradies and colleagues that shows ALCAR stabilizes the inner mitochondrial membrane, increases cardiolipin levels in the heart, and reverses the decline in activity of a number of mitochondrial translocases and of cytochrome c oxidase (11, 13, 15, 16). It is presently not known how ALCAR exerts this effect; however, Gadaleta et al. (27) also showed that ALCAR supplementation reversed the age-related decline in mitochondrial DNA transcription. It has been suggested that ALCAR also exerts its effects by increasing energy production either directly or indirectly (25). ALCAR supplementation also prevents or slows age-related memory impairment by means of increased neurotransmitter production (16) and maintains synaptic contacts (29) and levels of certain hormonal receptors (30). Maintenance of these systems may also be related to overall increased ATP production, which would allow greater synthesis and repair of damaged biomolecules. Measurement of ATP production and metabolite turnover studies in cells after ALCAR supplementation will be necessary to discern whether ALCAR affects these parameters.
When compared with young rats, the aged rat shows a remarkable decline in ambulatory activity. This decline in old rats reflects a true loss in activity because the calculated speeds of the animals, when they moved, were not significantly different from those of young rats (9 ± 0.5 cm/s). ALCAR supplementation improved ambulatory activity in young rats where activity increased 31% (Fig. 4) and markedly increased the ambulatory activity of old rats 2-fold. That ALCAR supplementation can restore significantly overall activity levels to old animals suggests that the decline in physiological activity with age may be the result of mitochondrial decay.
This report also shows that ALCAR supplementation increases the rate of oxidant production and decreases antioxidant levels. However, because ALCAR may also increase defenses such as DNA repair, it is not clear whether this small, but significant, increase in oxidant flux and antioxidant decrease translates into increased oxidative damage to macromolecules. It will be necessary to measure indices of cellular oxidative damage to assess the impact that ALCAR supplementation has on oxidative injury to the cell. In other work, we show that N-tert-butyl-α-phenylnitrone (31) or (R)-lipoic acid administration (unpublished data) decreases the rate of oxidant production in cells from old rats, even when co-administered with ALCAR. Long-term administration of this compound to animals is thus warranted to discern whether it may ameliorate mitochondrial decay with age and enhance the overall quality of life for a longer period of time.
Acknowledgments
We thank Drs. K. Beckman, M. N. Gadaleta, G. Paradies, M. K. Shigenaga, and B. P. Yu for critical review of this manuscript and Sigma Tau for a gift of ALCAR. This work was supported by a grant from the Sandoz Gerontological Foundation (T.M.H.), and by National Cancer Institute Outstanding Investigator Grant CA 39910 and National Institute on Environmental Health Sciences Center Grant ES01896 (B.N.A.).
ABBREVIATIONS
- R123
rhodamine 123
- ALCAR
acetyl-l-carnitine
- GSH
glutathione
- DCFH
reduced 2′,7′-dichlorofluorescin diacetate
References
- 1.Harman D. Age. 1983;6:86–94. [Google Scholar]
- 2.Hagen T M, Yowe D L, Bartholomew J C, Wehr C M, Do K L, Park J-Y, Ames B N. Proc Natl Acad Sci USA. 1997;94:3064–3069. doi: 10.1073/pnas.94.7.3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shigenaga M K, Hagen T M, Ames B N. Proc Natl Acad Sci USA. 1994;91:10771–10778. doi: 10.1073/pnas.91.23.10771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Erdincler D S, Seven A, Inci F, Beager T, Candan G. Clin Chim Acta. 1997;265:77–84. doi: 10.1016/s0009-8981(97)00106-x. [DOI] [PubMed] [Google Scholar]
- 5.Sanz N, Diez-Fernandez C, Alvarez A, Cascales M. J Hepatol. 1997;27:525–534. doi: 10.1016/s0168-8278(97)80358-3. [DOI] [PubMed] [Google Scholar]
- 6.Sohal R S, Weindruch R. Science. 1996;273:59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beckman K B, Ames B N. Physiol Rev. 1998;78:547–581. doi: 10.1152/physrev.1998.78.2.547. [DOI] [PubMed] [Google Scholar]
- 8.Berlett B S, Stadtman E R. J Biol Chem. 1997;272:20313–20316. doi: 10.1074/jbc.272.33.20313. [DOI] [PubMed] [Google Scholar]
- 9.Yu B P, Suescun E A, Yang S Y. Mech Ageing Dev. 1992;65:17–33. doi: 10.1016/0047-6374(92)90123-u. [DOI] [PubMed] [Google Scholar]
- 10.Hoch F L. Biochim Biophys Acta. 1992;1113:71–133. doi: 10.1016/0304-4157(92)90035-9. [DOI] [PubMed] [Google Scholar]
- 11.Paradies G, Ruggiero F M, Petrosillo G, Gadaleta M N, Quagliariello E. Mech Ageing Dev. 1995;84:103–112. doi: 10.1016/0047-6374(95)01636-8. [DOI] [PubMed] [Google Scholar]
- 12.Maccari F, Arseni A, Chiodi P, Ramacci M T, Angelucci L. Exp Gerontol. 1990;25:127–134. doi: 10.1016/0531-5565(90)90043-2. [DOI] [PubMed] [Google Scholar]
- 13.Paradies G, Ruggiero F M, Gadaleta M N, Quagliariello E. Biochim Biophys Acta. 1992;1103:324–326. doi: 10.1016/0005-2736(92)90103-s. [DOI] [PubMed] [Google Scholar]
- 14.Villa R F, Turpeenoja L, Benzi G, Giuffrida Stella A M. Neurochem Res. 1998;10:909–916. doi: 10.1007/BF00970761. [DOI] [PubMed] [Google Scholar]
- 15.Paradies G, Ruggiero F M, Petrosillo G, Gadaleta M N, Quagliariello E. FEBS Lett. 1994;350:213–215. doi: 10.1016/0014-5793(94)00763-2. [DOI] [PubMed] [Google Scholar]
- 16.Carta A, Calvani M, Bravi D, Bhuachalla S N. In: Alzheimer’s Disease: Amyloid Precursor Proteins, Signal Transduction and Neuronal Transplantation. Wurtman R J, Corkin S, Growdon J H, Nitsch R M, editors. Vol. 695. New York: New York Academy of Sciences; 1993. pp. 324–326. [Google Scholar]
- 17.Moldéus P, Högberg J, Orrenius S. Methods Enzymol. 1978;52:60–71. doi: 10.1016/s0076-6879(78)52006-5. [DOI] [PubMed] [Google Scholar]
- 18.Steinkamp J A, Fulwyler M J, Coulter J R, Hiebert R D, Horney J L, Mullancy P F. Rev Sci Instrum. 1973;44:1301–1310. doi: 10.1063/1.1686375. [DOI] [PubMed] [Google Scholar]
- 19.Gunasekar P G, Kanthasamy A G, Borowitz J L, Isom G E. J Neurosci Methods. 1995;61:15–21. doi: 10.1016/0165-0270(95)00018-p. [DOI] [PubMed] [Google Scholar]
- 20.Kooy N W, Royall J A, Ischiropoulos H. Free Radical Res. 1997;27:245–254. doi: 10.3109/10715769709065763. [DOI] [PubMed] [Google Scholar]
- 21.Gabriel C, Camins A, Sureda F X, Aquirre L, Escubedo E, Pallas M, Camarasa J. J Pharmacol Toxicol Methods. 1997;38:93–98. doi: 10.1016/s1056-8719(97)00066-x. [DOI] [PubMed] [Google Scholar]
- 22.LeBel C P, Ischiropoulos H, Bondy S C. Chem Res Toxicol. 1992;5:227–231. doi: 10.1021/tx00026a012. [DOI] [PubMed] [Google Scholar]
- 23.Robinson N C. J Lipid Res. 1990;31:1513–1516. [PubMed] [Google Scholar]
- 24.Reed D J, Babson J R, Beatty P W, Brodie A E, Eillis W W, Potter D W. Anal Biochem. 1982;106:55–62. doi: 10.1016/0003-2697(80)90118-9. [DOI] [PubMed] [Google Scholar]
- 25.Lykkesfeldt J, Loft S, Poulsen H E. Anal Biochem. 1995;229:329–335. doi: 10.1006/abio.1995.1421. [DOI] [PubMed] [Google Scholar]
- 26.Wilson P D, Franks L M. Adv Exp Med Biol. 1975;53:171–183. doi: 10.1007/978-1-4757-0731-1_13. [DOI] [PubMed] [Google Scholar]
- 27.Gadaleta M N, Petruzzella V, Renis M, Fracasso F, Cantatore P. Eur J Biochem. 1990;187:501–506. doi: 10.1111/j.1432-1033.1990.tb15331.x. [DOI] [PubMed] [Google Scholar]
- 28.Siliprandi N, Siliprandi D, Ciman M. Biochem J. 1965;96:777–780. doi: 10.1042/bj0960777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bertoni-Freddari C, Fattoretti P, Casoli T, Spagna C, Casell U. Brain Res. 1994;656:359–366. doi: 10.1016/0006-8993(94)91480-x. [DOI] [PubMed] [Google Scholar]
- 30.Castornia M, Ambrosini A M, Pacific L, Ramacci M T, Angelucci L. Neurochem Res. 1994;19:795–798. doi: 10.1007/BF00967446. [DOI] [PubMed] [Google Scholar]
- 31.Hagen T M, Wehr C M, Ames B N. In: Toward Prolongation of the Healthy Life Span: Practical Approaches to Intervention. Harman D, Holliday R, Meydani M, editors. New York: New York Academy of Sciences; 1998. , in press. [PubMed] [Google Scholar]