Abstract
Sister chromatid cohesion in meiosis is established by cohesin complexes, including the Rec8 subunit. During meiosis I, sister chromatid cohesion is destroyed along the chromosome arms to release connections of recombined homologous chromosomes (homologues), whereas centromeric cohesion persists until it is finally destroyed at anaphase II. In fission yeast, as in mammals, distinct cohesin complexes are used depending on the chromosomal region; Rec8 forms a complex with Rec11 (equivalent to SA3) mainly along chromosome arms, while Psc3 (equivalent to SA1 and SA2) forms a complex mainly in the vicinity of the centromeres. Here we show that separase activation and resultant Rec8 cleavage are required for meiotic chromosome segregation in fission yeast. A non-cleavable form of Rec8 blocks disjunction of homologues at meiosis I. However, displacing non-cleavable Rec8 restrictively from the chromosome arm by genetically depleting Rec11 alleviated the blockage of homologue segregation, but not of sister segregation. We propose that the segregation of homologues at meiosis I and of sisters at meiosis II requires the cleavage of Rec8 along chromosome arms and at the centromeres, respectively.
Keywords: cohesin/homologue segregation/meiosis/separase/sister chromatid cohesion
Introduction
In eukaryotes, sister chromatid cohesion is established during S phase and maintained throughout G2 until M phase. This cohesion is mediated by a conserved multisubunit complex, cohesin (Uhlmann, 2001; Hirano, 2002; Nasmyth, 2002). The cohesin complex in mitosis is composed of at least four subunits in fission yeast, Rad21, Psc3, Psm1 and Psm3 (equivalent to Scc1, Scc3, Smc1 and Smc3, respectively, in budding yeast) (Toth et al., 1999; Tomonaga et al., 2000). Chromosome segregation during mitosis is triggered by the dissolution of sister chromatid cohesion along the entire chromosome length. In yeast this dissociation is triggered by the proteolytic cleavage of cohesin subunit Rad21/Scc1 by a specialized endopeptidase separase. The separase (Cut1/Esp1 in fission/budding yeast) forms a complex with a guardian protein, securin (Cut2/Pds1), and this complex is transferred to the nucleus and spindle microtubules where the separase must be activated. But the proteolytic activity of separase is inhibited by securin until the onset of anaphase when the anaphase-promoting complex (APC) promotes the degradation of securin. In this way, separase is properly activated at metaphase–anaphase transition to cleave the cohesin in the chromatin (Kumada et al., 1998; Yanagida, 2000; Jensen et al., 2001; Hornig et al., 2002). In vertebrate mitosis, however, cohesin is lost in two steps. A non-proteolytic mechanism, presumably the phosphorylation of cohesin by a Polo-like kinase and of histone by Aurora kinase, removes the cohesin along chromosome arms during prophase, while the proteolytic cleavage of Rad21/Scc1 by separase removes centromeric cohesin at metaphase–anaphase transition (Losada et al., 1998; Hauf et al., 2001; Nasmyth, 2001; Losada et al., 2002; Sumara et al., 2002).
During meiosis, the cohesin subunit Rad21/Scc1 is largely replaced by its meiotic counterpart, Rec8 (Molnar et al., 1995; Klein et al., 1999; Watanabe and Nurse, 1999; Pasierbek et al., 2001; Eijpe et al., 2003; Lee et al., 2003). In the first meiotic division (meiosis I), recombination occurs between homologous chromosomes, resulting in the formation of chiasmata. The chiasmata are the sites at which one sister chromatid from one homologue is covalently attached to a sister chromatid from the other homologue, thereby providing a physical link between the homologous chromosomes. Consequently in order for homologues to segregate at meiosis I, sister chromatid cohesion along the chromosome arms must be released to resolve the chiasmata. During homologue segregation, sister chromatid cohesion at the centromeres is retained until the metaphase–anaphase II transition, when sister chromatids move to opposite poles in the equational division. Thus meiotic divisions require sister chromatid cohesion to be released in two steps (Orr-Weaver, 1999; Petronczki et al., 2003).
The localization of the Rec8 protein on meiotic chromosomes and its genetic analysis in various organisms support a central role for Rec8 in the meiosis-specific release of arm cohesion and the retention of centromeric cohesion of sister chromatids at meiosis I (Molnar et al., 1995; Klein et al., 1999; Watanabe and Nurse, 1999; Pasierbek et al., 2001). In budding yeast, homologue segregation at meiosis I is triggered by the separase-mediated cleavage of Rec8 along chromosome arms (Buonomo et al., 2000). A similar mechanism might work in Caenorhabditis elegans, because the inactivation of separase blocked meiosis I (Siomos et al., 2001). In Xenopus oocytes, however, injection of antibodies or antisense RNA directed against the APC activator protein Fizzy (Cdc20) failed to block meiosis I (Peter et al., 2001; Taieb et al., 2001). Moreover, neither antibodies against Cdc27, a core APC subunit, nor a non-degradable form of securin prevented homologue segregation at meiosis I (Peter et al., 2001). This raises the possibility that chiasmata in vertebrates might be resolved by a mechanism that requires neither the APC nor separase; for example, by the mechanism that removes most cohesin from the chromosome arms during mitotic prophase. However, the uncertainties surrounding the use of antibodies and antisense RNA might cast doubt on the Xenopus results (Nasmyth, 2001; Petronczki et al., 2003).
In comparison with budding yeast or C.elegans, several chromosomal features of fission yeast, for example centromere structure, seem more similar to those of higher eukaryotes (Pidoux and Allshire, 2000; Wood et al., 2002). In studying the mechanism of the arm-specific release of sister chromatid cohesion during meiosis I, it is especially noteworthy that a meiosis-specific Scc3-like molecule, SA3, exists that is detected along chromosome arms only until metaphase I and disappears during anaphase I (Prieto et al., 2001). Schizosaccharomyces pombe also has a presumptive SA3 homologue, Rec11, which is a partner of Rec8 mainly along the chromosome arms (Kitajima et al., 2003). The Rec8–Rec11 complex disappears from the chromosome arms when homologues separate at anaphase I; however, centromeric Rec8 persists until metaphase II by forming a complex mainly with another Scc3-like molecule, Psc3. Thus, an analysis of fission yeast meiosis might advance the understanding of a general feature as to how the dissociation of meiotic chromosomes is regulated in eukaryotes.
Here we show that the activation of separase Cut1 is required for homologue segregation at meiosis I in fission yeast. We identify two Cut1-dependent cleavage sites within Rec8 sequences and demonstrate that the homologue segregation at meiosis I is completely blocked by the expression of a non-cleavable form of Rec8. Moreover, we displaced non-cleavable Rec8 selectively from the arm regions using a deletion of rec11, a gene encoding an arm-biased Rec8 partner subunit, and demonstrate that such cells alleviate the blockage of homologue segregation, but not of sister segregation. These results, together with others, suggest that homologue segregation at meiosis I requires the cleavage of Rec8 along the chromosome arms and that sister separation at meiosis II requires the cleavage of centromeric Rec8.
Results
Separase Cut1 is required for Rec8 cleavage and for chromosome segregation during meiosis
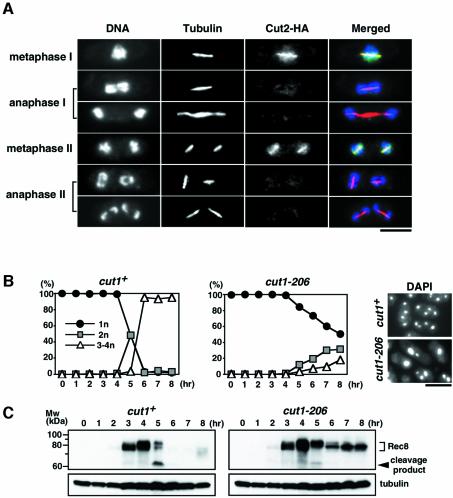
In fission yeast, the accumulation of Cut2 at the mitotic short spindle and its subsequent disappearance are good indicators of the transient activation of Cut1 during the cell cycle (Funabiki et al., 1996; Kumada et al., 1998). Here we examine Cut1 activation during two successive meiotic nuclear divisions by monitoring the Cut2 signal. In meiotic cells, Cut2 protein tagged with HA was clearly detected along the short spindles at metaphase I and metaphase II, but not at anaphase I, anaphase II or any other cell cycle stage of meiosis (Figure 1A). These results suggest that Cut1 loading and activation occurs transiently twice during meiotic division, once shortly before the first meiotic division, and a second time shortly before the second meiotic division.
Fig. 1. Rec8 degradation and meiotic nuclear division take place depending on separase activity. (A) Cells of a diploid cut2-HA strain (PY959) incubated in MM –N at 30°C for 5 h were fixed with formaldehyde and stained with anti-HA antibody, anti-tubulin antibody and DAPI. At this time, meiotic cells at all stages were present in the mixture. The stage of the meiotic cell cycle was judged by the DAPI and tubulin staining. (B) Cells of a diploid pat1-114/pat1-114 cut1-206/cut1-206 strain (PZ91, right) and its control cut1-206/cut1+ strain (PZ90, left) were synchronized in G1 by culturing in MM –N for 15 h at 25°C. The cells were then incubated with 0.1 g/l NH4Cl at 34°C to inactivate the Pat1 and Cut1 proteins simultaneously. The number of nuclei was counted by DAPI staining of samples collected every hour. Pictures of the cells at 8 h are shown. (C) Western blot analyses during a meiotic time courses with anti-Rec8 polyclonal antibodies (upper panel) and anti-tubulin antibody (lower panel) are shown. A probable cleavage product was detected at 5 h. Note that the migration of the Rad21/Rec8 subunit of cohesin is slower than expected (Birkenbihl and Subramani, 1995) (calculated molecular weight of Rec8 is 64.0 kDa). Bars, 5 µm.
To study the role of separase during meiosis, we transferred a cut1-206 temperature-sensitive (ts) mutation (Uzawa et al., 1990) to the pat1-114 ts background, which induces synchronous meiosis at restrictive temperatures (Iino and Yamamoto, 1985). We constructed a diploid strain, h+/h+ pat1-114/pat1-114 cut1-206/cut1-206 and its cut1+ version, and cultured them first in nitrogen-depleted medium at the permissive temperature of 25°C to synchronize them in G1 phase. By raising the temperature of the culture to 34°C, and thereby inactivating the meiotic inhibitor Pat1, we induced synchronous meiosis while simultaneously reducing the activity of separase Cut1. We then monitored the progression of meiotic nuclear divisions by DAPI staining. In cut1+ cells, successive first and second meiotic divisions occurred very synchronously between 4 and 6 h. In cut1-206 cells, however, 80% of the cells still had one nucleus even at 6 h, suggesting that Cut1 is required for the onset of the first meiotic division (Figure 1B). In budding yeast, separase Esp1 is required not only for the onset of anaphase, but also for spindle disassembly through the FEAR (Cdc14 early anaphase release) pathway in meiosis I (Buonomo et al., 2003). We investigated whether Cut1 could carry out the same tasks but could not find the accumulation of anaphase I spindle in the cut1 mutant (Supplementary data, available at The EMBO Journal Online). Thus, unlike Esp1, Cut1 appears to be dispensable for spindle disassembly at meiosis I.
Thinking that Rec8 might be the Cut1 substrate in meiosis, we next analysed the transition of the Rec8 protein level during synchronous meiosis by western blot analysis using anti-Rec8 antibodies. In cut1+ cells, the Rec8 protein accumulated with concomitant phosphorylation before meiosis I, and was then degraded quickly during two successive meiotic nuclear divisions (Figure 1C), as observed previously (Watanabe and Nurse, 1999). We noted that a presumable cleavage product appeared transiently when Rec8 was degraded (Figure 1C, 5 h). When the C-terminus of rec8+ was tagged with GFP or HA, no band was detected by anti-tag antibodies (Watanabe and Nurse, 1999), but a band with the same size was detected by anti-Rec8 antibodies (Figure 2C), suggesting that the cleavage product derives from the N-terminal fragment of Rec8 (see Discussion). In cut1-206 cells, however, Rec8 degradation and the production of the cleavage fragment was greatly inhibited (Figure 1C). We conclude that the onset of meiotic nuclear division and the concomitant degradation of Rec8 occur depending on separase Cut1 activity.
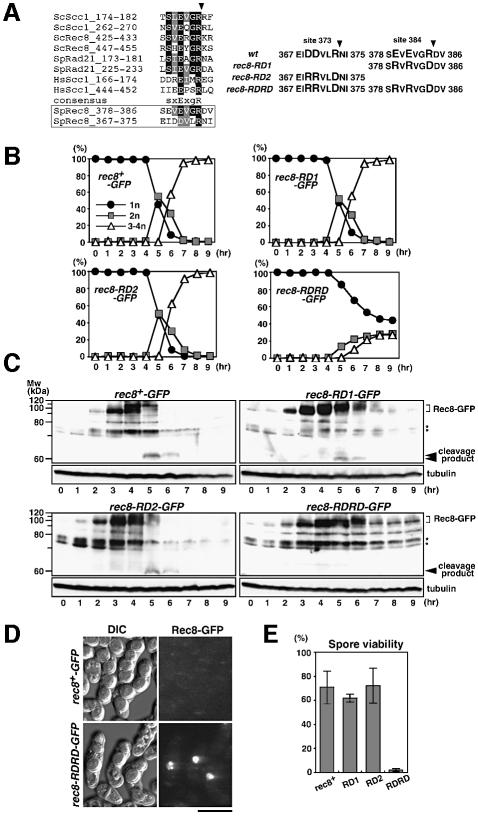
Fig. 2. Rec8–RDRD is a non-cleavable form of Rec8 and inhibits meiotic nuclear division. (A) Sequence alignment of known and putative cohesin cleavage sites. The arrowhead indicates the cleavage position. Amino acid changes in three Rec8 mutants (RD1, RD2, RDRD) are also shown. (B) Cells of a rec8-RD1-GFP strain (PZ45, top right), a rec8-RD2-GFP strain (PZ50, bottom left), a rec8-RDRD-GFP strain (PZ47, bottom right) and a control rec8+-GFP strain (PZ44, top left) were subjected to synchronous meiosis by inactivating pat1-114. Meiotic nuclear divisions monitored by DAPI staining. (C) Western blot analysis during the same time course as (B) was performed with either anti-Rec8 polyclonal antibodies (upper panel) or anti-tubulin antibody TAT-1 (control; lower panel). In all strains except rec8-RDRD, a putative cleavage product was detected at 5–6 h when meiotic nuclear divisions occurred. Asterisks indicate antibody cross-reacting bands. (D) Spore formation and Rec8–GFP fluorescence were simultaneously observed in an h90 rec8-RDRD-GFP strain (PY990, bottom) and a control rec8+-GFP strain (PY929, top). Bar, 5 µm. (E) Spore viability was measured by random spore analysis in the indicated strains, PY929 (rec8+), PY964 (RD1), PZ52 (RD2) and PY990 (RDRD). Four independent examinations were performed.
Rec8 cleavage at residues 373 and 384 is required for meiotic nuclear divisions
Separase recognizes a protein through a specific amino acid sequence and cleaves it there. Schizosaccharomyces pombe Rec8 has an obvious separase consensus site at position 384 (Figure 2A). To examine whether this site is indeed the cleavage site in vivo, we constructed rec8 carrying mutations at this sequence and expressed it in meiosis. Because arginine at site 384 and glutamic acid at site 381 are well conserved, and glutamic acid at site 379 corresponds to the conserved serine that is phosphorylated in Scc1 of budding yeast (Alexandru et al., 2001), we replaced these three amino acids with glutamic acid, arginine and arginine, respectively, to construct the rec8-RD1 allele (Figure 2A). Unexpectedly, the rec8-RD1-GFP cells underwent normal meiotic nuclear divisions (Figure 2B) and produced fully viable spores (Figure 2E). In synchronous meiosis, Rec8–RD1–GFP was degraded during nuclear divisions; however, the kinetics of degradation was slightly delayed compared with wild-type Rec8–GFP (Figure 2C). Moreover, we noticed that the migration of the putative cleavage product was somewhat faster in the rec8-RD1-GFP strain (Figure 2C; data not shown). From these observations, we speculated that a second cleavage site might be located nearby on the N-terminal side of the 384 site, producing a slightly shorter N-terminal cleavage product if the primary site is protected. We assumed the second cleavage site to lie at residue 373, because a highly conserved arginine residue is found only here within the N-terminal >50 amino acid sequence next to site 384 (Figure 2A). To test this assumption, we inserted a mutation at this putative secondary site 373 (RD2) of rec8-RD1 by replacing the glutamic acid residues at 369 and 370 and the arginine at 373 with arginine, arginine and glutamic acid, respectively, and constructed the double site mutation rec8-RDRD (Figure 2A). By replacing the endogenous rec8+ with this allele, we constructed h90 rec8-RDRD-GFP cells, and examined their meiosis. Consequently, the mutant cells produced irregular spores with low viability (Figure 2D and E). Moreover, most zygotes showed bright GFP fluorescence signals within one or two spores (Figure 2D), suggesting that degradation of the Rec8–RDRD–GFP protein was inhibited during meiosis. We then analysed the degradation of Rec8–RDRD–GFP protein and nuclear divisions in further detail using synchronous meiosis. The accumulation and phosphorylation of Rec8–RDRD–GFP occurred with normal time course, but its degradation was significantly inhibited, with no cleavage product produced (Figure 2C). Concomitantly, meiotic nuclear divisions were significantly prevented (Figure 2B), although abortive nuclear division was also observed within some populations with delayed time course (see below). Cells expressing the single site mutation rec8-RD2 underwent normal meiosis with degradation of the mutant Rec8 protein as rapidly as the wild-type protein (Figure 2B and C). Taken together, we conclude that proteolysis at either of the two cleavage sites is both necessary and sufficient for Rec8 degradation and the normal progression of meiotic nuclear divisions. Apparently, Rec8–RDRD levels decreased to some extent without cleavage during the meiotic time course (Figures 1C and 2C), suggesting the existence of a residual Rec8 degradation apart from the rapid and active cleavage pathway. This may partly reflect the dissociation of cohesin from chromatin during the condensation of chromosomes at metaphase I.
Non-cleavable Rec8-RDRD is functional except for its susceptibility to separase
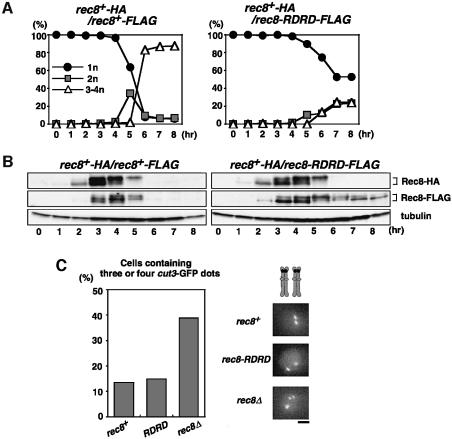
We next compared a diploid strain (rec8+-HA/rec8-RDRD-FLAG) expressing a FLAG-tagged Rec8–RDRD protein and an HA-tagged wild-type Rec8 protein with a diploid strain (rec8+-HA/rec8+-FLAG) expressing FLAG- and HA-tagged wild-type proteins. Nuclear divisions were greatly inhibited in rec8+-HA/rec8-RDRD-FLAG cells, indicating that Rec8–RDRD has a dominant effect on chromosome segregation (Figure 3A). Wild-type Rec8-HA and Rec8-FLAG proteins were degraded with similar kinetics in rec8+-HA/rec8+-FLAG and rec8+-HA/rec8-RDRD-FLAG cells; however, the Rec8–RDRD-FLAG protein failed to be degraded even when wild-type Rec8-HA protein expressed in the same cells underwent degradation with normal kinetics (Figure 3B). These results indicate that all of the meiotic signals needed for Rec8 cleavage are not blocked in rec8-RDRD cells.
Fig. 3. Rec8–RDRD is non-cleavable even when wild-type Rec8 has been cleaved in the same cells. (A) Nuclear divisions during synchronous meiosis in PZ86 (pat1-114/pat1-114 rec8+-HA/rec8+-FLAG) and PZ89 (pat1-114/pat1-114 rec8+-HA/rec8-RDRD-FLAG, right) were assayed by DAPI staining. (B) Cells in (A) were subjected to western blot analysis by anti-HA 12CA5 (upper panel), anti-FLAG M2 (middle panel) or anti-tubulin TAT-1 (lower panel) antibody. (C) cut3-GFP signals were observed in the mei4-arrested h90 cells of rec8+ (PZ137), rec8-RDRD (PZ138) and rec8Δ (PZ116). The percentage of cells containing more than two GFP dots, representing the split of sister cut3 sequences at either or both homologous chromosomes, is shown. Bar, 1 µm.
To examine whether Rec8–RDRD carries the proper Rec8 function, we first assayed meiotic recombination in which Rec8 plays a crucial role (DeVeaux and Smith, 1994). We examined intragenic recombination between ade6-M26 and ade6-469, and intergenic recombination between the mat1-leu1 loci (Supplementary data). Although spore viability was quite low in rec8-RDRD cells, the frequency of recombination was similar to that of rec8+ cells in both assays. We next examined sister chromatid cohesion during prophase I by monitoring GFP signals associated with the cut3 locus (at the middle of the left arm of chromosome II). As shown in Figure 3C, sister chromatid cohesion was normal in rec8-RDRD cells. Arm cohesion due to Rec8–RDRD depends on another cohesin subunit, Rec11 (see below), as does that due to wild-type Rec8, indicating that Rec8–RDRD works as a conventional cohesin complex. All these results suggest that the function of Rec8 other than its susceptibility to separase is not altered in the non-cleavable Rec8.
Non-cleavable Rec8 blocks homologue segregation at meiosis I
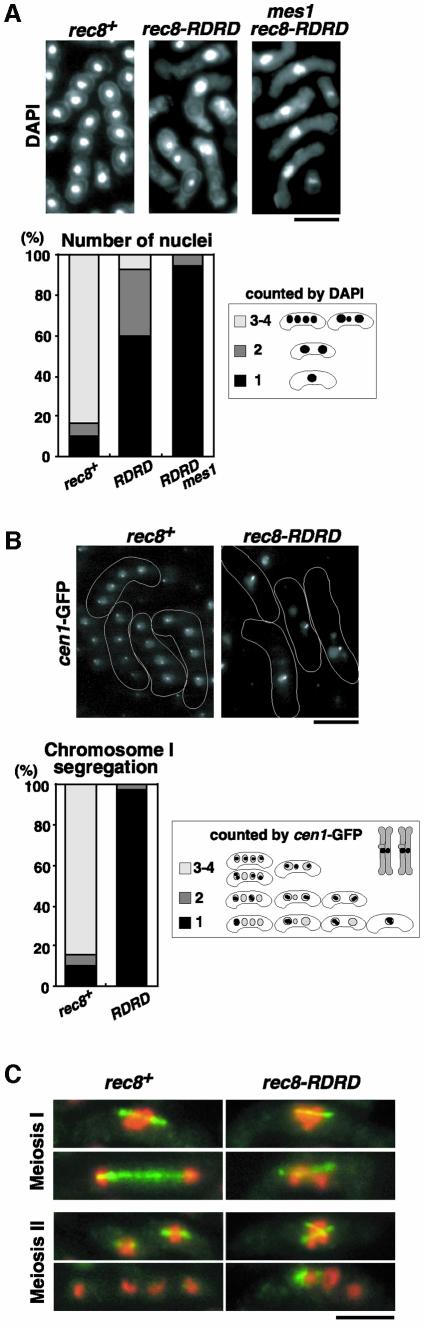
Meiotic nuclear divisions in rec8-RDRD cells were greatly inhibited, but segregated nuclei were also observed in some cells, especially at later time points of meiosis (Figures 2B and 4A). To investigate this phenomenon in more detail, we stained microtubules of rec8-RDRD cells undergoing meiosis and compared them with those of rec8+ cells. In rec8+ cells, a meiosis I spindle is usually observed in cells with one or two nuclei, corresponding, respectively, to metaphase I or anaphase I. In rec8-RDRD cells, however, we found a meiosis I spindle only in mononuclear cells, but never in the cells with two or more nuclei, and could not find proper meiosis II spindles in any multinuclear cells (Figure 4C). Moreover, the abortive nuclear segregation in rec8-RDRD cells was almost blocked if combined with a mes1 mutation, which arrests the meiotic cell cycle after the first division (Figure 4A). These results suggest that the multiple nuclei in rec8-RDRD cells were produced by nuclear fragmentation, presumably during an attempt to form meiosis II spindles. We next examined the segregation pattern of chromosome I by monitoring GFP fluorescence associated with the lys1 locus near the centromere (cen1-GFP). In rec8+ cells, two meiotic nuclear divisions took place properly, producing four equal nuclei with one cen1-GFP dot in each. In contrast, most of the rec8-RDRD cells showed all of the cen1-GFP dots in one nucleus, even in multinuclear cells, indicating that no segregation of either homologues or sister chromatids occurred during meiosis (Figure 4B). This result suggests that the observed segregated nuclei mostly represent one of the three bivalents (S.pombe has three pairs of homologues) instead of a chromosomal segregant. We conclude that the disjunction of homologues at meiosis I was almost completely blocked by the expression of non-cleavable Rec8. Similarly, homologue disjunction was also prohibited in the cut1-206 mutant, although the blockage was leaky compared with rec8-RDRD cells (data not shown). The leakiness of the cut1-206 cells could be explained by the residual separase activity, because a small amount of the Rec8 cleavage product was still detected in this mutant (Figure 1C). In summary, the phenotype of cells expressing non-cleavable Rec8 resembles that of cells with low separase activity. All the results suggest that one crucial function of separase during meiosis I is to cleave Rec8 and thereby to trigger the separation of recombined homologues.
Fig. 4. Non-cleavable Rec8 completely inhibits the segregation of homologues. (A) Cells of an h90 cen1-GFP rec8+ strain (PZ94, left), a rec8-RDRD strain (PZ97, middle) and a rec8-RDRD mes1 strain (PZ110, right) were incubated on sporulation-inducing medium SPA at 26.5°C for 1 day. The number of nuclei stained by DAPI was determined. (B) The segregation pattern of chromosome I was determined by observing cen1-GFP signals after completion of meiosis in the rec8+ (PZ94, left) and rec8-RDRD (PZ97, right) cells. All of the cen1-GFP dots packed into one nucleus indicate the non-disjunction of all the chromatids at both meiosis I and meiosis II. (C) Meiotic cells of PY929 (rec8+) and PY990 (rec8-RDRD) were fixed and stained with anti-tubulin TAT-1 (green) and DAPI (red). Representative cells are shown. Bars, 5 µm.
Mutation of an arm-specific cohesin partner of Rec8 restores disjunction of homologues, but not of sisters, in rec8-RDRD cells
If Rec8 cleavage by separase is necessary for separating sister chromatid cohesion along the arm regions and thereby for resolving the chiasmata, then the elimination of recombination should suppress the non-disjunction of homologues in cells expressing non-cleavable Rec8. This idea has indeed been verified in budding yeast (Buonomo et al., 2000), and we wished to determine whether this is also true in fission yeast. It is known that rec12+ (SPO11 homologue) encodes an endonuclease that generates double-strand breaks that initiate meiotic recombination, and that therefore rec12Δ cells neither process recombination nor form chiasmata (Steiner et al., 2002). We then examined h90 rec8-RDRD rec12Δ cut3-GFP cells, in which the cut3 sequence is marked with GFP, to monitor the segregation of chromosome II and sister chromatid cohesion along the arm region. As expected, rec12Δ relieved the block in homologue segregation of rec8-RDRD cells; nearly 60% of cells underwent homologue disjunction (Figure 5A and B, rec12Δ). The imperfect disjunction of homologues can be explained by the random segregation of unconnected homologues. Thus, these results suggest that the inability of resolving chiasmata along the chromosome arms is the main reason that non-cleavable Rec8 blocks homologue disjunction at meiosis I.
Fig. 5. Defect of recombination or arm cohesion restores disjunction of homologues, but not sisters, in rec8-RDRD cells. (A) h90 cut3-GFP rec8-RDRD cells of either wild-type (PZ134), rec12Δ (PZ146) or rec11Δ (PZ140) background were incubated on sporulation-inducing medium SPA at 26.5°C for 1 day. The nuclear diffusing signal of chromatin-unassociated LacI-GFP-NLS and the dots signal of cut3-GFP were observed, and the segregation patterns of nuclei and cut3-GFP dots were classified. The corresponding cells with cen3-GFP instead of cut3-GFP are shown undergoing meiosis I and meiosis II by staining tubulin and DNA. Bar, 5 µm. (B) Using h90 cut3-GFP rec8-RDRD cells as in (A), the percentage of cells containing three or four GFP dots, representing the split of sister cut3 sequences at either or both homologues, was measured. Representative cells are shown at the left. Arrowheads, paired GFP dots; barbed arrowheads, separated GFP dots. (C) h90 cut3-GFP rec8-RDRD mes1 cells of either wild-type (PZ136), rec12Δ (PZ148) or rec11Δ (PZ142) were incubated on sporulation-inducing medium SPA at 26.5°C for 1 day. The number nuclei and cut3-GFP segregation pattern were examined. (D) An h+ rec8Δ cen2-GFP strain was mixed with an h– rec8-RDRD strain, both carrying either wild type (PZ167 and PZ170), rec11Δ (PZ169 and PZ172), rec12Δ (PZ168 and PZ171) or rec11Δ psc3-2T (PZ992 and PZ962), and incubated on SPA at 26.5°C for 1 day. As a control, h– rec8+ (PZ166) cells mixed with h+ rec8Δ cen2-GFP (PZ170) cells were examined similarly. Cells containing two cen2-GFP dots only in one nucleus (black bar) indicate non-disjunction of sister chromatids.
We next investigated the regional requirement of Rec8 cleavage for meiotic chromosome segregation. For this assay, we took advantage of the discovery that the partner molecule of Rec8 is different at the centromeres and along the chromosome arms in fission yeast. Cohesin subunit Rec11 is a partner of Rec8, especially along arm regions, whereas Psc3, another partner of Rec8, works mainly in the vicinity of centromeres, and rec11Δ cells lose Rec8 location restrictively from the arm regions but not the centromeres in meiotic prophase nuclei (Kitajima et al., 2003). We then examined h90 rec11Δ rec8-RDRD cut3-GFP cells for meiotic chromosome segregation and arm cohesion by monitoring cut3-GFP. After the completion of meiosis, the cut3-GFP dots were frequently seen as more than three separated dots, indicating that sister chromatid cohesion along arm regions is indeed dissolved by rec11Δ (Figure 5B, note that the cohesion was intact in wild type and rec12Δ). Remarkably, a significant number of cells segregated the cut3-GFP dots into two nuclei, suggesting that the non-disjunction of homologous chromosomes was suppressed (Figure 5A and B, rec11Δ). If we examined the segregation pattern of cen2-GFP marked on one of the two pairs of sister chromatids, all the cen2-GFP dots were confined in one nucleus (Figure 5D), indicating that the disjunction of sister chromatids never occurs in rec11Δ rec8-RDRD cells. Thus we conclude that what segregates in rec11Δ rec8-RDRD cells are indeed homologous chromosomes. The homologue disjunction in rec11Δ rec8-RDRD cells is less frequent compared with rec12Δ rec8-RDRD cells, which was more evident if they were combined with the mes1 mutation, which blocks meiosis II (Figure 5C). This is reconcilable with the fact that Psc3, a preferential centromeric partner of Rec8, assists arm cohesion at residual levels in rec11Δ cells (Kitajima et al., 2003).
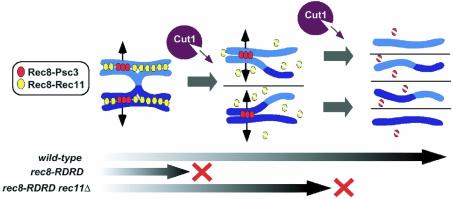
It is noteworthy that rec11Δ cells undergo significant levels of recombination in chromosome II except in the central chromosome region (DeVeaux and Smith, 1994; Krawchuk et al., 1999; Parisi et al., 1999); therefore, the average number of crossovers occurring along the whole chromosome length can be calculated to be roughly half that in rec11+ cells. Because the number of meiotic crossover events in chromosome II is estimated to be 15 (Egel, 1989), rec11Δ cells presumably undergo approximately seven or eight crossovers on chromosome II during one round of meiosis. This evaluation indicates that most of the disjunction observed in rec11Δ rec8-RDRD cells is not caused by a lack of crossovers as in rec12Δ cells, but presumably arises due to a loss of sister chromatid cohesion along the chromosome arms, which leads to premature resolution of crossover or chiasmata. Strikingly, even though meiosis II spindles were indeed observed in rec11Δ rec8-RDRD cells (Figure 5A), the segregation of sister chromatids was completely prevented among them (Figure 5D). Moreover, the introduction of a ts mutation of psc3, encoding a centromeric partner of Rec8, substantially allowed the rec11Δ rec8-RDRD cells to segregate sister chromatids (Figure 5D). Thus, these results underscore the requirement for the cleavage of Rec8 residing in the vicinity of contrivers for triggering sister chromatid segregation during meiosis (Figure 6).
Fig. 6. During meiotic prophase, cohesion between sister chromatids is established by cohesin Rec8, which forms complexes with Psc3 mainly at the centromeres and with Rec11 mainly along the chromosome arms. Sister chromatid cohesion maintains tension between homologous chromosomes through chiasmata. Separase Cut1 is first activated during meiosis I, and cleaves Rec8 along chromosome arms to segregate homologous chromosomes. Centromeric Rec8 somehow escapes from cleavage by Cut1 during meiosis I, presumably until cleaved by re-activated Cut1 during meiosis II. The expression of non-cleavable Rec8 (RDRD) blocks disjunction of homologous chromosomes during meiosis I. Depletion of Rec8–RDRD from the arm regions by rec11Δ, however, restores disjunction of homologous chromosomes but not of sister chromatids, presumably because centromeric cohesion between sisters is still preserved by Psc3-associating Rec8–RDRD. Thus it is likely that Cut1-dependent cleavage of Rec8 triggers two successive chromosome segregations in meiosis I and II.
Discussion
If sister chromatid cohesion mediated by the cohesin complex is responsible for the ability of chiasmata to hold homologues together in meiosis, then the dissociation of the cohesin complex will trigger homologue segregation at meiosis I (Moore and Orr-Weaver, 1998). Once the meiosis-specific cohesin subunit Rec8 was characterized, it was shown that Rec8 is dissociated stepwise from the arm regions at meiosis I and from the centromeres at meiosis II (Klein et al., 1999; Watanabe and Nurse, 1999; Pasierbek et al., 2001). This observation strongly supports the above hypothesis. Recent progress in yeast and human mitosis has revealed that the cleavage of cohesin Rad21/Scc1 by separase Cut1/Esp1 triggers cohesin disruption and thus chromosome separation at the metaphase–anaphase transition (Uhlmann et al., 1999, 2000; Hauf et al., 2001). Therefore, an obvious question is whether Rec8, a meiosis-specific counterpart of Rad21, is similarly disrupted by separase to trigger chromosome segregation during meiosis. An elegant study in budding yeast has suggested strongly that the cleavage of Rec8 by separase triggers the resolution of chiasmata and thereby meiosis I (Buonomo et al., 2000).
Schizosaccharomyces pombe, like Saccharomyces cerevisiae, is a very powerful model organism for the analysis of meiosis by molecular genetics. However, these two yeasts present several distinct features in their organization and regulation of chromatin. First, fission yeast centromeres are composed of repeated DNA structures and are associated heterochromatin protein complexes, whereas the budding yeast centromere has neither and the worm chromosome is holocentric (Pidoux and Allshire, 2000). Recent studies by us and others in fission yeast indicate that heterochromatin plays a crucial role in enriching the cohesin at the centromeres to ensure kinetochore cohesion and chromosome segregation in mitosis and meiosis II (Bernard et al., 2001; Watanabe et al., 2001; Nonaka et al., 2002; Kitajima et al., 2003). It is tempting to speculate, but not proven, that a similar mechanism might mediate the centromere-specific retention of cohesin and cohesion on chromosomes in metaphase-arrested vertebrate cells, and to determine the reason why cohesin dissociation is protected more in the vicinity of centromeres than along the chromosome arms. More directly related to the mechanism of how cohesin dissociates from chromosome arms in meiosis is that the partner of Rec8 in the cohesin complex is differentiated along chromosome arms and at centromeres in fission yeast (Kitajima et al., 2003). This feature is apparently conserved in mammals, but not in S.cerevisiae or C.elegans. Therefore, the results obtained here in S.pombe may have relevance to the mechanism by which homologue segregation is triggered during meiosis in mammals.
Proteolytic cleavage of Rec8 by separase triggers homologue segregation in fission yeast
Our present results in fission yeast support the notion that the proteolytic cleavage of Rec8 by separase triggers homologue segregation at meiosis I. The evidence can be summarized as follows. (i) The Rec8 protein is degraded around the time of meiosis I, producing a cleavage product. (ii) A separase mutant fails to cleave and degrade Rec8, preventing homologue segregation at meiosis I. (iii) Securin (Cut2), an inhibitor of separase Cut1, is degraded at anaphase I around the time that Rec8 starts to degrade. (iv) Rec8 contains one obvious consensus separase cleavage site and another less conserved but potential site. (v) Simultaneous mutations at these sites, but not at just one, blocked Rec8 degradation and the production of its cleavage product in vivo, and concomitantly prevented homologue segregation at meiosis I. (vi) Amino acid changes in Rec8 do not interfere with either recombination or with the cleavage of wild-type Rec8 protein on the same chromosome, suggesting that the function of Rec8 other than its susceptibility to separase is not altered in the non-cleavable Rec8. (vii) Eliminating recombination (and thereby chiasmata) by rec12Δ suppresses the non-disjunction of homologues caused by non-cleavable Rec8. (viii) Genetic depletion of cohesin subunit Rec11, a partner of Rec8 mainly along the chromosome arms, alleviated the blockage of homologue segregation, but not of sister segregation. All these data consistently support the notion that homologue segregation at meiosis I is triggered when Rec8 is cleaved by separase along the chromosome arms.
Conservation of cleavage sites in Rec8
Here we found two potential separase cleavage sites within the Rec8 sequence. The 384 site fits well with the consensus separase cleavage sequences as revealed in the yeast and human cohesin subunit, but the 373 site is atypical. Recent identifications of cleavage sites within the budding yeast Slk19 protein sequence and the human separase protein sequence, however, suggest that the 373 site also fits a more loosely defined consensus sequences (Nasmyth, 2001; Sullivan et al., 2001; Waizenegger et al., 2002). These cleavage sites in S.pombe Rec8 are located close to positions of the corresponding S.cerevisiae Rec8 cleavage sites, suggesting that the manner of cleavage may be well conserved. Western blotting of meiotic extracts using anti-Rec8 antibodies detected the N-terminal cleavage product of Rec8 but not the C-terminal one. The presumed N-terminal end residue (asparatic acid at 374 or asparagine at 385) of the C-terminal Rec8 cleavage fragment is a good substrate of the N-end rule pathway, as demonstrated in budding yeast cohesin Scc1 and Rec8 (Buonomo et al., 2000; Rao et al., 2001). Thus, rapid degradation may explain the inability to detect the C-terminal cleavage fragment of Rec8.
Anaphase II presumably requires the cleavage of Rec8 at centromeres
After meiosis I, a small pool of Rec8 persists in the vicinity of centromeres, suggesting that centromeric Rec8 is protected from degradation during anaphase I. This residual Rec8 presumably plays a crucial role in preserving centromeric cohesion, because selective removal of Rec8 from the pericentromeric regions results in precocious separation of sister centromeres after meiosis I (Kitajima et al., 2003). Then a crucial question is whether centromeric Rec8 is cleaved by separase at meiosis II. Experiments in grasshoppers demonstrated that sister chromatids in meiosis II can disjoin when placed on a meiosis I spindle, also suggesting that chromosome segregation is similarly triggered in meiosis II as in meiosis I (Nicklas, 1977). We have shown that the destruction of securin Cut2 occurs at the onset of anaphase II as well as anaphase I, as previously observed in budding yeast (Salah and Nasmyth, 2000). In Xenopus oocytes, the injection of non-cleavable securin prevents the onset of anaphase II (Peter et al., 2001). These observations are consistent with the notion that separase Cut1 is reactivated at the metaphase–anaphase II transition to cleave residual Rec8 at the centromeres. Finally, we demonstrate here that the disruption of rec11, a gene that encodes an arm-biased cohesin partner of Rec8, allows rec8-RDRD cells to restore the disjunction of homologues but not of sisters. This result further supports the notion that the Rec8 protein must be cleaved by separase, not only along the chromosome arms, but also at the centromeres, in order for sister chromatids to segregate at meiosis II (Figure 6).
Materials and methods
Yeast strains, genetic procedures and media
Schizosaccharomyces pombe strains used in this study are listed in Table I. All media and growth conditions unless otherwise stated were as described previously (Moreno et al., 1991). Complete medium (YE), minimal medium (SD), minimal medium (MM) and sporulation-inducing medium (SPA) were used.
Table I. Strain list.
| PY929 | h90 rec8::kanr<<ura4+<<Prec8-rec8+-GFP<<kanr ura4-D18 |
| PY964 | h90 rec8::kanr<<ura4+<<Prec8-rec8-RD1-GFP<<kanr ura4-D18 |
| PY959 | h+/h– cut2+-HA<<LEU2+/cut2+ ade6-M210/ade6-M216 leu1/leu1 pat1-114/pat1+ |
| PY990 | h90 rec8::kanr<<ura4+<<Prec8-rec8-RDRD-GFP<<kanr ura4-D18 |
| PZ44 | h–/h– pat1-114/pat1-114 rec8::kanr<<ura4+<<Prec8-rec8+-GFP<<kanr/rec8::kanr<<ura4+<<Prec8-rec8+-GFP<<kanr ade6-M210/ade6-M216 ura4-D18/ura4-D18 |
| PZ45 | h–/h– rec8::kanr<<ura4+<<Prec8-rec8-RD1-GFP<<kanr/rec8::kanr<<ura4+<<Prec8-rec8-RD1-GFP<<kanr pat1-114/pat1-114 ade6-M210/ade6-M216 ura4-D18/ura4-D18 |
| PZ47 | h–/h– rec8::kanr<<ura4+<<Prec8-rec8-RDRD-GFP<<kanr/rec8::kanr<<ura4+<<Prec8-rec8-RDRD-GFP<<kanr pat1-114/pat1-114 ade6-M210/ade6-M216 ura4-D18/ura4-D18 |
| PZ50 | h–/h– rec8::kanr<<ura4+<<Prec8-rec8-RD2-GFP<<kanr/rec8::kanr<<ura4+<<Prec8-rec8-RD2-GFP<<kanr pat1-114/pat1-114 ade6-M210/ade6-M216 ura4-D18/ura4-D18 |
| PZ52 | h90 rec8::kanr<<ura4+<<Prec8-rec8-RD2-GFP<<kanr ura4-D18 |
| PZ86 | h+/h+ rec8+-HA<<kanr/rec8::kanr<<ura4+<<Prec8-rec8+-FLAG<<kanr pat1-114/pat1-114 lys1+<<lacO/lys1+<<lacO his7+<<Pdis1-GFP-lacI-NLS/his7+<<Pdis1-GFP-lacI-NLS ade6-M210/ade6-M216 leu1/leu1+ ura4-D18/ura4-D18 |
| PZ89 | h+/h+ rec8+-HA<<kanr/rec8::kanr<<ura4+<<Prec8-rec8-RDRD-FLAG<<kanr pat1-114/pat1-114 lys1+<<lacO/lys1+<<lacO his7+<<Pdis1-GFP-lacI-NLS/his7+<<Pdis1-GFP-lacI-NLS ade6-M210/ade6-M216 leu1/leu1+ ura4-D18/ura4-D18 |
| PZ90 | h+/h+ cut1-206/cut1+ pat1-114/pat1-114 lys1+<<lacO/lys1+<<lacO his7+<<Pdis1-GFP-lacI-NLS/his7+<<Pdis1-GFP-lacI-NLS ade6-M210/ade6-M216 leu1/leu1+ ura4-D18/ura4+ |
| PZ91 | h+/h+ cut1-206/cut1-206 pat1-114/pat1-114 lys1+<<lacO/lys1+<<lacO his7+<<Pdis1-GFP-lacI-NLS/his7+<<Pdis1-GFP-lacI-NLS ade6-M210/ade6-M216 leu1/leu1+ ura4-D18/ura4+ |
| PZ94 | h90 rec8::kanr<<ura4+<<Prec8-rec8+-FLAG<<kanr lys1+<<lacO his7+<<Pdis1-GFP-lacI-NLS ade6-M216 leu1 ura4-D18 |
| PZ97 | h90 rec8::kanr<<ura4+<<Prec8-rec8-RDRD-FLAG<<kanr lys1+<<lacO his7+<<Pdis1-GFP-lacI-NLS ade6-M216 leu1 ura4-D18 |
| PZ110 | h90 rec8::kanr<<ura4+<<Prec8-rec8-RDRD-FLAG<<kanr lys1+<<lacO his7+<<Pdis1-GFP-lacI-NLS mes1-B44 ade6-M216 leu1 ura4-D18 |
| PZ116 | h90 rec8::kanr cut3+<<lacO his7+<<Pdis1-GFP-lacI-NLS mei4-P572 ade6 leu1 ura4-D18 |
| PZ134 | h90 rec8::kanr<<ura4+<<Prec8-rec8-RDRD-FLAG<<kanr cut3+<<lacO his7+<<Pdis1-GFP-lacI-NLS ade6 leu1 ura4-D18 |
| PZ136 | h90 rec8::kanr<<ura4+<<Prec8-rec8-RDRD-FLAG<<kanr cut3+<<lacO his7+<<Pdis1-GFP-lacI-NLS mes1-B44 ade6 leu1 ura4-D18 |
| PZ137 | h90 rec8::kanr<<ura4+<<Prec8-rec8+-FLAG<<kanr cut3+<<lacO his7+<<Pdis1-GFP-lacI-NLS mei4-P572 ade6 leu1 ura4-D18 |
| PZ138 | h90 rec8::kanr<<ura4+<<Prec8-rec8-RDRD-FLAG<<kanr cut3+<<lacO his7+<<Pdis1-GFP-lacI-NLS mei4-P572 ade6 leu1 ura4-D18 |
| PZ140 | h90 rec8::kanr<<ura4+<<Prec8-rec8-RDRD-FLAG<<kanr cut3+<<lacO his7+<<Pdis1-GFP-lacI-NLS rec11-156::LEU2+ ade6 leu1 ura4-D18 |
| PZ142 | h90 rec8::kanr<<ura4+<<Prec8-rec8-RDRD-FLAG<<kanr cut3+<<lacO his7+<<Pdis1-GFP-lacI-NLS rec11-156::LEU2+ mes1-B44 ade6 leu1 ura4-D18 |
| PZ146 | h90 rec8::kanr<<ura4+<<Prec8-rec8-RDRD-FLAG<<kanr cut3+<<lacO his7+<<Pdis1-GFP-lacI-NLS rec12-152::LEU2+ ade6 leu1 ura4-D18 |
| PZ148 | h90 rec8::kanr<<ura4+<<Prec8-rec8-RDRD-FLAG<<kanr cut3+<<lacO his7+<<Pdis1-GFP-lacI-NLS rec12-152::LEU2+ mes1-B44 ade6 leu1 ura4-D18 |
| PZ166 | h– rec8::kanr<<ura4+<<Prec8-rec8+-FLAG<<kanr leu1 ura4-D18 |
| PZ167 | h– rec8::kanr<<ura4+<<Prec8-rec8-RDRD-FLAG<<kanr leu1 ura4-D18 |
| PZ168 | h– rec8::kanr<<ura4+<<Prec8-rec8-RDRD-FLAG<<kanr rec12-152::LEU2+ leu1 ura4-D18 |
| PZ169 | h– rec8::kanr<<ura4+<<Prec8-rec8-RDRD-FLAG<<kanr rec11-156::LEU2+ leu1 ura4-D18 |
| PZ170 | h+ rec8::kanr cen2<<lacO<<ura4+<<kanr his7+<<Pdis1-GFP-lacI-NLS leu1 |
| PZ171 | h+ rec8::kanr rec12-152::LEU2+ cen2<<lacO<<ura4+<<kanr his7+<<Pdis1-GFP-lacI-NLS leu1 |
| PZ172 | h+ rec8::kanr rec11-156::LEU2+ cen2<<lacO<<ura4+<<kanr his7+<<Pdis1-GFP-lacI-NLS leu1 |
| PZ962 | h+ rec8::kanr rec11-156::LEU2+ psc3-2T-HA<<kanr cen2<<lacO<<ura4+<<kanr his7+<<Pdis1-GFP-lacI-NLS leu1 |
| PZ972 | h90 rec8::kanr<<ura4+<<Prec8-rec8+-FLAG<<kanr ade6::lacO<<LEU2+ his7+<<Pdis1-GFP-lacI-NLS leu1 ura4-D18 |
| PZ976 | h90 rec8::kanr<<ura4+<<Prec8-rec8+-FLAG<<kanr ade6::lacO<<LEU2+ his7+<<Pdis1-GFP-lacI-NLS rec11-156::LEU2+ leu1 ura4-D18 |
| PZ980 | h90 rec8::kanr<<ura4+<<Prec8-rec8+-FLAG<<kanr ade6::lacO<<LEU2+ his7+<<Pdis1-GFP-lacI-NLS rec12-152::LEU2+ leu1 ura4-D18 |
| PZ992 | h– rec8::kanr<<ura4+<<Prec8-rec8-RDRD-FLAG<<kanr rec11-156::LEU2+ psc3-2T-HA<<kanr leu1 ura4-D18 |
Construction of rec8 mutant strains
See Supplementary data.
Synchronous meiosis in pat1-114 diploid cells
pat1-114/pat1-114 diploid cells were cultured to log phase in MM +N medium at 25°C. The cells were then washed and transferred to MM-N at a density of 2–3 × 106 cells/ml. After 15.5 h at 25°C, most cells were arrested in G1. The culture was then shifted to 34°C in the presence of 0.1 g/l NH4Cl to inactivate the meiotic inhibitor Pat1, thereby inducing synchronous meiosis. Aliquots of cells were collected and fixed with methanol at 1 h intervals during a meiotic time course, and stained with DAPI to visualize the DNA.
Protein preparation and western blot analysis
Cells (∼2 × 108) were suspended in HB buffer, and boiled for 5 min. Approximately 0.5 ml of glass beads (∼500 µm in diameter) were added and the cells were broken by vigorous vortexing. A total of 25 µg of protein from each sample was electrophoresed in SDS-polyacrylamide gels and immunoblotted. Rec8 and Rec8–GFP were detected using anti-Rec8 polyclonal antibodies at 1:1000. Rec8-HA was detected using anti-HA 12CA5 (Roche) antibody at 1:2000, and Rec8-FLAG with anti-FLAG M2 (Sigma) antibody at 1:2500. Tubulin was detected using anti-tubulin antibody TAT-1 (a gift from Dr Keith Gull) at 1:5000.
Observation of chromosomes marked with GFP
To observe whether homologous chromosomes were segregated or not, h90 cells bearing cen1-GFP or cut3-GFP were cultured to log phase, collected by centrifugation, and then spotted on an SPA plate. cen2-GFP cells were used to observe whether sister chromatids were segregated or not. In this case, opposite mating type cells, one marked with GFP and the other not, were mixed prior to spotting on SPA. After incubation for 1 day at 26.5°C, the zygotes were monitored for GFP. Images were taken using a microscope (Axioplan2; Zeiss) equipped with a cooled CCD camera (Quantix; Photometrics) and Metamorph software (Universal Imaging Corporation). Seven Z-sections for GFP signals were converted to single two-dimensional images by taking the maximum signal at each pixel position in the images.
Preparation of antiserum
See Supplementary data.
Immunostaining
Cut2-HA staining was performed by the method described previously (Funabiki et al., 1996) with modifications. The cut2-HA diploid cells (5 × 107) cultured in MM-N for 5 h were resuspended in 1 ml of PEM (100 mM PIPES pH 6.9, 5 mM EGTA, 5 mM MgCl2), fixed in 3.7% para-formaldehyde for 45 min at 30°C, washed, and treated with 0.1 mg/ml zymolyase 20T (Seikagaku) in PEMS (PEM plus 1.2 M Sorbitol) for 10 min at 37°C. To stain tubulin in zygotes, cells incubated on SPA at 26.5°C for 8 h were used. Cut2-HA was detected using rabbit anti-HA Y-11 (Santa Cruz) at 1:100 and Alexa488-conjugated anti-rabbit antibody (Molecular Probes) at 1:100. Tubulin was detected using TAT-1 antibody at 1:200 and Cy3-tagged anti-mouse antibody (Chemicon) at 1:2000. Cells were counterstained with DAPI to visualize the DNA.
Supplementary data
Supplementary data are available at The EMBO Journal Online.
Acknowledgments
Acknowledgements
We are grateful to Drs G.R.Smith, P.Nurse and M.Yanagida for providing cell strains. We also thank all the members of our laboratory for their help and discussion. This work was supported in part by grants from the Ministry of Education, Science and Culture of Japan.
References
- Alexandru G., Uhlmann,F., Mechtler,K., Poupart,M.A. and Nasmyth,K. (2001) Phosphorylation of the cohesin subunit Scc1 by Polo/Cdc5 kinase regulates sister chromatid separation in yeast. Cell, 105, 459–472. [DOI] [PubMed] [Google Scholar]
- Bernard P., Maure,J.F., Partridge,J.F., Genier,S., Javerzat,J.P. and Allshire,R.C. (2001) Requirement of heterochromatin for cohesion at centromeres. Science, 294, 2539–2542. [DOI] [PubMed] [Google Scholar]
- Birkenbihl R.P. and Subramani,S. (1995) The rad21 gene product of Schizosaccharomyces pombe is a nuclear, cell cycle-regulated phosphoprotein. J. Biol. Chem., 270, 7703–7711. [DOI] [PubMed] [Google Scholar]
- Buonomo S.B., Clyne,R.K., Fuchs,J., Loidl,J., Uhlmann,F. and Nasmyth,K. (2000) Disjunction of homologous chromosomes in meiosis I depends on proteolytic cleavage of the meiotic cohesin Rec8 by separin. Cell, 103, 387–398. [DOI] [PubMed] [Google Scholar]
- Buonomo S.B., Rabitsch,K.P., Fuchs,J., Gruber,S., Sullivan,M., Uhlmann,F., Petronczki,M., Toth,A. and Nasmyth,K. (2003) Division of the nucleolus and its release of CDC14 during anaphase of meiosis I depends on separase, SPO12 and SLK19. Dev. Cell, 4, 727–739. [DOI] [PubMed] [Google Scholar]
- DeVeaux L.C. and Smith,G.R. (1994) Region-specific activators of meiotic recombination in Schizosaccharomyces pombe. Genes Dev., 8, 203–210. [DOI] [PubMed] [Google Scholar]
- Egel R. (1989) Mating-type genes, meiosis and sporulation. In Nasim,A., Young,P. and Johnson,B.F. (eds), Molecular Biology of the Fission Yeast. Academic Press Inc., San Diego, CA, pp. 31–73. [Google Scholar]
- Eijpe M., Offenberg,H., Jessberger,R., Revenkova,E. and Heyting,C. (2003) Meiotic cohesin REC8 marks the axial elements of rat synaptonemal complexes before cohesins SMC1β and SMC3. J. Cell Biol., 160, 657–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funabiki H., Yamano,H., Kumada,K., Nagao,K., Hunt,T. and Yanagida,M. (1996) Cut2 proteolysis required for sister-chromatid separation in fission yeast. Nature, 381, 438–441. [DOI] [PubMed] [Google Scholar]
- Hauf S., Waizenegger,I.C. and Peters,J.M. (2001) Cohesin cleavage by separase required for anaphase and cytokinesis in human cells. Science, 293, 1320–1323. [DOI] [PubMed] [Google Scholar]
- Hirano T. (2002) The ABCs of SMC proteins: two-armed ATPases for chromosome condensation, cohesion and repair. Genes Dev., 16, 399–414. [DOI] [PubMed] [Google Scholar]
- Hornig N.C., Knowles,P.P., McDonald,N.Q. and Uhlmann,F. (2002) The dual mechanism of separase regulation by securin. Curr. Biol., 12, 973–982. [DOI] [PubMed] [Google Scholar]
- Iino Y. and Yamamoto,M. (1985) Negative control for the initiation of meiosis in Schizosaccharomyces pombe. Proc. Natl Acad. Sci. USA, 82, 2447–2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen S., Segal,M., Clarke,D.J. and Reed,S.I. (2001) A novel role of the budding yeast separin Esp1 in anaphase spindle elongation: evidence that proper spindle association of Esp1 is regulated by Pds1. J. Cell Biol., 152, 27–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitajima T.S., Yokobayashi,S., Yamamoto,M. and Watanabe,Y. (2003) Distinct cohesin complexes organize meiotic chromosome domains. Science, 300, 1152–1155. [DOI] [PubMed] [Google Scholar]
- Klein F., Mahr,P., Galova,M., Buonomo,S.B.C., Michaelis,C., Nairz,K. and Nasmyth,K. (1999) A central role for cohesins in sister chromatid cohesion, formation of axial elements and recombination during yeast meiosis. Cell, 98, 91–103. [DOI] [PubMed] [Google Scholar]
- Krawchuk M.D., DeVeaux,L.C. and Wahls,W.P. (1999) Meiotic chromosome dynamics dependent upon the rec8+, rec10+ and rec11+ genes of the fission yeast Schizosaccharomyces pombe. Genetics, 153, 57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumada K., Nakamura,T., Nagao,K., Funabiki,H., Nakagawa,T. and Yanagida,M. (1998) Cut1 is loaded onto the spindle by binding to Cut2 and promotes anaphase spindle movement upon Cut2 proteolysis. Curr. Biol., 8, 633–641. [DOI] [PubMed] [Google Scholar]
- Lee J., Iwai,T., Yokota,T. and Yamashita,M. (2003) Temporally and spatially selective loss of Rec8 protein from meiotic chromosomes during mammalian meiosis. J. Cell Sci., 116, 2781–2790. [DOI] [PubMed] [Google Scholar]
- Losada A., Hirano,M. and Hirano,T. (1998) Identification of Xenopus SMC protein complexes required for sister chromatid cohesion. Genes Dev., 12, 1986–1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losada A., Hirano,M. and Hirano,T. (2002) Cohesin release is required for sister chromatid resolution, but not for condensin-mediated compaction, at the onset of mitosis. Genes Dev., 16, 3004–3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar M., Bahler,J., Sipiczki,M. and Kohli,J. (1995) The rec8 gene of Schizosaccharomyces pombe is involved in linear element formation, chromosome pairing and sister-chromatid cohesion during meiosis. Genetics, 141, 61–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore D.P. and Orr-Weaver,T.L. (1998) Chromosome segregation during meiosis: building an unambivalent. In Hamdel,M.A. (ed.), Meiosis and Gametogenesis. Academic Press, San Diego, CA, pp. 263–299. [DOI] [PubMed] [Google Scholar]
- Moreno S., Klar,A. and Nurse,P. (1991) Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol., 194, 795–823. [DOI] [PubMed] [Google Scholar]
- Nasmyth K. (2001) Disseminating the genome: joining, resolving and separating sister chromatids during mitosis and meiosis. Annu. Rev. Genet., 35, 673–745. [DOI] [PubMed] [Google Scholar]
- Nasmyth K. (2002) Segregating sister genomes: the molecular biology of chromosome separation. Science, 297, 559–565. [DOI] [PubMed] [Google Scholar]
- Nicklas R.B. (1977) Chromosome distribution: experiments on cell hybrids and in vitro. Philos. Trans. R. Soc. Lond. B Biol. Sci., 277, 267–276. [DOI] [PubMed] [Google Scholar]
- Nonaka N., Kitajima,T., Yokobayashi,S., Xiao,G., Yamamoto,M., Grewal,S.I. and Watanabe,Y. (2002) Recruitment of cohesin to heterochromatic regions by Swi6/HP1 in fission yeast. Nat. Cell Biol., 4, 89–93. [DOI] [PubMed] [Google Scholar]
- Orr-Weaver T.L. (1999) The ties that bind: localization of the sister-chromatid cohesin complex on yeast chromosomes. Cell, 99, 1–4. [DOI] [PubMed] [Google Scholar]
- Parisi S. et al. (1999) Rec8p, a meiotic recombination and sister chromatid cohesion phosphoprotein of the Rad21p family, conserved from fission yeast to humans. Mol. Cell. Biol., 19, 3515–3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasierbek P., Jantsch,M., Melcher,M., Schleiffer,A., Schweizer,D. and Loidl,J. (2001) A Caenorhabditis elegans cohesion protein with functions in meiotic chromosome pairing and disjunction. Genes Dev., 15, 1349–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter M., Castro,A., Lorca,T., Le Peuch,C., Magnaghi-Jaulin,L., Doree,M. and Labbe,J.C. (2001) The APC is dispensable for first meiotic anaphase in Xenopus oocytes. Nat. Cell Biol., 3, 83–87. [DOI] [PubMed] [Google Scholar]
- Petronczki M., Siomos,M.F. and Nasmyth,K. (2003) Un menage a quatre: the molecular biology of chromosome segregation in meiosis. Cell, 112, 423–440. [DOI] [PubMed] [Google Scholar]
- Pidoux A.L. and Allshire,R.C. (2000) Centromeres: getting a grip of chromosomes. Curr. Opin. Cell Biol., 12, 308–319. [DOI] [PubMed] [Google Scholar]
- Prieto I., Suja,J.A., Pezzi,N., Kremer,L., Martinez,A.C., Rufas,J.S. and Barbero,J.L. (2001) Mammalian STAG3 is a cohesin specific to sister chromatid arms in meiosis I. Nat. Cell Biol., 3, 761–766. [DOI] [PubMed] [Google Scholar]
- Rao H., Uhlmann,F., Nasmyth,K. and Varshavsky,A. (2001) Degradation of a cohesin subunit by the N-end rule pathway is essential for chromosome stability. Nature, 410, 955–959. [DOI] [PubMed] [Google Scholar]
- Salah S.M. and Nasmyth,K. (2000) Destruction of the securin Pds1p occurs at the onset of anaphase during both meiotic divisions in yeast. Chromosoma, 109, 27–34. [DOI] [PubMed] [Google Scholar]
- Siomos M.F., Badrinath,A., Pasierbek,P., Livingstone,D., White,J., Glotzer,M. and Nasmyth,K. (2001) Separase is required for chromosome segregation during meiosis I in Caenorhabditis elegans. Curr. Biol., 11, 1825–1835. [DOI] [PubMed] [Google Scholar]
- Steiner W.W., Schreckhise,R.W. and Smith,G.R. (2002) Meiotic DNA breaks at the S. pombe recombination hot spot M26. Mol. Cell, 9, 847–855. [DOI] [PubMed] [Google Scholar]
- Sullivan M., Lehane,C. and Uhlmann,F. (2001) Orchestrating anaphase and mitotic exit: separase cleavage and localization of Slk19. Nat. Cell Biol., 3, 771–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumara I., Vorlaufer,E., Stukenberg,P.T., Kelm,O., Redemann,N., Nigg,E.A. and Peters,J.M. (2002) The dissociation of cohesin from chromosomes in prophase is regulated by Polo-like kinase. Mol. Cell, 9, 515–525. [DOI] [PubMed] [Google Scholar]
- Taieb F.E., Gross,S.D., Lewellyn,A.L. and Maller,J.L. (2001) Activation of the anaphase-promoting complex and degradation of cyclin B is not required for progression from meiosis I to II in Xenopus oocytes. Curr. Biol., 11, 508–513. [DOI] [PubMed] [Google Scholar]
- Tomonaga T. et al. (2000) Characterization of fission yeast cohesin: essential anaphase proteolysis of Rad21 phosphorylated in the S phase. Genes Dev., 14, 2757–2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth A., Ciosk,R., Uhlmann,F., Galova,M., Schleiffer,A. and Nasmyth,K. (1999) Yeast Cohesin complex requires a conserved protein, Eco1p(Ctf7), to establish cohesion between sister chromatids during DNA replication. Genes Dev., 13, 320–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlmann F. (2001) Chromosome cohesion and segregation in mitosis and meiosis. Curr. Opin. Cell Biol., 13, 754–761. [DOI] [PubMed] [Google Scholar]
- Uhlmann F., Lottspeich,F. and Nasmyth,K. (1999) Sister-chromatid separation at anaphase onset is promoted by cleavage of the cohesin subunit Scc1. Nature, 400, 37–42. [DOI] [PubMed] [Google Scholar]
- Uhlmann F., Wernic,D., Poupart,M.A., Koonin,E.V. and Nasmyth,K. (2000) Cleavage of cohesin by the CD clan protease separin triggers anaphase in yeast. Cell, 103, 375–386. [DOI] [PubMed] [Google Scholar]
- Uzawa S., Samejima,I., Hirano,T., Tanaka,K. and Yanagida,M. (1990) The fission yeast cut1+ gene regulates spindle pole body duplication and has homology to the budding yeast ESP1 gene. Cell, 62, 913–925. [DOI] [PubMed] [Google Scholar]
- Waizenegger I., Gimenez-Abian,J., Wernic,D. and Peters,J. (2002) Regulation of human separase by securin binding and autocleavage. Curr. Biol., 12, 1368. [DOI] [PubMed] [Google Scholar]
- Watanabe Y. and Nurse,P. (1999) Cohesin Rec8 is required for reductional chromosome segregation at meiosis. Nature, 400, 461–464. [DOI] [PubMed] [Google Scholar]
- Watanabe Y., Yokobayashi,S., Yamamoto,M. and Nurse,P. (2001) Pre-meiotic S phase is linked to reductional chromosome segregation and recombination. Nature, 409, 359–363. [DOI] [PubMed] [Google Scholar]
- Wood V. et al. (2002) The genome sequence of Schizosaccharomyces pombe. Nature, 415, 871–880. [DOI] [PubMed] [Google Scholar]
- Yanagida M. (2000) Cell cycle mechanisms of sister chromatid separation; roles of Cut1/separin and Cut2/securin. Genes Cells, 5, 1–8. [DOI] [PubMed] [Google Scholar]