Abstract
Epithelial tubulogenesis involves complex cell rearrangements that require control of both cell adhesion and migration, but the molecular mechanisms regulating these processes during tubule development are not well understood. Interactions of the cytoplasmic protein, β-catenin, with several molecular partners have been shown to be important for cell signaling and cell–cell adhesion. To examine if β-catenin has a role in tubulogenesis, we tested the effect of expressing NH2-terminal deleted β-catenins in an MDCK epithelial cell model for tubulogenesis. After one day of treatment, hepatocyte growth factor/scatter factor (HGF/ SF)-stimulated MDCK cysts initiated tubulogenesis by forming many long cell extensions. Expression of NH2-terminal deleted β-catenins inhibited formation of these cell extensions. Both ΔN90 β-catenin, which binds to α-catenin, and ΔN131 β-catenin, which does not bind to α-catenin, inhibited formation of cell extensions and tubule development, indicating that a function of β-catenin distinct from its role in cadherin-mediated cell–cell adhesion is important for tubulogenesis. In cell extensions from parental cysts, adenomatous polyposis coli (APC) protein was localized in linear arrays and in punctate clusters at the tips of extensions. Inhibition of cell extension formation correlated with the colocalization and accumulation of NH2-terminal deleted β-catenin in APC protein clusters and the absence of linear arrays of APC protein. Continued HGF/ SF treatment of parental cell MDCK cysts resulted in cell proliferation and reorganization of cell extensions into multicellular tubules. Similar HGF/SF treatment of cysts derived from cells expressing NH2-terminal deleted β-catenins resulted in cells that proliferated but formed cell aggregates (polyps) within the cyst rather than tubules. Our results demonstrate an unexpected role for β-catenin in cell migration and indicate that dynamic β-catenin–APC protein interactions are critical for regulating cell migration during epithelial tubulogenesis.
Morphogenetic movements of cells are critical to normal development of organs and tissues (Trinkaus, 1984). During embryogenesis, folding and rearrangement of sheets of cells are required for gastrulation and neurulation (Keller and Winklbauer, 1992; Keller et al., 1992a , b ). During organogenesis, cell rearrangements have a central role in branching morphogenesis and tubulogenesis, processes that are essential for formation of many organs, including lung, kidney, pancreas, and mammary gland (Spooner and Wessells, 1970; Wessells, 1970; Wessells, 1977; Hogg et al., 1983; Coleman et al., 1988; Saxen, 1987; Hisaoka et al., 1992, 1993). Cell rearrangements require coordinate regulation of cell–cell adhesion and cell migration through extracellular matrix (Gumbiner, 1992). To understand the molecular mechanisms involved in cell rearrangements during development, it is important to define molecules that regulate and coordinate cell adhesion and migration during morphogenesis.
β-catenin/armadillo plays a critical role in morphogenesis during embryonic development (McCrea et al., 1993; Peifer et al., 1993b ; Funayama et al., 1995; Haegel et al., 1995; Cox et al., 1996). Originally, β-catenin was identified in a complex with cadherins, a family of Ca2+-dependent cell adhesion proteins (Nagafuchi and Takeichi, 1989; Ozawa et al., 1989; McCrea and Gumbiner, 1991; McCrea et al., 1991). β-catenin binds to the cytoplasmic domain of cadherins and to another cytoplasmic protein, α-catenin, which in turn links the cadherin–catenin complex to the actin cytoskeleton (Aberle et al., 1994; Hulsken et al., 1994; Jou et al., 1995; Rimm et al., 1995). Disruption of β-catenin binding to cadherin (Ozawa et al., 1990; Oyama et al., 1994), or deletion of the β-catenin gene (Peifer et al., 1993b ; Haegel et al., 1995; Cox et al., 1996) results in loss of cell–cell adhesion and disorganization of cells and tissues. β-catenin is also part of a signal transduction pathway initiated by the extracellular ligand, wingless/Wnt (McCrea et al., 1993; Peifer et al., 1993a ; Noordermeer et al., 1994; Siegfried and Perrimon, 1994; Siegfried et al., 1994). Activation of this pathway results in localization of β-catenin to the nucleus, indicating a role for β-catenin in regulating gene expression (Funayama et al., 1995; Gumbiner, 1995). Recently, β-catenin has been shown to bind to LEF-1, a DNA-binding transcription factor, and affects LEF-1–induced DNA bending (Behrens et al., 1996; Huber et al., 1996; Molenaar et al., 1996). β-catenin also binds to the product of the adenomatous polyposis coli (APC)1 tumor suppressor gene in a complex that is independent of β-catenin binding to E-cadherin (Hulsken et al., 1994; Rubinfeld et al., 1995). Mutations in the APC gene constitute the genetic defect in the inherited colon cancer syndrome, familial adenomatous polyposis, and represent an early event in a high percentage of sporadic colon cancers (Polakis, 1995). Mutations in APC protein result in accumulation of enterocytes near the crypt–villus boundary in the colon (Polakis, 1995). Näthke et al. (1996) showed that APC protein is localized in punctate clusters near the ends of microtubules that protrude into actively migrating membrane structures, suggesting a role for APC protein in cell migration.
Binding of β-catenin to cadherin, APC protein, and LEF-1 indicates divergent functions of β-catenin. However, these interactions are interrelated. Participation of β-catenin in signal transduction is dependent on a high cytoplasmic level of β-catenin that may be regulated, in part, by cadherin and APC protein (Hulsken et al., 1994). Binding of β-catenin to cadherin antagonizes β-catenin signaling function by sequestering β-catenin to the cadherin complex (Heasman et al., 1994; Fagotto et al., 1996). Binding of β-catenin to APC protein has been suggested to increase β-catenin turnover, thereby decreasing β-catenin levels (Munemitsu et al., 1995). That β-catenin forms complexes with both cadherin and APC protein also raises the possibility that β-catenin is involved in regulating functions of both proteins during epithelial morphogenesis.
To examine the function(s) of β-catenin in epithelial morphogenesis, we expressed mutant β-catenins containing NH2-terminal deletions of 90 (ΔN90) or 131 (ΔN131) amino acids in MDCK epithelial cells. We have determined recently that in MDCK cells grown as monolayers, ΔN90 and ΔN131 β-catenin are significantly more stable and strongly colocalize in complexes with APC protein at the tips of membrane protrusions. Colocalization of mutant β-catenin and APC protein correlated with a more fibroblastic cell morphology and a delay in colony formation (Barth et al., 1997). In the present study, we examined the effects of mutant β-catenin on tubulogenesis in an in vitro assay for epithelial tubule development. This assay provides an experimentally tractable system with which to analyze the molecular mechanisms underlying the role of β-catenin in cell adhesion and migration during morphogenetic cell rearrangements. Our results reveal that stable interaction of mutant β-catenin with APC protein inhibits cell migration during tubulogenesis. We provide evidence that dynamic interaction of β-catenin with APC protein is modulated by the NH2-terminal domain of β-catenin and may regulate directed cell movements that are critical for tubulogenesis.
Materials and Methods
Cell Culture
The clones of MDCK cells used in this study are described in detail in Barth et al. (1997). The parental (T23) MDCK cell clone was derived from a polymeric immunoglobulin receptor-expressing cell line (Mostov et al., 1987) that was cotransfected with plasmid pUHD15-1 coding for expression of the tetracycline-repressible transactivator, tTA (Gossen and Bujard, 1992), and a plasmid pSV2-puro, containing a gene for resistance to puromycin. The parental T23 cell line was cotransfected with an expression vector under the control of a tetracycline-repressible promoter for full length β-catenin (β-cat*), luciferase (Lu), or β-catenin mutant proteins containing NH2-terminal deletions of either 90 (ΔN90) or 131 (ΔN131) amino acids (Barth et al., 1997) and plasmid pHMR272 carrying a gene for resistance to hygromycin (Gossen and Bujard, 1992). The following clones of MDCK cells were used in this study: parental (T23); β-cat*–10; luciferase-C and -D; ΔN90-2 and -A; ΔN131-5, -7, and -D. Multiple clones were used to ensure that our results are not due to clonal variation. All cells were passaged at subconfluent density on plastic petri dishes in minimal essential medium (MEM) containing Earle's balanced salt solution (MEM–EBSS; Cellgro; Mediatech, Inc., Hendron, VA) supplemented with 5% FCS (Hyclone, Logan, UT), 100 U/ml penicillin, and 100 mg/ml streptomycin in 5% CO2/95% air. Cells were grown in the presence or absence of Doxycyclin (Dox; 20 ng/ml; Sigma Chemical Co., St. Louis, MO). For growth in three-dimensional collagen gels, cells were isolated with trypsin and triturated into a single-cell suspension. Cells were diluted to ∼2 × 104 cells/ml in a type I collagen solution containing 66% Vitrogen 100 (3 mg/ml; Celtrix, Palo Alto, CA), 1 × MEM, 2.35 mg/ml NaHCO3, 0.02 M Hepes, pH 7.6, and 12% dH2O. The cell suspension was pipetted onto NUNC filters (10 mm, 0.02–0.2 μm pore size; 162243; Applied Scientific, So. San Francisco, CA). The culture was incubated at 37°C, 5% CO2/95% air to allow the type I collagen solution to gel and then overlayered with medium. The medium was changed every 2–3 d. Cultures were grown for 10–12 d, at which time cysts comprising a closed monolayer of cells surrounding a fluid-filled lumen had formed.
Production of MRC5 Fibroblast-Conditioned Medium Containing Hepatocyte Growth Factor/Scatter Factor
MRC5 human lung fibroblasts (CCL171; American Type Culture Collection, Rockville, MD) were grown to confluence in DME containing 10% FBS, 100 U/ml penicillin, 100 mg/ml streptomycin, in tissue culture flasks (T75; Corning, Acton, MA). The medium was replaced with 30 ml fresh medium, and cells were cultured for 3 d. Conditioned medium was collected, centrifuged to remove cell debris, and stored frozen at −20°C.
Stimulation of Tubulogenesis in MDCK Cysts with Hepatocyte Growth Factor/ Scatter Factor–Conditioned Medium
Medium was removed from three-dimensional collagen gels containing MDCK cysts and replaced with MRC5-conditioned medium diluted 1:1 with MEM–EBSS, 5% FBS. Treatment was continued for different periods (see Fig. 1), during which time cultures were refed daily with freshly diluted, conditioned medium. (Virtually identical results were obtained using purified recombinant hepatocyte growth factor/scatter factor [HGF/ SF], a kind gift from R. Schwall, Genentech, So. San Fransisco, CA). Nomarski interference contrast images of cysts and developing tubules were obtained using a microscope (BH2-RFCA; Olympus Corp., Park Success, NY) fitted with a 20× objective (LWD CD Plan; Olympus) and connected to an MTI-CCD video camera. Images were captured with Scion 1.59 onto a Power MacIntosh (7500; Apple Computers, Cupertino, CA) and processed using NIH Image 1.6 and Adobe Photoshop 3.0.5 (Adobe Systems Inc., Mountain View, CA). For quantifying tubule formation, phase contrast images of 24-h cultures ±HGF/SF and ±Dox were photographed at 20 to 35 random sites throughout each culture dish using a microscope (Axiovert 35; Zeiss, Inc., Thornwood, NY) with a 20× LP Acrostigmat objective; focus was adjusted to the midline of cysts in the field so that the monolayer of cells in the cyst wall was in focus. Three different cultures were analyzed from each of two independent experiments for each clone ±HGF/SF and ±Dox. Images were printed, and the number of cell extensions from each cyst in the field was counted manually, binned, and tabulated. Pooled counts from three cultures were used to determine the percentage of cysts with a certain number of extensions within each bin or the average number of extensions per cyst; statistical significance was calculated by Student's t test.
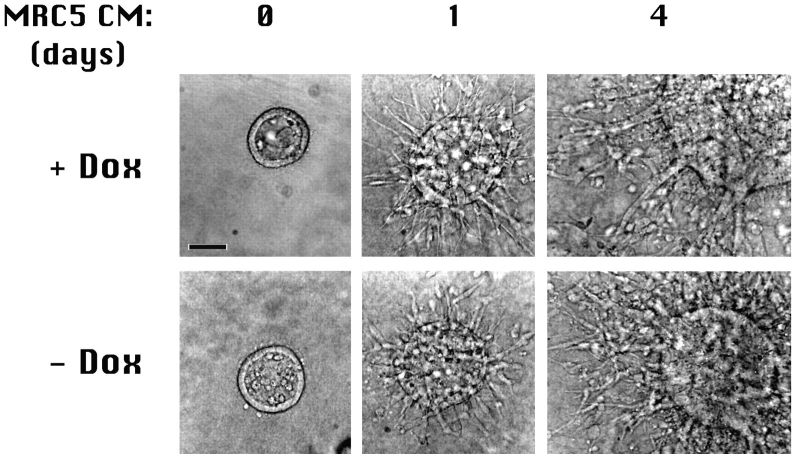
Figure 1.
Tubulogenesis is not affected by Dox. Cysts derived from the parental (T23) clone of MDCK cells were grown and treated with HGF/SF in the presence (+ Dox) or absence (− Dox) of doxycycline. Cultures grown (0 d) and treated for either 1 or 4 d with HGF/SF demonstrate that Dox had no effect on cyst formation or tubule development. Bar, 50 μm.
Immunofluorescence Confocal Microscopy
MDCK cysts were rinsed with PBS, pH 7.4, containing 1 mM CaCl2, 0.5 mM MgCl2 (PBS+), fixed for 20 min with 4% paraformaldehyde in PBS+ and 10 min with 4% paraformaldehyde in PBS+/0.025% saponin, permeabilized for 30 min with 0.025% saponin in PBS+, rinsed with PBS+, and quenched for 10 min with 75 mM NH4Cl, 20 mM glycine in PBS+, pH 8.0. Nonspecific binding sites for antibodies were blocked by incubating collagen gels for 30 min in blocking buffer (BB) containing PBS+, 0.025% saponin, 0.7% fish skin gelatin, followed by 10 min in BB containing 0.1 mg/ ml boiled RNase A. Samples were then rocked for 1–3 d at 4°C in primary antibody diluted in BB containing 0.02% Sodium–azide. Primary antibodies included: rr1 mAb supernatant (anti–E-cadherin; Gumbiner and Simons, 1986); affinity purified KT3 mAb supernatant (KT3 epitope tag on mutant β-catenins; Barth et al., 1997); and a mouse mAb to β-catenin (Transduction Labs, Lexington, KY). Rabbit polyclonal antibodies to APC protein were kindly provided by P. Polakis (ONYX Pharmaceuticals, Richmond, CA) and I. Näthke (Stanford University, Stanford, CA; Näthke et al., 1996). Collagen gels were washed extensively in PBS+/saponin and BB and incubated for 18 h at 4°C in BB containing FITC- or Texas red-conjugated secondary antibody (Jackson ImmunoResearch Laboratories, West Grove, PA; 1:100 dilution) and/or propidium iodide (ppI; Sigma Chemical Co.; 1:1,000 dilution from a 3–4 mg/ml stock). After extensive washing with PBS+/saponin and BB, collagen gels were postfixed with 4% paraformaldehyde in 0.1 M cacodylate buffer, pH 7.4, and mounted in Vectashield (Vector Labs, Burlingame, CA). Confocal images were collected using a krypton–argon laser with K1 and K2 filter sets coupled to a confocal head (MRC600; BioRad, Richmond, CA) and a Nikon microscope (Optiphot II) with a Plan Apo 60× 1.4 NA objective. Individual sections were selected from sets of serial sections collected in the x–y plane that completely spanned through cysts. Data were analyzed using Cosmos software and NIH Image 1.6. Images were converted to TIFF format, contrast levels of the images adjusted, and composites of contrast-corrected images were prepared using Adobe Photoshop (Adobe Co.) on a Power Macintosh (7200/75; Apple Computers).
Results
MDCK cells seeded in a collagen gel matrix grow clonally to form cysts that comprise a monolayer of polarized cells that surround a fluid-filled lumen (Wang et al., 1990; Montesano et al., 1991b ; Santos et al., 1993; Fig. 1, 0 d). In the presence of conditioned medium from MRC5 cells that contains HGF/SF, MDCK cysts are stimulated to form branching tubules (Montesano et al., 1991a ,b; Fig. 1, 1 and 4 d). Tubulogenesis of MDCK cells in vitro can be subdivided into 4 major stages: (1) extension of cell processes from the wall of the cysts; (2) formation of cell chains that remain in contact with the cyst; (3) lengthening and conversion of chains of cells to thickened cords; and (4) formation of long, blind-ended tubules that connect with the lumen of the cyst (Pollack, A.L., K.E. Mostov, manuscript submitted for publication). These events require localized changes in cell adhesion and migration through which cells emigrate and rearrange into new structures without loss of cell–cell contact (Pollack, A.L., and K.E. Mostov, manuscript submitted for publication). To investigate the role of β-catenin in tubule development we analyzed two time periods after addition of HGF/SF (Fig. 1): day 1 (stages 1 and 2), when cell extensions form and chains of cells are initiated; and day 4 (stages 3 and 4), when cords of cells have been established and some tubule lumens have formed.
To examine functions of β-catenin in MDCK cell tubulogenesis, we expressed mutant β-catenins with NH2-terminal deletions of either 90 (ΔN90) or 131 (ΔN131) amino acids. ΔN90 β-catenin retains binding sites for α-catenin, E-cadherin, and APC protein, whereas ΔN131 β-catenin binds E-cadherin and APC protein but not α-catenin (Barth et al., 1997). For comparison, we also examined tubulogenesis of MDCK cysts derived from parental (T23) cells, cells expressing β-cat*, and cells expressing a control protein, luciferase. Expression of exogenous genes was under the control of the Dox repressible transactivator; in the presence of Dox, gene expression was repressed, and in the absence of Dox β-cat*, mutant β-catenins or luciferase was expressed (for details of β-catenin expression see Barth et al., 1997).
We first examined whether addition of Dox affected tubulogenesis. MDCK parental cell line (T23) cysts were grown and stimulated with MRC5 cell-conditioned medium in the presence or absence of Dox. Nomarski interference contrast images show that formation of initial cell extensions (day 1), complex reorganization of cells into cords (day 4), and development of tubules (data not shown) is similar for parental (T23) cells in the presence or absence of Dox (Fig. 1). Thus, Dox does not affect the timing or extent of morphological changes in cell organization during tubulogenesis.
Expression of β-catenin Mutants (ΔN90, ΔN131) Decreases HGF/SF-stimulated Cell Extension and Cell Chain Formation (Day 1)
The following clones of MDCK cells were seeded in collagen gel matrices in the presence or absence of Dox: parental cell line (T23), luciferase-D (Lu-D), ΔN90-A β-catenin, and ΔN131-D β-catenin. MDCK cysts formed from cells expressing mutant β-catenin or luciferase appear morphologically identical to cysts derived from parental cells; phase contrast microscopy shows that all cysts comprise a closed monolayer of cells surrounding a central lumen (data not shown). To stimulate tubulogenesis, cyst medium was exchanged with conditioned medium from MRC5 cells containing HGF/SF, and cysts were examined after 1 d by Nomarski interference contrast microscopy (Fig. 2).
Figure 2.
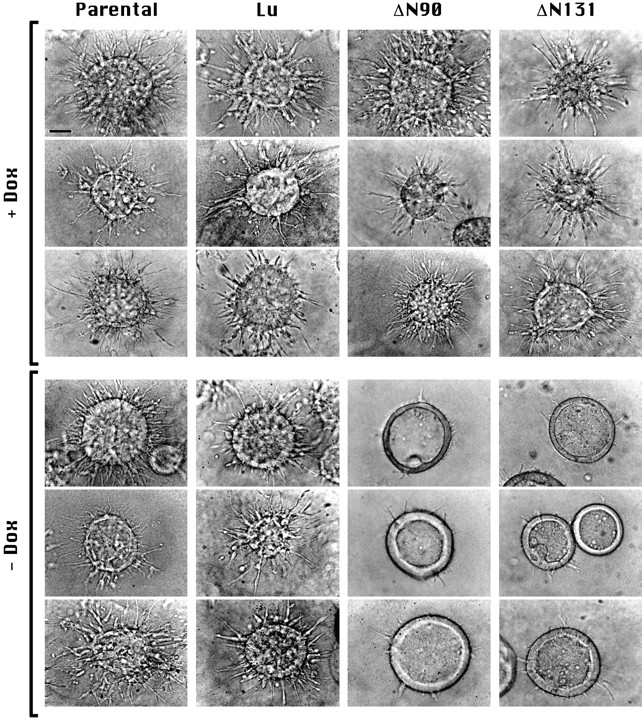
Expression of NH2-terminal deleted β-catenin inhibits early stages of tubulogenesis. Cysts were grown in the presence or absence of Dox from T23 (Parental) MDCK cells or from T23 cells transfected with Dox- repressible expression vectors for Lu or mutant β-catenins that contain NH2-terminal deletions of 90 (ΔN90) or 131 (ΔN131) amino acids. Nomarski interference contrast microscopy images of cysts treated 1 d with HGF/SF are shown. Top images (+ Dox) show typical examples of cysts in which expression of transfected proteins was inhibited with Dox. Bottom images (− Dox) show cysts in which Lu, ΔN90, or ΔN131 are expressed. Note that cysts expressing ΔN90 or ΔN131 β-catenins have fewer extensions. Representative images are shown. Experiments were repeated two to four times for each clone. Bar, 50 μm.
To determine if expression of an exogenous neutral protein affected tubulogenesis, we examined cysts formed from cells expressing Lu. Lu-D cysts cultured in either the presence or absence of Dox formed multiple cell extensions and cell chains when stimulated for 1 d with HGF/SF (Fig. 2, Lu, compare +Dox with −Dox), similar to the parental (T23) cyst response (Fig. 2, Parental, + and −Dox). Cysts derived from cells overexpressing exogenous β-cat* also formed multiple cell extensions that were indistinguishable from those formed by parental (T23) cells (data not shown; Fig. 3); note, however, that cells accumulate higher amounts of mutant β-catenins than full length β-catenin, because deletion of the NH2-terminus of β-catenin results in increased protein stability (Barth et al., 1997). Cysts derived from ΔN90-A or ΔN131-D cells, in which mutant β-catenin expression was repressed by Dox, also formed numerous cell extensions similar to those of parental (T23) and Lu-D cysts (Fig. 2, +Dox, compare Lu with ΔN90 and ΔN131). In contrast, cysts derived from cells expressing ΔN90 or ΔN131 β-catenin in the absence of Dox had significantly fewer and shorter cell extensions and chains compared to the same clones grown in the presence of Dox (Fig. 2, ΔN90 and ΔN131, compare +Dox with −Dox), suggesting that extension formation was inhibited in the presence of mutant β-catenin. Note that expression of either ΔN90 or ΔN131 β-catenin inhibited tubulogenesis, demonstrating that the inhibitory effect was independent of the ability of mutant β-catenins to bind α-catenin.
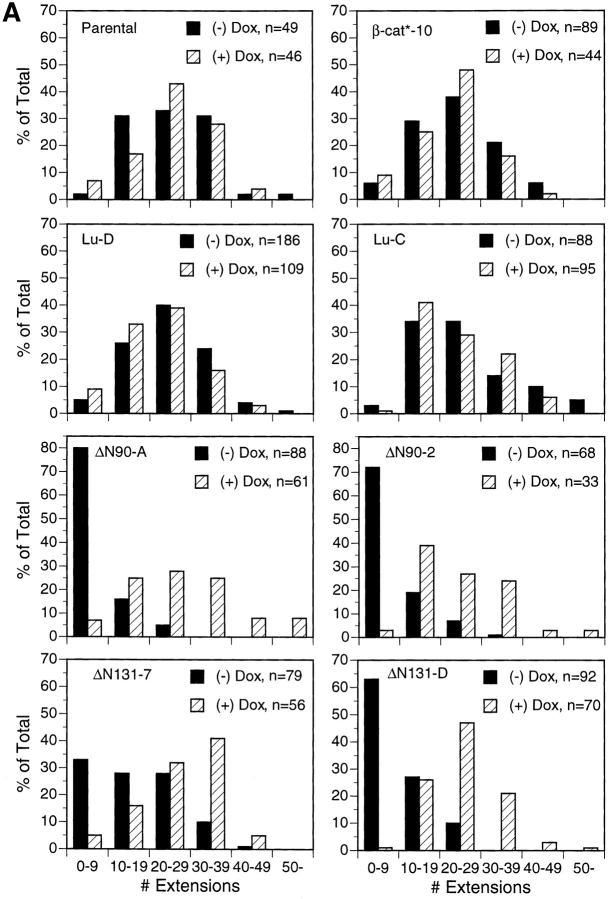
Figure 3.
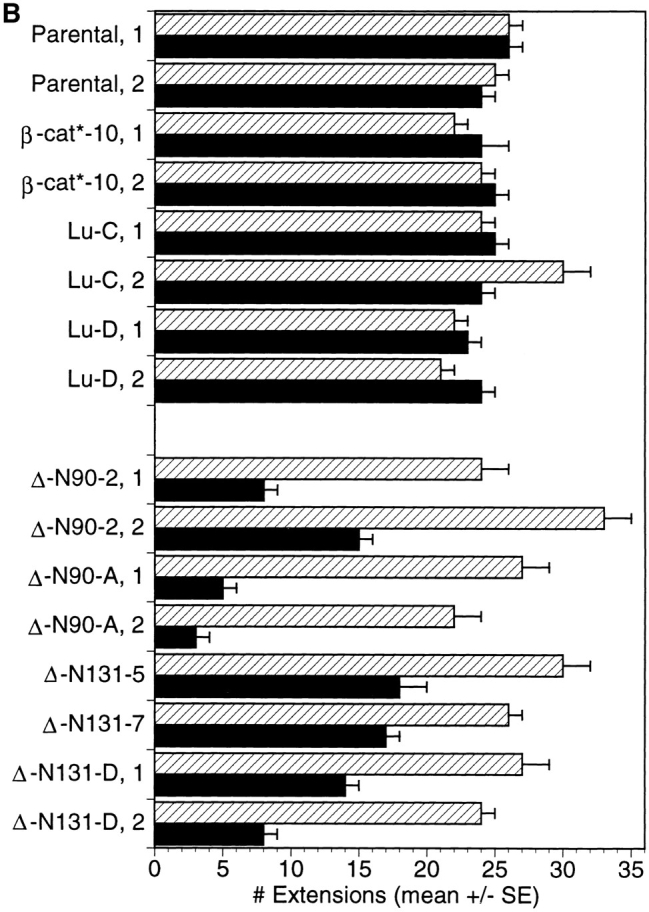
Quantification of tubulogenetic response after 1 d of treatment with HGF/SF. Cysts derived from T23 (Parental) MDCK cells, clone β-cat*–10 expressing full length β-catenin, two different luciferase clones (Lu-C and Lu-D), two different ΔN90 clones (ΔN90-A and -2), and three different clones of ΔN131 (ΔN131-D, -5, -7) were grown and treated for 1 d in the presence or absence of Dox. The number of extensions per cyst for each clone, ±Dox were counted in the midline focal plane of each cyst. Histograms in A show binned numbers of extensions per cyst. A representative experiment for each clone is shown. n, total number of cysts counted for each clone and experimental condition. The graph in B shows the average number of extensions per cyst (mean ± SEM). Quantification was obtained from two experiments for each clone. ▨ , + Dox; ▪, − Dox.
To quantify numbers of cell extensions in cysts derived from different MDCK clones, randomly selected cysts throughout each culture were photographed with the midline plane of cells in the cyst wall in focus, and the number of cell extensions in this focal plane was counted. Cysts were examined from parental (T23) cells, clone β-cat*–10 expressing full length β-catenin, Lu-C and -D clones, two independent clones of cells expressing ΔN90 β-catenin (ΔN90-2 and -A), and three independent clones of cells expressing ΔN131 β-catenin (ΔN131-D, -5, and -7). For each clone, data were compiled from the analysis of ∼25–30 cysts/culture in three separate cultures for each of two independent experiments; the data were binned and plotted as histograms (Fig. 3 A). Detailed analysis of numbers of cell extensions in cysts derived from parental (± Dox), β-cat*–10 (± Dox), Lu-C and -D (± Dox), and ΔN90 (+ Dox) and ΔN131 (+ Dox) clones reveals that the majority of cysts contained >20 cell extensions, and very few had <9 cell extensions (Fig. 3 A). In contrast, detailed analysis of the numbers of cell extensions in cysts derived from cells expressing ΔN90 and ΔN131 β-catenin (i.e., in the absence of Dox) reveals that the majority of cysts had <9 cell extensions, and very few had >20 cell extensions (Fig. 3 A).
The mean number of extensions for each condition (± Dox) and clone was calculated (Fig. 3 B). Results show that control cysts from parental cells, β-cat*–10, and Lu clones that were formed and stimulated in the presence of Dox had pooled averages of 26 ± 1, 23 ± 1, and 24 ± 2 cell extensions, respectively. In the absence of Dox, parental cells, β-cat*–10, and Lu clones had pooled averages of 25 ± 1, 25 ± 1, and 24 ± 1 cell extensions, respectively. This confirms quantitatively that neither Dox, full length β-catenin expression, nor Lu expression affected the formation of cell extensions during initial stages of HGF/SF-induced tubulogenesis. Similarly, cysts derived from ΔN90 (ΔN90 plus Dox) and ΔN131 (ΔN131 plus Dox) cells in which mutant β-catenin expression was repressed with Dox had pooled averages of 27 ± 2 and 27 ± 1 extensions, respectively. However, cysts derived from cells expressing ΔN90 β-catenin (ΔN90 minus Dox) had a pooled average of 8 ± 3 extensions, and cysts derived from cells expressing ΔN131 β-catenin (ΔN131 minus Dox) had a pooled average of 14 ± 2 extensions. The difference in number of cell extensions from cysts expressing mutant β-catenin compared to controls is significant (P < 0.01; Student's t test). These results demonstrate that expression of mutant β-catenins containing NH2-terminal deletions inhibits cell extension and chain formation.
Inhibition of Tubulogenic Activity Is Affected by the Level of Mutant β-Catenin Expression
Mutant β-catenin expression in cysts stimulated for 1 d with HGF/SF was analyzed by confocal immunofluorescence microscopy. Mutant β-catenin was distinguishable from endogenous β-catenin, because both ΔN90 and ΔN131 mutants were tagged with an epitope recognized specifically by the KT3 monoclonal antibody. In the presence of Dox, expression of ΔN90 and ΔN131 β-catenin was completely repressed, and we observed diffuse background staining with KT3 antibody (KT3; Fig. 4, A and F); as expected, these cysts formed many cell extensions, as detected by staining with propidium iodide (ppI; Fig. 4, A′ and F′). In the absence of Dox, cells within cysts had heterogeneous levels of mutant β-catenin expression; in some cells KT3 staining was strong (KT3; Fig. 4, B, C, G, and H, closed arrowheads) and in other cells, levels of KT3 staining were low (KT3; Fig. 4, B, C, G and H, open arrowheads). The reasons for heterogeneity in expression of mutant β-catenin are unknown. We note, however, that heterogeneity of expression was observed only with polarized cells in monolayer cultures (data not shown) or cysts (Fig. 4) but not in low density cultures of the same clones (Barth et al., 1997). Thus, heterogeneity in exogenous gene expression appears to be related to the differentiation status of the cells.
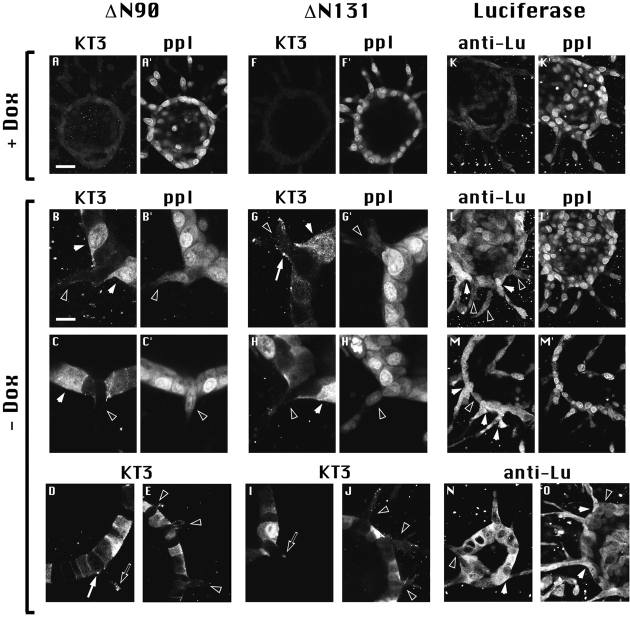
Figure 4.
Expression of ΔN90 or ΔN131 β-catenin inhibits formation of cell extensions. Cysts derived from ΔN90, ΔN131, and luciferase clones were grown and treated for 1 d with HGF/SF in the presence (A, F, and K) or absence (B–E, G–J, and L–O) of Dox and processed for double immunofluorescence labeling of KT3 and ppI (A– C′ and F–H′) or anti-luciferase (anti-Lu) and ppI (K–M′), KT3 alone (D, E, I, and J), or anti-Lu alone (N and O). In A, F, and K, the weak KT3 and anti-Lu staining shows that Dox inhibits expression of mutant β-catenins and luciferase. ppI staining in A′, F′, and K′ shows that cysts from all clones treated with HGF/SF plus Dox formed multiple extensions. Cultures in B–J and L–O were treated with HGF/ SF minus Dox. Staining with KT3 or anti-Lu detects cells with high (closed arrowheads) and low (open arrowheads) ΔN90, ΔN131, and luciferase expression. (B–J) Cells expressing high amounts of mutant β-catenins do not form extensions. Extensions formed from cells with low mutant β-catenin expression are detected with ppI. Subcellular clusters of mutant β-catenins are shown at tips of cells adjacent to extending cells (D and G; closed arrows) and at tips of cell extensions (D and I; open arrows). (L–O) Cells with both high and low luciferase expression form extensions. Bars: (A, E, F, J–O) 20 μm; (B–D and G–I) 10 μm.
The heterogeneity in exogenous gene expression proved useful in assessing the effects of mutant β-catenin expression on formation of cell extensions. All cells expressing high levels of ΔN90 or ΔN131 β-catenin did not form cell extensions. However, cells that expressed much lower levels of ΔN90 or ΔN131 β-catenin formed cell extensions (compare KT3 and ppI; Fig. 4, B, B′, C, C′, G, G′, H, and H′). An even stronger correlation between expression of mutant β-catenins and lack of formation of cell extensions is shown in slightly lower magnification images (Fig. 4, E and J). At multiple sites along each cyst wall, extensions formed from cells that had very low ΔN90 or ΔN131 β-catenin expression (Fig. 4, E and J, open arrowheads) but did not form from any of the adjacent cells expressing high levels of ΔN90 or ΔN131 β-catenin.
To determine if the inverse correlation between protein expression levels and inhibition of tubulogenesis was specific for mutant β-catenins, we examined expression levels of a control protein, Lu, versus formation of HGF/SF- induced cell extensions. Fig. 4 shows representative examples of cysts derived from a Lu-expressing clone that had been stimulated with HGF/SF for 1 d in the presence (Fig. 4, K and K′) or absence (Fig. 4, L–O) of Dox. Cysts formed multiple cell extensions when grown in either the presence or absence of Dox (Figs. 2 and 3). Immunofluorescence staining revealed that Lu expression levels in individual cells from cysts cultured in the absence of Dox are quite heterogeneous; some cells exhibit high levels of staining (Fig. 4, L–O, closed arrowheads), while others exhibit low, background levels of staining (Fig. 4, L–O, open arrowheads). Note that there is no correlation between the level of Lu expression and formation of cell extensions; cell extensions formed from cells that express high, medium, or low levels of Lu. These results establish that: (a) the factor(s) causing heterogeneous gene expression in these cysts do not affect formation of cell extensions; and (b) tubulogenic activity is specifically affected by the level of expression of NH2-terminal–deleted β-catenins. Thus, some function of the NH2-terminal region of endogenous β-catenin is essential to support early stages of tubulogenesis.
ΔN90 and ΔN131 β-Catenins Colocalize with APC Protein at the Tips of Cell Extensions
We have shown previously that in MDCK cell monolayers ΔN–β-catenin accumulates in APC protein clusters at the tips of extending cell membranes (Barth et al., 1997). In addition, results from Näthke et al. (1996) suggest that these APC protein clusters have a function in the formation of stable, microtubule-containing membrane extensions. Close inspection of the subcellular distribution of ΔN90 and ΔN131 β-catenin during early stages of tubulogenesis revealed that mutant β-catenins are localized in punctate clusters at the tips of the short cell extensions formed from cells expressing low levels of mutant β-catenin (Fig. 4, D and I; open arrows). Therefore, we sought to determine if mutant β-catenin and APC protein colocalize in these punctate structures and if their codistribution correlated with observed defects in the development of cell extensions during HGF/SF-induced tubulogenesis.
Cysts from parental, ΔN90- and ΔN131-expressing clones were grown in the absence of Dox and treated for 1 d with HGF/SF. In parental cells, immunofluorescence staining of APC protein revealed diffuse, finely granular staining in cells of the cyst wall (Fig. 5 A′), intense linear staining within cell extensions (Fig. 5 A′, open arrows), and localized accumulation in puncta at the tip of cell extensions (Fig. 5, B and C; closed arrowheads). Double immunofluorescence revealed little or no overlap in the staining patterns of endogenous β-catenin and APC protein at either cell–cell contacts or along the shaft of cell extensions (Fig. 5 A′′). Colocalization of APC protein and endogenous β-catenin was observed occasionally in puncta at the tip of cell extensions (Fig. 5 A′′, open arrowhead), although the majority of APC protein clusters was free of detectable endogenous β-catenin (Fig. 5, B and C).
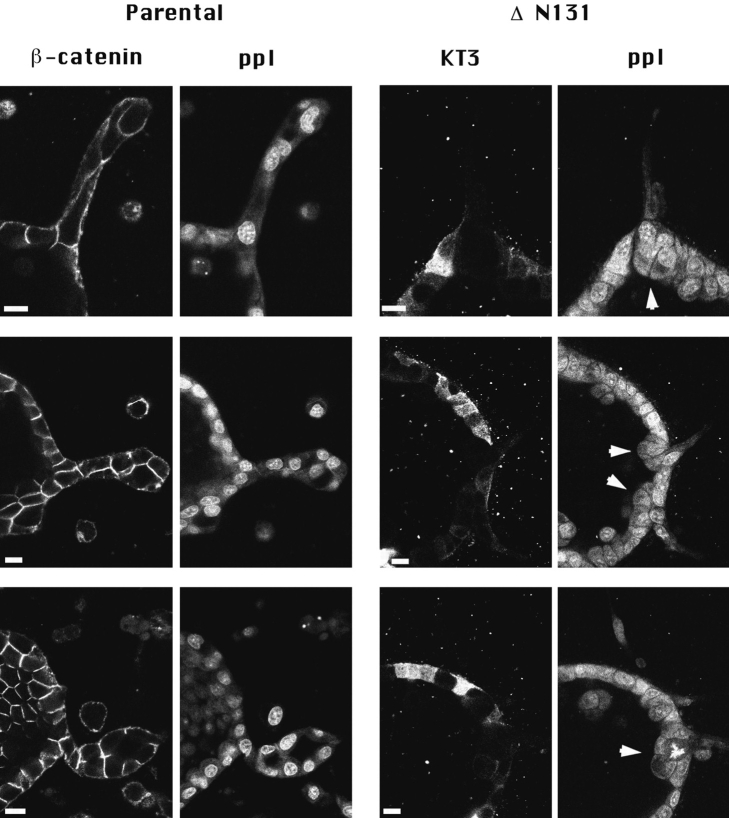
Figure 5.
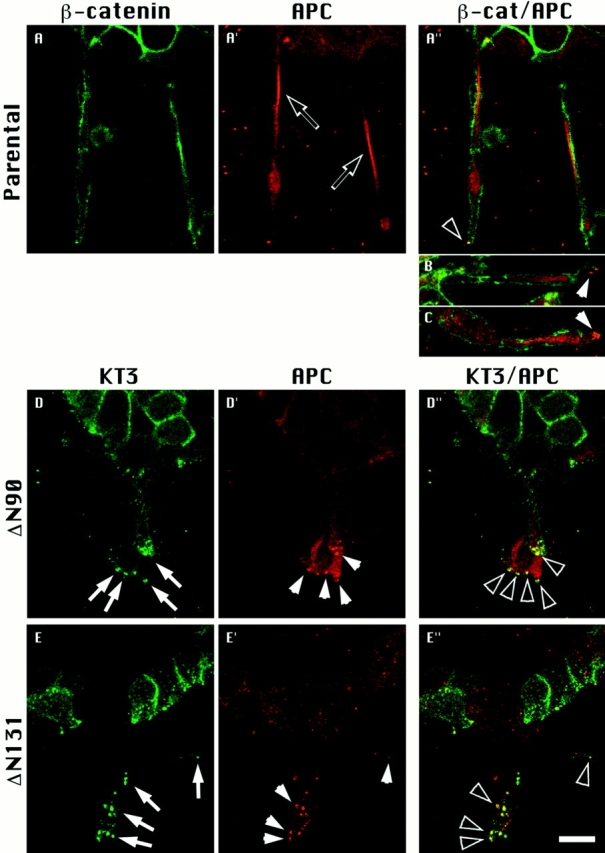
ΔN90 and ΔN131 β-catenins accumulate in clusters with APC protein. Parental cell cysts (A, A′, A′′, B, and C) and cysts expressing either ΔN90 (D, D′, D′′) or ΔN131 (E, E′, E′′) β-catenin were treated for 1 d with HGF/SF. Cultures were double labeled for APC protein (A′, D′, and E′) and either endogenous β-catenin (A) or KT3 (D and E). Merged images are shown in A′′, B, C, D′′, and E′′. Extensions from parental cysts contained bright, linear staining of APC (A′, open arrows) that did not colocalize with endogenous β-catenin (A′, B, C). Punctate APC protein staining is sometimes detected at tips of parental cell extensions (B and C, closed arrowheads) and occasionally colocalizes with endogenous β-catenin (A, open arrowheads). Punctate clusters of KT3 staining (D and E, closed arrows) are strikingly detected at tips of extensions of cells expressing ΔN90 and ΔN131 β-catenin. Punctate clusters of APC protein detected in D′ and E′ (closed arrowheads) colocalize with clusters of mutant β-catenin staining (D′′ and E′′, open arrowheads). Bar, 10 μm.
APC protein staining in cysts derived from cells expressing ΔN90 and ΔN131 β-catenin was diffuse in the cytoplasm of cells within the cyst wall and was localized in punctate clusters at the tips of short cell extensions (Fig. 5, D′ and E′, closed arrowheads), similar to APC protein staining in parental cells. Double immunofluorescence revealed that ΔN90 and ΔN131 β-catenin (Fig. 5, D and E; closed arrows) colocalized with APC protein in intensely stained punctate clusters at the tips of cell extensions (Fig. 5, D′′ and E′′, open arrowheads). In contrast to APC protein staining in parental cell extensions, staining of linear arrays of APC protein was absent in short extensions formed from cells expressing mutant β-catenins (Fig. 5, D′ and E′). Linear staining in control extensions might be due to the association of APC with a cellular structure (e.g., cytoskeleton) or may result from confinement of diffuse APC protein within long, narrow extensions. We note, however, that in control cell extensions, linear APC protein staining can be distinguished from diffuse staining near the cell membrane (Fig. 5 A′′, and data not shown), suggesting that APC protein is associated with a distinct cellular structure. The lack of linear arrays of APC protein in short extensions formed from mutant β-catenin–expressing cells likely reflects perturbation of these arrays. In addition, these results show that accumulation of ΔN90 and ΔN131 β-catenin in the APC protein clusters correlated with the inhibition of formation of long membrane extensions.
Formation of Cell Aggregates (Polyps) in the Absence of Formation of Long Cell Extensions in Cysts Stimulated with HGF/SF for Four Days
4 d after addition of HGF/SF to parental cells, developing tubules consist of multilayered cords of cells that have begun to rearrange into lumen-containing tubules (Fig. 6, left; Pollack, A.L., and K.E. Mostov, manuscript submitted for publication); the bottom left panels of Fig. 6 show that β-catenin and ppI staining are not continuous in the center of the extension, indicating that a small lumen has formed between the layers of cells. Endogenous β-catenin staining is strongly localized to cell–cell contacts within the cyst wall and between cells of the cords.
Figure 6.
Stimulation of tubulogenesis from cysts, in which cell extension is inhibited, results in the formation of cell aggregates (polyps). Cysts derived from Parental (left column) and ΔN131 β-catenin clones (right column) were stimulated for 4 d with HGF/SF in the absence of Dox. Double immunofluorescence images of endogenous β-catenin and ppI or KT3 and ppI show that cells accumulate in aggregates at the base of short cell extensions formed from cells expressing low levels of ΔN131 β-catenin (arrowheads) but not at the base of tubules developing from parental cysts. Bars, 10 μm.
In contrast to parental cells, cysts derived from cells expressing ΔN131 (Fig. 6, right), ΔN90 (data not shown), and β-catenin formed short cell extensions that had not developed further into multilayered cords of cells after 4 d in the presence of HGF/SF. These extensions were formed from cells that expressed low levels of mutant β-catenin, whereas cells expressing relatively high levels of mutant β-catenin did not form extensions (Fig. 4). In cysts formed from cells expressing ΔN131 β-catenin, we noticed the occasional accumulation of cells that were not integrated into the wall of the cyst but instead appeared to form grape-like aggregates (polyps) within the wall of the cyst (Fig. 6, right, arrowheads). These cell clusters were invariably found at the base of cells that were forming short plasma membrane extensions into the surrounding collagen gel matrix. In some cases, these cell aggregates protruded into the lumenal space of the cyst. Occasionally, cells in mitosis were detected in these cell aggregates (Fig. 6, ppI, bottom right). These cell aggregates were never found in control cysts (Fig. 6, Parental, left), nor in cysts derived from cells expressing ΔN90 β-catenin (data not shown).
Discussion
Tubulogenesis involves complex cellular rearrangements that are important for the formation of many organs and tissues during embryogenesis. For example, after mesenchymal to epithelial conversion in kidney development, cell aggregates reorganize into long tubules. These tubules are comprised of a closed epithelial monolayer surrounding a fluid-filled lumen and develop into differentiated nephrons (Saxen and Sariola, 1987). Cellular rearrangements during tubulogenesis likely involve the coordinate regulation of cell–cell interactions and cell migration (Trinkaus, 1984; Gumbiner, 1992). In this study, we demonstrate for the first time that β-catenin, a cytoplasmic protein involved in both cell–cell adhesion and cell signaling, has a role in regulating tubulogenesis. Although β-catenin binds to functionally divergent proteins, including E-cadherin, α-catenin, APC protein, fascin, and LEF-1 (Nagafuchi and Takeichi, 1989; Ozawa et al., 1989; McCrea and Gumbiner, 1991; McCrea et al., 1991; Rubinfeld et al., 1993; Behrens et al., 1996; Tao et al., 1996), we have shown that expression of NH2-terminal deleted β-catenin inhibits tubulogenesis coincident with the colocalization and accumulation of mutant β-catenin with APC protein. We suggest that dynamic interaction of β-catenin with APC protein is regulated by the NH2-terminal domain of β-catenin and is essential for normal tubule development.
To analyze the role(s) of β-catenin in epithelial tubulogenesis we expressed mutant β-catenins that contain a deletion of the NH2 terminus either before (ΔN90 β-catenin) or including (ΔN131 β-catenin) the binding site for α-catenin. Binding sites for E-cadherin and APC protein were retained in these mutants (Barth et al., 1997). Mutant β-catenin expression was controlled by the Dox-repressible transactivator (Gossen and Bujard, 1992), which allowed us to examine the same clone of cells with (− Dox) or without (+ Dox) mutant β-catenin expression. To examine stages of tubule development we exploited an in vitro tubulogenesis assay in which three-dimensional cysts formed from kidney epithelial MDCK cells are induced with HGF/SF. This assay has been shown previously to mimic cellular rearrangements that accompany tubulogenesis in vivo (Montesano et al., 1991b ; Gumbiner, 1992).
Cysts formed from cells expressing ΔN90 and ΔN131 β-catenin were similar in size and structure to cysts derived from parental (T23) cells and “control” cysts derived from Lu or full length β-catenin–expressing cells and ΔN90- or ΔN131–β-catenin cells grown under Dox repression. Control cysts and cysts derived from cells expressing ΔN90 or ΔN131 β-catenin had similar growth rates, dimensions, and comprised a closed monolayer of cells surrounding a fluid-filled lumen. Thus, expression of neither ΔN90 nor ΔN131 β-catenin affected formation of a polarized cell monolayer during cyst development. ΔN90 β-catenin binds to both E-cadherin and α-catenin and, therefore, may not interfere with E-cadherin–based adhesion. ΔN131 β-catenin, on the other hand, binds E-cadherin and not α-catenin and might be expected to disrupt binding of the cadherin complex to the actin cytoskeleton. However, levels of ΔN131 β-catenin expression may not be sufficient to compete with and disrupt the function of endogenous β-catenin in E-cadherin–based adhesion.
Although neither ΔN90 nor ΔN131 β-catenin had detectable effects on cell–cell adhesion in polarized cysts, both mutant β-catenins had strong inhibitory effects on tubulogenesis. Tubulogenesis begins with directed, protrusive plasma membrane activity of individual cells of the cyst wall, such that long membrane extensions formed as cell processes migrate in the direction of subsequent tubule development (Montesano et al., 1991b ; Pollack, A.L., and K.E. Mostov, manuscript submitted for publication). Cysts derived from cells expressing ΔN90 and ΔN131 β-catenin had significantly fewer cell extensions than control cysts after 1 d, and many cysts had little or no evidence of migration of cell processes from the cyst wall. The few cell extensions that formed from cysts expressing ΔN90 and ΔN131 β-catenin were initiated exclusively from cells that expressed low levels of mutant β-catenin. Note that inhibition of cell extensions by these mutant β-catenins is independent of their ability to bind α-catenin. Therefore, NH2-terminal deletions of β-catenin disrupt a function of β-catenin in tubulogenesis that is distinct from its role as a functional link between E-cadherin, α-catenin, and the actin cytoskeleton.
How does expression of ΔN90 and ΔN131 β-catenin inhibit directed cell extension and migration from MDCK cysts? Recently, β-catenin has been shown to bind to a transcription factor (Behrens et al., 1996; Huber et al., 1996; Molenaar et al., 1996). It is possible that mutant β-catenins interfere with the cellular response to HGF/SF by altering the expression of genes that are important for signal transduction. However, our results indicate that mutant β-catenin inhibits the motogenic but not mitogenic response to HGF/SF. Taken together with the distinctive subcellular localization of mutant β-catenin (see below), we suggest that these mutant β-catenins affect a structural component of cell migration that is required for tubulogenesis.
The localization of APC protein in clusters at the tip of actively protruding membranes has led to the suggestion that APC protein may have a function in cell migration (Näthke et al., 1996). Accordingly, during tubulogenesis we observed that APC protein was prominently localized in clusters at the tips of membrane extensions, in cells that were actively protruding from the cyst wall and their nearest neighbors but not in cells of the cyst wall that were not forming extensions. In our previous study (Barth et al., 1997) we showed that both ΔN90 and ΔN131 β-catenin were significantly more stable in APC protein complexes than full length β-catenin. This increased stability resulted in the accumulation of mutant β-catenin within APC protein clusters at the tips of membrane processes. Endogenous β-catenin and overexpressed full length β-catenin were rarely found in these APC protein clusters (Näthke et al., 1996; Barth et al., 1997). During tubulogenesis, full length β-catenin was only occasionally found colocalized in distinct clusters with APC protein in cell extensions from control cysts. Inhibition of cell extension formation during tubulogenesis, however, correlated with the accumulation of mutant β-catenin in APC protein clusters and the absence of linear cytoplasmic arrays of APC protein in the few short extensions that did form from cells expressing mutant β-catenin. Therefore, we suggest that β-catenin binding to APC protein regulates a function of APC protein that is important in the development of cell extensions during tubulogenesis.
What function of APC protein is regulated by β-catenin? It is noteworthy that in actively migrating epithelial cells, bundles of microtubules invade cell extensions and coalesce at clusters of APC protein that are localized at the leading edge of cell protrusions (Näthke et al., 1996). In vitro, APC protein binds to and bundles microtubules (Munemitsu et al., 1994), and in transfected cells, exogenous APC protein codistributes along the length of microtubules (Smith et al., 1994). Significantly, addition of nocodazole to cells results in disruption of both microtubules and APC protein localization to the tips of membrane extensions and inhibition of directed cell migration (Näthke et al., 1996). An interesting corollary to these observations is that during the formation of stable extensions in growth cone outgrowth, individual microtubules actively invade cell protrusions and are subsequently organized into bundles that stabilize the direction of migration (Sabry et al., 1991; Tanaka and Kirschner, 1991; Tanaka and Sabry, 1995). Formation of cell extensions during epithelial tubulogenesis may involve similar processes in which establishing and stabilizing the direction of migration involves the reorganization and stabilization of microtubules. As evidence of this, disruption of microtubule organization or dynamics, respectively, with colcemid or taxol, inhibits elongation and directed cell migration during HGF/SF- induced tubulogenesis (Dugina et al., 1995). We suggest that β-catenin regulates a function of APC protein in organizing microtubules that is required for the formation and/ or stabilization of cell extensions during tubulogenesis. We propose that β-catenin–APC protein interactions are normally transient, thereby allowing APC protein to function. Stabilization of the β-catenin–APC complex perturbs APC protein function, resulting in inhibition of cell migration.
After prolonged treatment with HGF/SF (4 d), cysts expressing mutant β-catenin formed aggregates (polyps) that accumulated within the cyst wall subjacent to cell extensions that were inhibited in migration. A few mitotic cells were observed in these aggregates as well as in normally developing tubules of control cysts in response to mitogenic signaling by HGF/SF. We suggest that a normal proliferative response of mutant β-catenin–expressing cells to HGF/SF, in the absence of cell migration, results in the formation of cell aggregates rather than tubules. This phenotype has an intriguing parallel to intestinal epithelium organization after mutations in APC protein. During differentiation of mammalian enterocytes, cells migrate from the crypt to the villus as a sheet of cells and are then sloughed from the villus tip (Gordon and Hermiston, 1994). However, mutations in APC protein that delete the microtubule binding site result in the accumulation of cells in polyps at the crypt/villus boundary (Polakis et al., 1995). Our results indicate that altering the function of APC protein in cell migration, through stabilization of dynamic interactions of APC protein with β-catenin, inhibits cell rearrangements during tubulogenesis. Furthermore, in the presence of continued proliferation, inhibition of cell migration may lead to the piling up of cells and the formation of cell aggregates (polyps). Taken together with the results of other studies, we suggest that in vivo disruption of the function of APC protein, either through abnormalities in binding to microtubules or alterations in the dynamics of β-catenin–APC protein interactions, may modify the timing and progression of cell rearrangements that affect the final outcome of epithelial morphogenesis.
Acknowledgments
We thank Dr. Ray Runyan (University of Arizona, Tucson, AZ) for help with digital capture of Nomarski images. We are also thankful to Drs. Inke Näthke (Stanford University, Stanford, CA) and Paul Polakis, (ONYX Pharmaceuticals, Richmond, CA) for providing APC protein antisera and for helpful discussions, to Dr. Rolf Kemler (Max Planck Institute for Immunobiology, Frieburg, Germany) for providing the β-catenin cDNA, to Dr. Hermann Bujard, (ZMDH, Heidelberg, Germany) for providing the plasmids for the tetracycline repressible expression system, and to Dr. Gernot Walter (University of California, San Diego, CA) for providing the monoclonal antibody KT3.
This work was supported by grants to W.J. Nelson from the National Institutes of Health (DK45573) and The Mathers Charitable Foundation, and by grants to K.E. Mostov from the National Institutes of Health (AI25144, AI36953, HL55980). A. Barth was supported by a North Atlantic Treaty Organization (NATO) fellowship from the Deutscher Akademischer Austauschdienst and an American Heart Association fellowship.
Abbreviations used in this paper
- APC
adenomatous polyposis coli
- β-cat*
full length β-catenin
- Dox
doxycycline
- HGF/SF
hepatocyte growth factor/scatter factor
- Lu
luciferase
- ppI
propidium iodide
Footnotes
A.L. Pollack and A.I.M. Barth contributed equally to this paper.
Please address all correspondence to Angela I.M. Barth, Department of Molecular and Cellular Physiology, Stanford University School of Medicine, Stanford, CA 94305-5426; Tel.: (415) 725-7596; Fax: (415) 725-8021; E-mail: angelab@leland.stanford.edu
A.L. Pollack's present address is Department of Cell Biology and Anatomy, University of Arizona, 1501 North Campbell Road, Tucson, AZ 85724.
References
- Aberle H, Butz S, Stappert J, Weissig H, Kemler R, Hoschuetzky H. Assembly of the cadherin-catenin complex in vitro with recombinant proteins. J Cell Sci. 1994;107:3655–3663. doi: 10.1242/jcs.107.12.3655. [DOI] [PubMed] [Google Scholar]
- Barth AIM, Pollack AL, Altschuler Y, Mostov KE, Nelson WJ. NH2-terminal deletion of β-catenin results in stable colocalization of mutant β-catenin with APC protein and altered MDCK cell adhesion. J Cell Biol. 1997;136:693–706. doi: 10.1083/jcb.136.3.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens J, von Kries JP, Kuhl M, Bruhn L, Wedlich D, Grosschedl R, Birchmeier W. Functional interaction of β-catenin with the transcription factor LEF-1. Nature (Lond) 1996;382:638–642. doi: 10.1038/382638a0. [DOI] [PubMed] [Google Scholar]
- Coleman S, Silberstein GB, Daniel CW. Ductal morphogenesis in the mouse mammary gland: evidence supporting a role for epidermal growth factor. Dev Biol. 1988;127:304–315. doi: 10.1016/0012-1606(88)90317-x. [DOI] [PubMed] [Google Scholar]
- Cox RT, Kirkpatrick C, Peifer M. Armadillo is required for adherens junction assembly, cell polarity, and morphogenesis during Drosophilaembryogenesis. J Cell Biol. 1996;134:133–148. doi: 10.1083/jcb.134.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugina VB, Alexandrova AY, Lane K, Bulanova E, Vasiliev JM. The role of the microtubular system in the cell response to HGF/SF. J Cell Sci. 1995;108:1659–1667. doi: 10.1242/jcs.108.4.1659. [DOI] [PubMed] [Google Scholar]
- Fagotto F, Funayama N, Gluck U, Gumbiner BM. Binding to cadherins antagonizes the signaling activity of β-catenin during axis formation in Xenopus. . J Cell Biol. 1996;132:1105–1114. doi: 10.1083/jcb.132.6.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funayama N, Fagotto F, McCrea P, Gumbiner BM. Embryonic axis induction by the armadillo repeat domain of β-catenin: evidence for intracellular signaling. J Cell Biol. 1995;128:959–968. doi: 10.1083/jcb.128.5.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon JI, Hermiston ML. Differentiation and self-renewal in the mouse gastrointestinal epithelium. Curr Opin Cell Biol. 1994;6:795–803. doi: 10.1016/0955-0674(94)90047-7. [DOI] [PubMed] [Google Scholar]
- Gossen M, Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci USA. 1992;89:5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumbiner BM. Epithelial morphogenesis. Cell. 1992;69:385–387. doi: 10.1016/0092-8674(92)90440-n. [DOI] [PubMed] [Google Scholar]
- Gumbiner BM. Signal transduction of β-catenin. Curr Opin Cell Biol. 1995;7:634–640. doi: 10.1016/0955-0674(95)80104-9. [DOI] [PubMed] [Google Scholar]
- Gumbiner B, Simons K. A functional assay for proteins involved in establishing an epithelial occluding barrier: identification of a uvomorulin-like polypeptide. J Cell Biol. 1986;102:457–468. doi: 10.1083/jcb.102.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haegel H, Larue L, Ohsugi M, Fedorov L, Herrenknecht K, Kemler R. Lack of β-catenin affects mouse development at gastrulation. Development. 1995;121:3529–3537. doi: 10.1242/dev.121.11.3529. [DOI] [PubMed] [Google Scholar]
- Heasman J, Crawford A, Goldstone K, Garner-Hamrick P, Gumbiner B, McCrea P, Kintner C, Noro CY, Wylie C. Overexpression of cadherins and underexpression of β-catenin inhibit dorsal mesoderm induction in early Xenopusembryos. Cell. 1994;79:791–803. doi: 10.1016/0092-8674(94)90069-8. [DOI] [PubMed] [Google Scholar]
- Hisaoka M, Haratake J, Yamamoto O. Morphological development of the rat fetal pancreas. Sangyo Ika Daigaku Zasshi. 1992;14:1–12. doi: 10.7888/juoeh.14.1. [DOI] [PubMed] [Google Scholar]
- Hisaoka M, Haratake J, Hashimoto H. Pancreatic morphogenesis and extracellular matrix organization during rat development. Differentiation. 1993;53:163–172. doi: 10.1111/j.1432-0436.1993.tb00705.x. [DOI] [PubMed] [Google Scholar]
- Hogg NA, Harrison CJ, Tickle C. Lumen formation in the developing mouse mammary gland. J Embryol Exp Morphol. 1983;73:39–57. [PubMed] [Google Scholar]
- Huber O, Bierkamp C, Kemler R. Cadherins and catenins in development. Curr Opin Cell Biol. 1996;8:685–691. doi: 10.1016/s0955-0674(96)80110-4. [DOI] [PubMed] [Google Scholar]
- Hulsken J, Birchmeier W, Behrens J. E-cadherin and APC compete for the interaction with β-catenin and the cytoskeleton. J Cell Biol. 1994;127:2061–2069. doi: 10.1083/jcb.127.6.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jou TS, Stewart DB, Stappert J, Nelson WJ, Marrs JA. Genetic and biochemical dissection of protein linkages in the cadherin-catenin complex. Proc Natl Acad Sci USA. 1995;92:5067–5071. doi: 10.1073/pnas.92.11.5067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller R, Winklbauer R. Cellular basis of amphibian gastrulation. Curr Top Dev Biol. 1992;27:39–89. doi: 10.1016/s0070-2153(08)60532-3. [DOI] [PubMed] [Google Scholar]
- Keller, R., J. Shih, and C. Domingo. 1992a. The patterning and functioning of protrusive activity during convergence and extension of the Xenopus organizer. Dev. Suppl. 81–91. [PubMed]
- Keller R, Shih J, Sater A. The cellular basis of the convergence and extension of the Xenopusneural plate. Dev Dyn. 1992b;193:199–217. doi: 10.1002/aja.1001930302. [DOI] [PubMed] [Google Scholar]
- McCrea PD, Gumbiner BM. Purification of a 92-kDa cytoplasmic protein tightly associated with the cell-cell adhesion molecule E-cadherin (uvomorulin). Characterization and extractability of the protein complex from the cell cytostructure. J Biol Chem. 1991;266:4514–4520. [PubMed] [Google Scholar]
- McCrea PD, Turck CW, Gumbiner B. A homolog of the armadillo protein in Drosophila(plakoglobin) associated with E-cadherin. Science (Wash DC) 1991;254:1359–1361. doi: 10.1126/science.1962194. [DOI] [PubMed] [Google Scholar]
- McCrea PD, Brieher WM, Gumbiner BM. Induction of a secondary body axis in Xenopusby antibodies to β-catenin. J Cell Biol. 1993;123:477–484. doi: 10.1083/jcb.123.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molenaar M, van de Wetering M, Oosterwegel M, Peterson-Maduro J, Godsave S, Korinek V, Roose J, Destree O, Clevers H. XTcf-3 transcription factor mediates β-catenin-induced axis formation in Xenopusembryos. Cell. 1996;86:391–399. doi: 10.1016/s0092-8674(00)80112-9. [DOI] [PubMed] [Google Scholar]
- Montesano R, Matsumoto K, Nakamura T, Orci L. Identification of a fibroblast-derived epithelial morphogen as hepatocyte growth factor. Cell. 1991a;67:901–908. doi: 10.1016/0092-8674(91)90363-4. [DOI] [PubMed] [Google Scholar]
- Montesano R, Schaller G, Orci L. Induction of epithelial tubular morphogenesis in vitro by fibroblast-derived soluble factors. Cell. 1991b;66:697–711. doi: 10.1016/0092-8674(91)90115-f. [DOI] [PubMed] [Google Scholar]
- Mostov KE, Breitfeld P, Harris JM. An anchor-minus form of the polymeric immunoglobulin receptor is secreted predominantly apically in Madin-Darby canine kidney cells. J Cell Biol. 1987;105:2031–2036. doi: 10.1083/jcb.105.5.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munemitsu S, Souza B, Muller O, Albert I, Rubinfeld B, Polakis P. The APC gene product associates with microtubules in vivo and promotes their assembly in vitro. Cancer Res. 1994;54:3676–3681. [PubMed] [Google Scholar]
- Munemitsu S, Albert I, Souza B, Rubinfeld B, Polakis P. Regulation of intracellular β-catenin levels by the adenomatous polyposis coli (APC) tumor-suppressor protein. Proc Natl Acad Sci USA. 1995;92:3046–3050. doi: 10.1073/pnas.92.7.3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagafuchi A, Takeichi M. Transmembrane control of cadherin- mediated cell adhesion: a 94 kDa protein functionally associated with a specific region of the cytoplasmic domain of E-cadherin. Cell Regul. 1989;1:37–44. doi: 10.1091/mbc.1.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Näthke IS, Adams CL, Polakis P, Sellin JH, Nelson WJ. The adenomatous polyposis coli tumor suppressor protein localizes to plasma membrane sites involved in active cell migration. J Cell Biol. 1996;134:165–179. doi: 10.1083/jcb.134.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noordermeer J, Klingensmith J, Perrimon N, Nusse R. dishevelled and armadillo act in the wingless signalling pathway in Drosophila. . Nature (Lond) 1994;367:80–83. doi: 10.1038/367080a0. [DOI] [PubMed] [Google Scholar]
- Oyama T, Kanai Y, Ochiai A, Akimoto S, Oda T, Yanagihara K, Nagafuchi A, Tsukita S, Shibamoto S, Ito F, et al. A truncated β-catenin disrupts the interaction between E-cadherin and α-catenin: a cause of loss of intercellular adhesiveness in human cancer cell lines. Cancer Res. 1994;54:6282–6287. [PubMed] [Google Scholar]
- Ozawa M, Baribault H, Kemler R. The cytoplasmic domain of the cell adhesion molecule uvomorulin associates with three independent proteins structurally related in different species. EMBO (Eur Mol Biol Organ) J. 1989;8:1711–1717. doi: 10.1002/j.1460-2075.1989.tb03563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa M, Ringwald M, Kemler R. Uvomorulin-catenin complex formation is regulated by a specific domain in the cytoplasmic region of the cell adhesion molecule. Proc Natl Acad Sci USA. 1990;87:4246–4250. doi: 10.1073/pnas.87.11.4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peifer, M., S. Orsulic, L.M. Pai, and J. Loureiro. 1993a. A model system for cell adhesion and signal transduction in Drosophila. Dev. Suppl. 163–176. [PubMed]
- Peifer M, Orsulic S, Sweeton D, Wieschaus E. A role for the Drosophila segment polarity gene armadilloin cell adhesion and cytoskeletal integrity during oogenesis. Development. 1993b;118:1191–1207. doi: 10.1242/dev.118.4.1191. [DOI] [PubMed] [Google Scholar]
- Polakis P. Mutations in the APC gene and their implications for protein structure and function. Curr Opin Genet Dev. 1995;5:66–71. doi: 10.1016/s0959-437x(95)90055-1. [DOI] [PubMed] [Google Scholar]
- Rimm DL, Koslov ER, Kebriaei P, Cianci CD, Morrow JS. α-1(E)-catenin is an actin-binding and -bundling protein mediating the attachment of F-actin to the membrane adhesion complex. Proc Natl Acad Sci USA. 1995;92:8813–8817. doi: 10.1073/pnas.92.19.8813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinfeld B, Souza B, Albert I, Muller O, Chamberlain SH, Masiarz FR, Munemitsu S, Polakis P. Association of the APC gene product with β-catenin. Science (Wash DC) 1993;262:1731–1734. doi: 10.1126/science.8259518. [DOI] [PubMed] [Google Scholar]
- Rubinfeld B, Souza B, Albert I, Munemitsu S, Polakis P. The APC protein and E-cadherin form similar but independent complexes with α-catenin, β-catenin, and plakoglobin. J Biol Chem. 1995;270:5549–5555. doi: 10.1074/jbc.270.10.5549. [DOI] [PubMed] [Google Scholar]
- Sabry JH, O'Connor TP, Evans L, Toroian-Raymond A, Kirschner M, Bentley D. Microtubule behavior during guidance of pioneer neuron growth cones in situ. J Cell Biol. 1991;115:381–395. doi: 10.1083/jcb.115.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos OF, Moura LA, Rosen EM, Nigam SK. Modulation of HGF-induced tubulogenesis and branching by multiple phosphorylation mechanisms. Dev Biol. 1993;159:535–548. doi: 10.1006/dbio.1993.1262. [DOI] [PubMed] [Google Scholar]
- Saxen, L. 1987. Organogenesis of the Kidney. Cambridge University Press, Cambridge, UK.
- Saxen L, Sariola H. Early organogenesis of the kidney. Pediatr Nephrol. 1987;1:385–392. doi: 10.1007/BF00849241. [DOI] [PubMed] [Google Scholar]
- Siegfried E, Perrimon N. Drosophilawingless: a paradigm for the function and mechanism of Wnt signaling. Bioessays. 1994;16:395–404. doi: 10.1002/bies.950160607. [DOI] [PubMed] [Google Scholar]
- Siegfried E, Wilder EL, Perrimon N. Components of wingless signalling in Drosophila. . Nature (Lond) 1994;367:76–80. doi: 10.1038/367076a0. [DOI] [PubMed] [Google Scholar]
- Smith KJ, Levy DB, Maupin P, Pollard TD, Vogelstein B, Kinzler KW. Wild-type but not mutant APC associates with the microtubule cytoskeleton. Cancer Res. 1994;54:3672–3675. [PubMed] [Google Scholar]
- Spooner BS, Wessells NK. Mammalian lung development: interactions in primordium formation and bronchial morphogenesis. J Exp Zool. 1970;175:445–454. doi: 10.1002/jez.1401750404. [DOI] [PubMed] [Google Scholar]
- Tanaka EM, Kirschner MW. Microtubule behavior in the growth cones of living neurons during axon elongation. J Cell Biol. 1991;115:345–363. doi: 10.1083/jcb.115.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka E, Sabry J. Making the connection: cytoskeletal rearrangements during growth cone guidance. Cell. 1995;83:171–176. doi: 10.1016/0092-8674(95)90158-2. [DOI] [PubMed] [Google Scholar]
- Tao YS, Edwards RA, Tubb B, Wang S, Bryant J, McCrea PD. β-catenin associates with the actin-bundling protein fascin in a noncadherin complex. J Cell Biol. 1996;134:1271–1281. doi: 10.1083/jcb.134.5.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinkaus, J.P. 1984. Cells into Organs; the Forces That Shape the Embryo. 2nd Edition. Prentice-Hall, Englewood Cliffs, N.J.
- Wang AZ, Ojakian GK, Nelson WJ. Steps in the morphogenesis of a polarized epithelium. I. Uncoupling the roles of cell-cell and cell-substratum contact in establishing plasma membrane polarity in multicellular epithelial (MDCK) cysts. J Cell Sci. 1990;95:137–151. doi: 10.1242/jcs.95.1.137. [DOI] [PubMed] [Google Scholar]
- Wessells NK. Mammalian lung development: interactions in formation and morphogenesis of tracheal buds. J Exp Zool. 1970;175:455–466. doi: 10.1002/jez.1401750405. [DOI] [PubMed] [Google Scholar]
- Wessells, N.K. 1977. Tissue Interactions and Development. Benjamin/Cummings, Menlo Park, CA.