Abstract
We expressed the human anti-apoptotic protein, Bcl-2, in Saccharomyces cerevisiae to investigate its effects on antioxidant protection and stationary phase survival. Yeast lacking copper-zinc superoxide dismutase (sod1Δ) show a profound defect in entry into and survival during stationary phase even under conditions optimal for survival of wild-type strains (incubation in water after stationary phase is reached). Expression of Bcl-2 in the sod1Δ strain caused a large improvement in viability at entry into stationary phase, as well as increased resistance to 100% oxygen and increased catalase activity. In addition, Bcl-2 expression reduced mutation frequency in both wild-type and sod1Δ strains. In another set of experiments, wild-type yeast incubated in expired minimal medium instead of water lost viability quickly; expression of Bcl-2 significantly delayed this stationary phase death. Our results demonstrate that Bcl-2 has activities in yeast that are similar to activities it is known to possess in mammalian cells: (a) stimulation of antioxidant protection and (b) delay of processes leading to cell death.
Bcl-2 is a human protein that was originally isolated based on its oncogenic properties (37). Unlike most carcinogens, which act by affecting cell proliferation, Bcl-2 acts by inhibiting cell death, but the precise mechanism of its action is unknown. Bcl-2 does appear to play an antioxidant role in some experimental systems, possibly by blocking the generation of oxygen radical species that play a role in apoptotic or necrotic processes (16).
Apoptosis is a type of programmed cell death, or cell suicide, in which a clearly defined and active pathway leads to an orderly death. Apoptosis has defining morphologic features in mammalian cells, but programmed cell death without some or all of these features occurs in some lower organisms (4, 28, 31).
Oxidative damage can cause cell death by either apoptosis or necrosis, depending on the degree of the damage. Expression of Bcl-2 in neuronal cells has been shown to result in decreased generation of reactive oxygen species, decreased lipid peroxidation, and decreased apoptosis (19). It has also been shown that Bcl-2 can inhibit necrotic death (death by catastrophe, which includes cell lysis and uncontrolled spillage of contents), as well as apoptotic death in mammalian neuronal cells (20). Bcl-2 was found to protect mammalian cells from death due to other oxidative insults as well, such as hypoxia/glycemia, ionizing radiation, and hydrogen peroxide (16, 32). On the other hand, it has recently been shown that Bcl-2 can inhibit oxygen-independent apoptosis (18, 33), suggesting that it can also act in a paradigm that does not require oxygen.
The yeast Saccharomyces cerevisiae is a highly studied unicellular eucaryote with some remarkable similarities to human cells at the macromolecular and organelle level. A number of yeast proteins, such as Ras (27) and superoxide dismutase (29), have been shown to be functionally interchangeable with the highly homologous human proteins. Furthermore, yeast can be easily manipulated genetically (12) and its genome has been completely sequenced (26); these features make this simple eukaryote an excellent host for study of the function of human proteins.
Yeast, like other eukaryotic cells, contain two unrelated superoxide dismutases (SODs):1 a copper- and zinc-containing form (CuZnSOD, product of the SOD1 gene) in the cytoplasm and a less abundant manganese containing form (MnSOD, product of the SOD2 gene) in the mitochondrial matrix. Yeast mutants lacking CuZnSOD (sod1Δ) are in a chronic state of oxidative stress while they are growing in air, are sensitive to hyperoxia, and are unable to grow in the absence of methionine and lysine (8). Yeast mutants lacking MnSOD (sod2Δ) have a less dramatic phenotype when grown in standard glucose media, but are sensitive to hyperoxia. The phenotype of the double mutant (sod1Δsod2Δ) is a summation of the single mutant phenotypes.
The growth cycle of a typical yeast culture in glucose medium follows a distinct pattern. First, there is a phase of logarithmic growth while cell density is relatively low and glucose is fermented. When glucose is used up the cells undergo a shift to respiratory metabolism and growth slows. Finally yeast enter stationary phase—there is no further growth, but they can survive for weeks without added nutrients, i.e., in water. Mutants lacking either SOD rapidly lose viability after reaching stationary phase, and reactive oxygen species originating from the mitochondria have been shown to play a major role in the death of these strains (24).
We previously studied Bcl-2 expressed in log phase sod1Δ yeast (deficient in CuZnSOD) and observed small improvements in the oxygen-sensitive phenotype (19). Thus, stationary phase in these strains, wherein yeast are dying due to oxidative stress, seemed an ideal one in which to study further the effects of Bcl-2 expression. While the death we observed may not be apoptotic nor necessarily due to any other form of programmed cell death, it is clearly a reproducible death pattern, and the effects we observe may therefore provide insight into the mechanism of Bcl-2 action.
In the present paper, we describe our investigation of the more dramatic effects of Bcl-2 expression on stationary phase yeast. The characteristic features of yeast in this phase (use of respiration rather than glycolysis for energy, lack of cell division, and prolonged survival time) make cells in this phase a suitable model system for higher organisms. In addition, cell death can be studied in this stationary phase model system; it is thus particularly suitable for studies of the effects of Bcl-2 expression since Bcl-2 exerts its effects in mammalian cells by preventing death rather than by promoting growth. Our goals for this study were the following: (a) to determine if Bcl-2 functions as an antioxidant in yeast; (b) to determine if Bcl-2 can block cell death in a single-celled eukaryote; and (c) to define a simple model system in which to study the mechanism of action of Bcl-2. We report here our findings that Bcl-2 reverses growth and survival defects of yeast lacking superoxide dismutase and that it substantially improves survival of wild-type S. cerevisiae under death-inducing conditions.
Materials and Methods
Yeast Strains
Strains of S. cerevisiae used in these studies are the following: EG103 (wild type) (DBY746; MATα leu2-3,112 his3Δ1 trp1-289 ura3-52 GAL +), EG118 (sod1Δ) (EG103 with sod1ΔA::URA3), and EG133 (sod1Δsod2Δ) (EG103 with sod1ΔA::URA3 sod2Δ::TRP1) (9, 23). In these strains the mutations in SOD1 and SOD2 are deletions of most of the coding region. In all the experiments reported in this paper, these strains were transformed with either plasmid pAD4, a multicopy yeast expression vector carrying the LEU2 selectable marker, or pAD4-bcl-2, the same vector carrying the human bcl-2 gene under the transcriptional control of the strong alcohol dehydrogenase (ADH1) promoter (19). Expression from this promoter is high during log phase growth and decreases at stationary phase. Transformations were carried out by the lithium acetate method (17). It should be pointed out that, as observed in our previous study (24), sod1 mutants transformed with plasmids lost viability somewhat less rapidly than untransformed mutants. This is a behavior that we have not yet explained, but which may be due to unavoidable selection for robustness during the transformation, or to an effect of the LEU2 marker, which was present in all the plasmids. To test the latter possibility, the experiments described herein were repeated with strains transformed with the plasmids pBMT116 (vector control) and pBMT116-bcl-2 (carrying human bcl-2 cDNA), which are similar to the pAD4 plasmids, except that they contain the TRP1 selectable marker. Results with these plasmids (data not shown) were similar to those reported in this paper using LEU2 plasmids.
The expression of Bcl-2 was verified by Western analysis. A 25-μg sample of total cellular protein was run on a 15% SDS–polyacrylamide gel. Purified human recombinant Bcl-2 was used as a control and Bcl-2 was detected by anti–Bcl-2 antibody (cat. # 1624 989; Boehringer Mannheim Biochemicals, Indianapolis, IN) using the Western-Light Plus Chemiluminescent Detection System (TROPIX, Inc., Bedford, MA). Bcl-2 antibody (primary antibody) was diluted 1:80 and anti–rabbit alkaline phosphatase– conjugated secondary antibody 1:10,000. In all cases where the bcl-2 gene was present (and in no cases where the gene was not present), protein that reacted with the anti–Bcl-2 antibody was detected.
Media and Growth Conditions
Unless stated otherwise, all experiments in liquid media were performed in synthetic dextrose complete media (SDC) with 2% glucose, and supplemented with amino acids, adenine and uracil as described (18) as well as a fourfold excess of the supplements Trp, Leu, His, and uracil. Strains were streaked from frozen stocks onto YPD (1% yeast extract, 2% peptone, 2% dextrose, 2% agar) plates. After 3 d of incubation, cells from a single colony on the YPD plates were transferred to 3 ml of SDC overnight medium lacking the appropriate supplement (Leu, ura, and/or Trp) to select for plasmid-containing cells (LEU +) with the correct SOD deletion (URA + for sod1Δ or TRP + for sod2Δ). For normal oxygen (high aeration) conditions, overnight cultures grown in selective media were inoculated at an OD600 of 0.01 into flasks with a flask volume/medium volume ratio of 5: 1 and grown at 30°C, shaking at 220 rpm. Growth was followed by monitoring the turbidity at 600 nm (OD600). An OD600 of 1 is equivalent to 107 cells per ml. For long-term stationary phase cultures, after 72 h, the cells were washed twice, resuspended in sterile distilled water, and incubation with shaking was continued. Cell viability was measured by plating serial dilutions of the yeast cultures onto YPD plates. Colonies formed were counted after 2 d of incubation at 30°C. Plates from cell lines lacking CuZnSOD were incubated in a low oxygen atmosphere generated using CampyPaks (BBL Scientific, Cockeysville, MD). All experiments were repeated at least three times with duplicate samples.
For experiments in 100% oxygen, cells were grown to stationary phase and on day 3 viability was measured as described above, except that plates were incubated for 48 h in glass dessicators filled with 100% oxygen, followed by incubation in air for the remainder of the time. The dessicator containing the plates was filled by evacuating and refilling it with oxygen two times. Alternatively, oxygen was gently blown in to the bottom of the dessicator for 15 min, allowing gas to escape through a small hole in the top. In either case, the container was then sealed and incubated at 30°C. Oxygen was replenished after 24 h. Identical control platings were always made and incubated in low oxygen (CampyPaks).
GSH Determination
Cells were grown as described above. At the desired time, 5 × 108 cells were pelleted at 3,000 g and the supernatant was carefully removed. The tubes were held on ice. 100 μl of 3.5% sulfosalicyclic acid and 100 μl of glass beads (0.5 μm, acid washed and dried) were added. Cells were vortexed for 1 min and then placed on ice for 1 min. This vortexing/cooling process was repeated six times. The samples were then centrifuged at 15,000 g for 3 min. The acid soluble fraction was used to determine glutathione concentration. Total glutathione was measured according to the method of Tietze (36). Oxidized glutathione (GSSG) was measured using the method of Griffith (11) in which 2-vinylpyridine was used to derivatize the reduced form of glutathione. Standard curves used in this assay contained all components used to derivatize the reduced form of the glutathione.
Enzyme Activities and Metal Ion Levels
Crude cellular protein extracts were prepared by glass bead lysis as follows. Cells were suspended in lysis buffer at 4°C (50 mM Tris 7.2, 150 mM NaCl, 5 mM EDTA, and 0.2 mM PMSF) with an equal volume of acid washed 0.5 mm glass beads, and vortexed for six to eight cycles of 30 s of vortexing followed by 30 s of cooling. The mixture was then microfuged for 2 min to remove the cellular debris and glass beads. The supernatant was frozen until assayed. The catalase activity was determined spectrophotometrically by monitoring the disappearance of hydrogen peroxide at 240 nm (25). SOD activity was determined by monitoring inhibition of the autoxidation of 6-hydroxydopamine (14, 15). Protein concentration was determined using the Bio Rad assay (Bio Rad Laboratories, Hercules, CA), with bovine serum albumin as protein standard.
Copper and manganese were determined by atomic absorption spectrophotometry, using a Varian SpectrAA (Varian Instr. Business, San Fernando, CA) equipped with graphite furnace. Whole cell samples were digested in 3% HNO3 at 100°C overnight, and then diluted to 0.1% HNO3 for analysis. Alternatively soluble protein extracts were made to 0.1% HNO3 and incubated overnight before analysis.
Mutation Frequencies
Mutation frequency was estimated by determining the number of cells able to form colonies on SDC plates lacking arginine and containing 60 μg/ml canavanine. (Resistance to canavanine, an arginine analogue, arises when mutations in the arginine permease occur.) Approximately 100 million cells were removed from cultures after 72 h of incubation and plated on canavanine plates. Colonies were counted after 4 d. Mutation frequencies are reported as mutants per 106 viable cells. Three separate experiments with duplicate platings of duplicate samples were performed.
Results
Bcl-2 Reversed Viability Defects of sod1Δ and sod1Δsod2Δ Mutants
To determine whether human Bcl-2 could overcome cell death associated with oxidative stress, we expressed Bcl-2 in strains lacking either or both SODs. In the paradigm used here (24), cells were grown to saturation in SDC medium. At 72 h, the cells were washed, resuspended in water, and the incubation continued. Under this treatment, strains lacking either SOD are known to lose viability rapidly (24), beginning at entrance into stationary phase, while wild-type cells survive up to 2 mo.
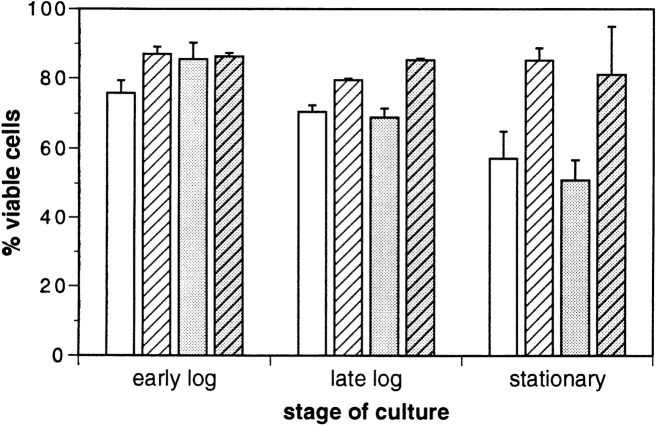
In the present study, sod1Δ and sod1Δsod2Δ mutants transformed with either the multicopy plasmid pAD4–bcl-2 or the vector control pAD4, were tested for survival. Early in the culture, when growth was logarithmic (early log phase, <2 × 107 cells/ml) Bcl-2 had little effect on the viability (Fig. 1). At late log phase (∼6 × 107 cells/ml, generally reached after 10–15 h of growth), differences began to be apparent. By the time stationary phase was reached at 24 h (10–13 × 107 cells/ml, no further growth), differences were quite obvious. At 24 h, sod1Δ and sod1Δsod2Δ strains expressing Bcl-2 maintained their viability, while the viability of strains carrying plasmid controls was markedly reduced (Fig. 1). Over a culture period of 26 d, viability for all strains continued to decrease and the strain expressing Bcl-2 continued to show a much higher survival rate than the control. Table I (first two columns) summarizes the survival of the sod1Δ and sod1Δsod2Δ strains with or without Bcl-2 expression at 26 d. Because of the large difference in viability already evident at day 1 (Fig. 1) we also show the data normalized to the viability on day 1 (Table I, last two columns). For the sod1Δ strain, the difference was statistically significant (P < 0.01 by the two-sided t test) in both cases (raw data as well as normalized). The difference for the sod1Δsod2Δ strain was not statistically significant, although the trend was always the same as for the single sod1Δ mutant. Nevertheless, the major effect of Bcl-2 expression on survival in these experiments clearly occurred at the time of entry into stationary phase, rather than in the later stages.
Figure 1.
Bcl-2 reversed stationary phase viability loss in strains lacking one or both SODs. Percent viability of sod1Δ (EG118) and sod1Δsod2Δ (EG133) strains with or without Bcl-2 expression at different stages of growth. Data are reported as percent viable cells, or colony forming units (live cells), per total cells (live and dead cells). Total cell number was determined by optical density (OD600) and confirmed by hemocytometer counting. Differences between control and Bcl-2–expressing strains at 24 h are significant (P < 0.01). (white bars) sod1Δ; (gray bars) sod1Δ- sod2Δ; (crosshatched pattern) strains expressing Bcl-2; (no pattern) strains harboring plasmid vector (pAD4).
Table I.
Long Term Viability of Strains Expressing Bcl-2
| Viable cells/ml (× 106) on day 26 | Data normalized to viability on day 1 (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Plasmid | pAD4 | pAD4-bcl-2 | pAD4 | pAD4-bcl-2 | ||||
| Host Strain | ||||||||
| Wild-type | 49.5 (0.7) | 43.0 (4.2) | 76 (1.1) | 64 (6.2) | ||||
| sod1Δ | 0.77 (1.07) | 10.9 (3.4)* | 2.5 (3.4) | 20 (6.4)* | ||||
| sod1Δsod2Δ | 0.16 (0.17) | 0.65 (0.07) | 0.5 (0.6) | 1.2 (0.1) | ||||
Cultures of wild-type (EG103) sod1Δ (EG118) and sod1Δsod2Δ (EG133) strains with and without Bcl-2 expression were grown, switched to water on day 3, and tested for viability at intervals, as described in Materials and Methods. The table shows the viability measured on day 26. Standard deviations are in parentheses. In the first two columns, total viable cells are reported. In last two columns, the same data are shown as a percent of the number of viable cells in the same culture on day 1 to correct for the loss of viability which occurred immediately upon entry into stationary phase. An asterisk marks data sets where the difference between with and without Bcl-2 was statistically significantly (P < 0.01).
Bcl-2 Improved Survival of sod1Δ Mutants in 100% Oxygen
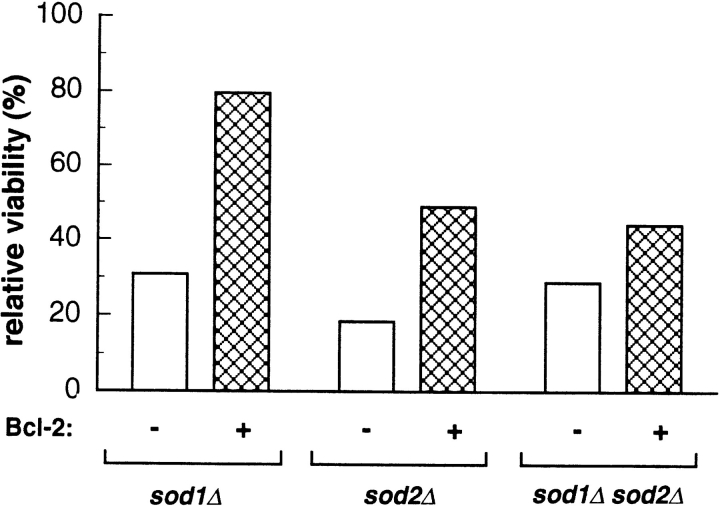
Somewhat surprisingly, wild-type yeast with or without Bcl-2 expression grew well in 100% oxygen, exhibiting 80– 100% viability relative to growth in air (data not shown). On the other hand, yeast lacking CuZnSOD and/or MnSOD were quite sensitive to 100% oxygen and were unable to form colonies under these conditions. To test the effect of Bcl-2 expression on the growth of sod1Δ, sod2Δ, and sod1Δsod2Δ mutants under this intense oxidative stress, we tested the ability of these strains to survive a 48 h exposure to 100% oxygen. As shown in Fig. 2, expression of Bcl-2 in SOD-deficient cells substantially improved their ability to form colonies after incubation in 100% oxygen. The effect was particularly pronounced for the sod1Δ single mutant, but was also evident for the sod2Δ single mutant and the double mutant.
Figure 2.
Bcl-2 expression allowed sod1Δ mutants to form colonies in 100% oxygen. sod1Δ (EG118), sod2Δ (EG110), and sod1Δsod2Δ (EG133) cells harboring pAD4–bcl-2 (crosshatched bars) or pAD4 (open bars), were grown to stationary phase and equal numbers of cells were plated on selective plates (SD–leu) and incubated in low aeration (CampyPaks) for 3 d, or in an atmosphere of 100% oxygen for 48 h followed by incubation in air. (Oxygen was replenished after 24 h.) Viability was recorded when colonies became big enough to count—at day 3 (EG118) or day 4 (EG110 and EG133)—and is reported as a percentage of the viability of the same strain in low aeration (relative viability). The experiment was performed with similar results at least four times for each strain, with two separate samples in each experiment. A representative experiment is shown.
Effects of Bcl-2 Are Not Due to Altered Copper or Manganese Metabolism
Culotta and co-workers (21–23) have recently identified several mutations that partially “rescue” the sod1Δ sod2Δ strain, and they showed that each of these mutations altered copper or manganese metabolism in some way. One of these, the pmr1 mutation, exerted its effects by increasing cellular manganese accumulation. (High intracellular manganese was previously shown to rescue sod1Δ mutants, probably by its own dismutase activity [2].) A side effect of the pmr1 mutation is an increased sensitivity to abnormally high manganese levels in the medium (21). We reasoned that, if Bcl-2 exerted its effects by a mechanism similar to that of pmr1, we should see increased sensitivity to manganese and increased manganese accumulation in strains expressing Bcl-2. In fact, we saw the opposite, expression of Bcl-2 in strains lacking SOD led to decreased sensitivity to elevated manganese levels as measured by cell survival (Fig. 3 A) and decreased manganese accumulation as measured by atomic absorption (Fig. 3 B). In other words, Bcl-2 expression caused the sod1Δ and sod1Δsod2Δ strains to exhibit a more wild-type–like phenotype.
Figure 3.
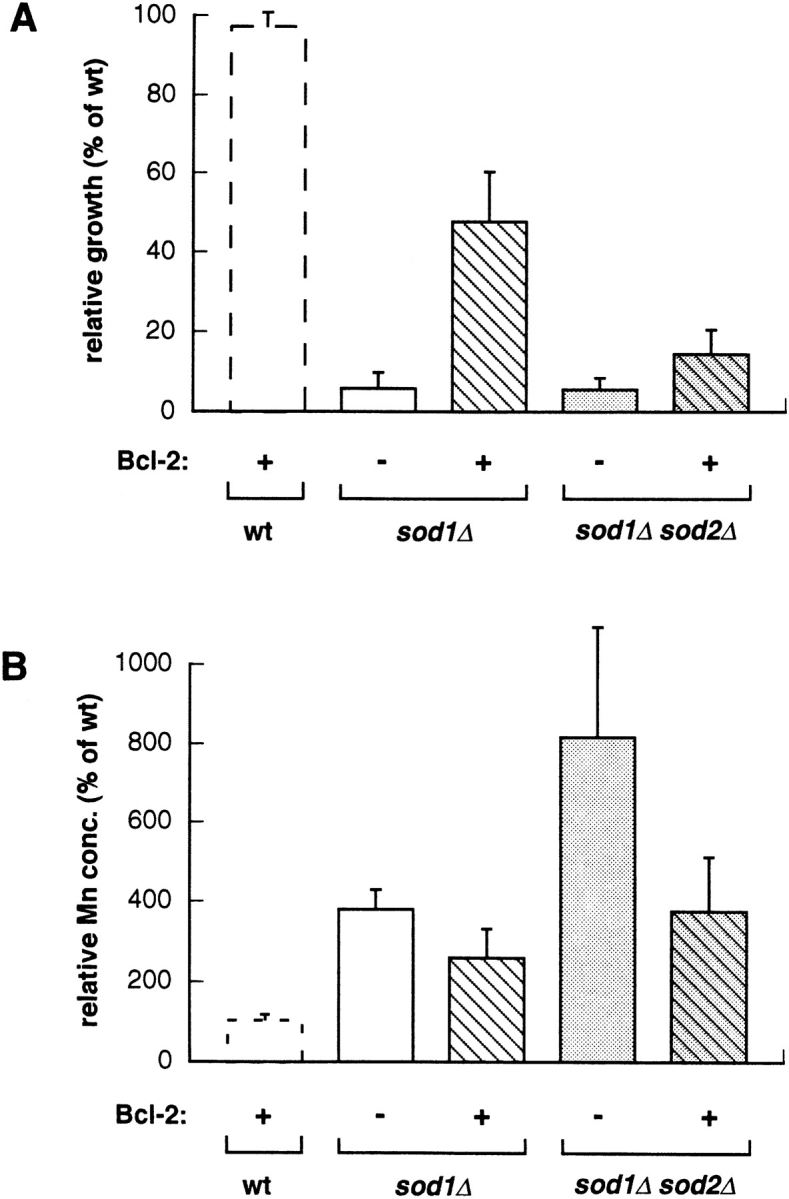
Bcl-2 expression and Mn metabolism. (A) Wild-type (EG103), sod1Δ (EG118), and sod1Δsod2Δ (EG133) cells transformed with pAD4 or pAD4–bcl-2 were inoculated at 106 cells/ml in SD–leu medium with or without 5 mM MnSO4 added, and incubated at 30°C and 220 rpm. OD600 was measured at 48 h. Experiments were performed three times with duplicate samples from two separate transformations. Results are reported relative to growth of wild type without Bcl-2 measured on the same day. (B) The same strains were analyzed for manganese accumulation by atomic absorption at stationary phase (after 3 d of incubation in SDC medium with normal levels of manganese). Experiments were repeated three times. Results are reported as percent of the manganese levels of the wild-type strain (lacking Bcl-2) measured on the same day.
Culotta and co-workers also observed that a mutation in the yeast BSD2 gene bypassed the growth requirement for SOD by altering copper homeostasis in a manner seemingly analogous to the pmr1 mutation. In this case, cells became hypersensitive to elevated copper concentrations (22). We tested sod1Δ and sod1Δsod2Δ strains expressing Bcl-2 for copper sensitivity and saw a slight, although not statistically significant, decrease in sensitivity in the Bcl-2 expressors (data not shown). This decrease came instead of the marked increase we would have expected if Bcl-2 were working through a mechanism like that of bsd2. We conclude that, unlike other suppressors of the sod1Δ phenotype, Bcl-2 does not work via effects on copper or manganese accumulation.
Indices of Antioxidant Protection in Cells Expressing Bcl-2
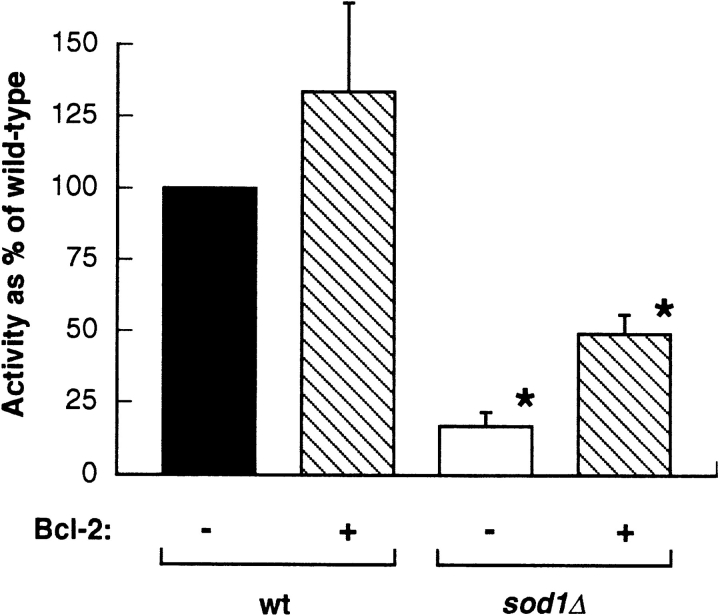
To determine if Bcl-2 had a direct effect on the status of other antioxidant proteins in yeast, we measured catalase activity in both wild-type and sod1Δ mutant cells expressing Bcl-2 (Fig. 4). Yeast has two catalases (Catalase A and Catalase T), but only the cytoplasmic Catalase T is expressed under our conditions. Catalase A is peroxisomal and only expressed under special conditions (8). We observed an increase in catalase activity for wild-type cells expressing Bcl-2 relative to vector-transformed controls. The sod1Δ mutant had a lower level of catalase than wild type. Bcl-2 expression caused an increase in catalase activity, but the activity was still lower than that of the wild-type strain (Fig. 4).
Figure 4.
Bcl-2 expression increased catalase activity in stationary phase yeast. Protein extracts were prepared from wild-type and sod1Δ cells transformed with pAD4 or pAD4–bcl-2 and grown 72 h (to stationary phase). Catalase activity was measured as described in Materials and Methods. Results are the average of four experiments and are reported as percent of wild-type activity; error bars represent standard deviation. The asterisks indicate data sets that are significantly different (P < 0.05 by the two-sided t test). The difference between wild-type and either sod1Δ strain was also statistically significant. Specific activity for wild type was 770 U/mg protein.
We also monitored levels of reduced and oxidized glutathione (GSH and GSSG, respectively) in wild-type cells with or without expression of Bcl-2. Total glutathione (GSH + GSSG) levels were unchanged by Bcl-2 expression, but the GSH:GSSG ratio increased from 85:1 for the vector-transformed control to 105:1 for the Bcl-2 expressor, indicating a slightly more reducing condition in the cells expressing Bcl-2. This result was significant to the P < 0.1 level.
Mutation Frequencies Increased in sod1Δ and sod1Δsod2Δ Mutants Compared to Wild Type, but Decreased in Yeast Expressing Bcl-2
Steinman reported that expression of Bcl-2 in Escherichia coli led to increased catalase expression and a higher mutation rate, and he therefore proposed a pro-oxidant mechanism for Bcl-2 in this system (33). To determine whether a similar phenomenon occurred in yeast expressing Bcl-2, we measured mutation frequencies, using forward mutation to canavanine resistance. As expected based on earlier work (9), mutation frequencies were clearly higher in sod1Δ and sod1Δsod2Δ mutants than in the wild-type parental strain. However, in all three cell lines, Bcl-2 expression decreased mutation frequencies (Table II). This result is consistent with an antioxidant activity for Bcl-2, since oxidative damage is believed to be the cause of much spontaneous mutation and is almost certain to be the cause of the excess spontaneous mutation found in strains lacking CuZnSOD.
Table II.
Mutation Rates
| Strain | Experiment 1 | Experiment 2 | Experiment 3 | Average (SD) | ||||
|---|---|---|---|---|---|---|---|---|
| wt/pAD4 | 2.8 | 5.0 | 3.3 | 3.7 (1.1) | ||||
| wt/pAD4-bcl-2 | 2.2 | 3.3 | 2.1 | 2.5 (0.7) | ||||
| sod1Δ/pAD4 | 6.5 | 6.3 | 6.5 | 6.4 (0.1) | ||||
| sod1Δ/pAD4-bcl-2 | 3.3 | 4.3 | 5.0 | 4.2 (0.8) | ||||
| sod1Δsod2Δ/pAD4 | 7.6 | 11.3 | 8.5 | 9.1 (1.9) | ||||
| sod1Δsod2Δ/pAD4-bcl-2 | 3.7 | 7.0 | 6.3 | 5.7 (1.7) |
Cells were grown for 72 h and then plated on plates containing canavanine. Mutation to canavanine resistance is expressed as resistant cells per 106 viable cells. Three separate experiments were performed and are reported separately. The average and standard deviation are also reported.
Bcl-2 Prolonged Wild-Type Yeast Survival in Spent Medium
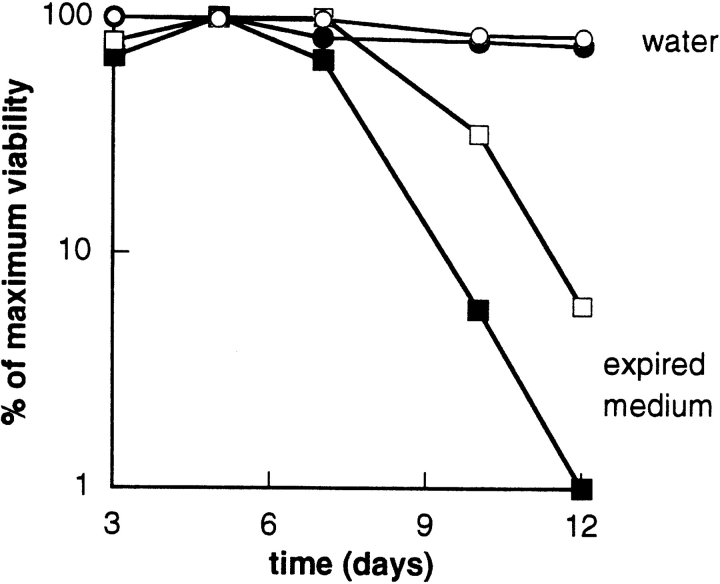
We recently observed that yeast cells left in expired synthetic glucose medium (SDC), but not in rich medium (YPD) or water, lost viability quickly (Longo, V.D., P. Morcos, T.E. Johnson, E.B. Gralla, and J.S. Valentine, manuscript submitted for publication). To determine whether Bcl-2 could affect this type of cell death, we studied survival in water and in expired SDC medium of wild-type yeast expressing Bcl-2. Wild-type yeast left in complete glucose medium (SDC) rapidly lost viability beginning at day 5. The viability loss was significantly delayed by expression of Bcl-2 such that there was a five- to sixfold difference in the number of survivors by day 10 (Fig. 5). To be sure that the viability loss was not caused by the nutritional auxotrophies of the EG103 wild-type strain, survival in spent medium was also measured for the prototrophic strains D27310b, which also lost viability prematurely (data not shown). By contrast, no effect of Bcl-2 was seen on survival of wild-type yeast under conditions optimal for stationary phase survival, i.e., switched to water at day 3, probably because survival was already very high (Fig. 5).
Figure 5.
Bcl-2 delayed viability loss in wild-type yeast. Wild-type yeast (EG103) transformed with pAD4 or pAD4–bcl-2 were grown in complete glucose medium (SDC), and left in the medium (□, ▪), or switched to water at 72 h (○, •). Viability was assayed by plating on YPD plates at the indicated times. The experiment was repeated four times with similar results. A representative experiment is shown. Note that the viability is reported on a log scale. pAD4 transformed (▪, •); pAD4–bcl-2 transformed (□, ○).
Discussion
The yeast sod1Δ and sod1Δ sod2Δ strains provide a simple model system for studies of the properties of Bcl-2. The presence of Bcl-2 prevented short-term death of both yeast sod1Δ and sod1Δ sod2Δ mutants at the point of entry into stationary phase (Fig. 1). In addition, it improved, although less dramatically, their long-term survival (Table II). Furthermore, Bcl-2 enabled sod1Δ, but not sod1Δsod2Δ, mutants to grow in 100% oxygen (Fig. 2) and increased indicators of antioxidant defense, i.e., catalase (Fig. 4) and reduced glutathione. These results confirm an antioxidant property for Bcl-2 and suggest that it functions in this regard by acting on basic intracellular mechanisms present in all eukaryotes (in human cells as well as in yeast).
Mechanisms of oxidative stress and antioxidant defenses in vivo are intimately connected with metal metabolism. Most pathways that have been proposed for generation of significant fluxes of reactive oxygen species in vivo involve participation of redox active metal ion catalysts (usually copper or iron ions). Defensive mechanisms also often involve metal ions, e.g., superoxide dismutase and catalase are metalloenzymes. Studies on sod1Δ yeast have demonstrated the importance of metal ions in oxidative stress resistance. First, high levels of extracellular manganese ion rescued sod1Δ strains, apparently because excess manganese accumulated in the cytoplasm and was able to function as a weak dismutase (2). In addition, sod1Δ strains have been shown to be partially rescued by excess copper in the medium. This rescue depended upon intracellular binding of the metal by the yeast copper metallothionein protein (34), rather than on a “free” metal ion as is postulated for manganese, but is otherwise similar.
Thus far, all mutations identified that reverse growth defects of sod1Δ and sod1Δ sod2Δ yeast appear to cause accumulation of manganese or copper in the cytosol. If Bcl-2 were involved in the accumulation of manganese or copper, as are the products of the PMR1 and BSD2 genes, respectively (21, 22), we would have expected a higher sensitivity to elevated concentrations of these metal ions in cells expressing the oncoprotein. We found instead that Bcl-2 improves the growth of sod1Δ and sod1Δ sod2Δ mutants in the presence of both metals but especially manganese (Fig. 3), indicating that it works in another fashion. We have not yet determined whether this improvement is due to a general decrease of the oxidative stress in the cells or to a direct involvement of Bcl-2 in manganese homeostasis or subcellular distribution.
Mammalian neural cells expressing Bcl-2 have recently been shown to contain increased concentrations of glutathione (5, 19). The expression of Bcl-2 in E. coli was shown to induce catalase expression without exposure to exogenous hydrogen peroxide. It also led to increased mutation rates, leading the author to conclude that this oncoprotein functions as a prooxidant (33). In yeast, we also observed an increase in catalase activity in strains expressing Bcl-2 (Fig. 4), but the spontaneous mutation frequency was reduced by Bcl-2 expression (Table II). In addition, wild-type yeast expressing Bcl-2 had slightly higher GSH/ GSSG ratios than wild-type controls, although no difference in total glutathione was observed. Taken together, these data argue against a prooxidant effect of this oncoprotein in yeast and support a role for Bcl-2 in promoting antioxidant defenses.
We observed that wild-type yeast kept in expired SDC medium (not switched to water) during stationary phase lost viability rapidly. While the death of sod1Δ and sod1Δ- sod2Δ strains is known to be oxidative (24) it is not clear to what to attribute the death of wild-type cells in expired medium. The death of wild-type cells in expired medium was delayed by the expression of human Bcl-2 (Fig. 5). The precipitous viability loss we observed for yeast in expired minimal medium was quite surprising, especially in view of the fact that yeast transferred to water show no such dramatic viability loss and instead survive for months. A possible explanation is based on the assumption that yeast are likely to encounter a nutrient-depleted environment quite often in the wild and therefore might be expected to recognize and respond to such a condition. It is thus possible that death could be an evolved response to such a situation. One's first notion is that in a unicellular organism such as yeast, each individual cell competes against all others. However, this approach may not be the most effective for maintenance of the species. Yeast grow in colonies that are generally isogenic, so survival of the genes of a single colony is guaranteed even if only a few organisms survive. The cell death we observed in expired medium could be explained by a specific cell suicide program, evolved to kill most members of a colony so that a few better-adapted individuals could survive and grow on the limited nutrients. This putative death program would increase the chance of survival of the species. The better adaptation of the lucky individual(s) that survived could be due either to mutations (leading to natural selection) or simply to random variation in the micro-environment. (Note that such a suicide response would not be expected to occur in pure water. Since there are no nutrients to be shared, the best response is for each cell to last as long as possible.)
Ras2 is one of two yeast Ras proteins that play roles in growth control and have structural and functional homology to the ras family of mammalian proto-oncogenes. In yeast, Ras is coupled to a signal cascade that involves intracellular cAMP and is sensitive to nutritional status (7, 35). In another study, we found that the viability loss of yeast in expired minimal medium was prevented by a null mutation in ras2, suggesting that Ras2 is involved in a cell death pathway (Longo, V.D., P. Morcos, T.E. Johnson, E.B. Gralla, and J.S. Valentine, manuscript submitted for publication). Interestingly, extracellular cAMP and starvation are involved in initiating the cell death program of stalk cells in the developmental cycle of the primitive eukaryote Dictyostelium (4). In human cells, Bcl-2 has been shown to co-precipitate with both R-Ras and p21-Ras (3, 6) both of which are highly homologous to yeast Ras2 (7). These observations raise the possibility that Bcl-2 may prevent cell death in yeast by acting through the Ras pathway.
Evidence is slowly accumulating that some lower eukaryotes, even unicellular ones, exhibit programmed cell death, which, however, may or may not share the morphological features that define apoptotic cell death (1). For example, a cell death program was demonstrated in E. coli, consisting of a plasmid-encoded DNA restriction/modification system that caused DNA cuts but not other typical apoptotic morphologies (28). Cell death during stalk formation in Dictyostelium followed a distinct program that included chromatin condensation, but not DNA fragmentation (4). Other, nonapoptotic forms of PCD may occur in higher eukaryotes as well. For example, none of the features characteristic of apoptosis were observed in the PCD of intersegmental muscle of the moth Manduca sexta at the end of metamorphosis (31).
The results presented here are consistent with the presence of a programmed cell death pathway in yeast, but they are equally consistent with an antioxidant role for Bcl-2 which could be acting on pathways or proteins that promote compensatory antioxidant defenses in sod null mutants as well as wild-type cells under certain circumstances. Our searches of the yeast whole genome database did not reveal the presence of homologues of pro-apoptotic proteins such as ICE or CED-3, suggesting that a programmed cell death in yeast, if it does indeed exist, may not involve the proteins and mechanisms that cause apoptosis in higher eukaryotes. No Bcl-2 homologues with high identity were found either, but we cannot exclude the possibility that functional rather than sequence homologues of Bcl-2 may be present in yeast, or that bcl-2 is a relatively new feature of apoptosis, added later in evolution.
There are only a few reports in the literature examining members of the bcl-2 gene family expressed in yeast (10, 13, 30). These papers are based on the finding that expression of Bax, a cell death–inducing Bcl-2 family member, is toxic to yeast. Using the two-hybrid system, Reed and co-workers (13, 30) showed that this Bax-induced cell death was inhibited by co-expression of Bcl-2 and some Bcl-2 homologues which are known to interact physically with Bax. However, since expression of foreign proteins fairly frequently causes cell death, it is not clear in these studies whether the death observed is a specific triggered cell death pathway or simply the result of a non-specific toxicity. If the latter is the case, then rescue by Bcl-2 could be explained simply as a case of Bcl-2/Bax dimerization masking the toxicity. Our results demonstrate an antideath activity for Bcl-2 in yeast in the absence of Bax, increasing the possibility that a real cell death program is being observed.
Recent work (10) using a similar system, demonstrated that Bax-related growth inhibition could be experimentally separated from cellular death in S. cerevisiae and that the presence of functional mitochondria was necessary for cell death to occur. These results make it appear more likely that Bax is acting on an endogenous system. In our work, both the oxidative stress-related death of sod1Δ and sod1Δ sod2Δ mutants in stationary phase and the death of wild-type cells in expired minimal medium occurred without introduction of any foreign genes, i.e., they are naturally occurring processes. That Bcl-2 is able to prevent both kinds of death is therefore strong evidence that it is acting on, or in concert with, an endogenous pathway that already exists in yeast.
In conclusion, our results demonstrate that Bcl-2 can provide antioxidant protection in yeast and that it can delay natural death in two separate paradigms. The fact that Bcl-2 exerts an antioxidant-type protective effect in yeast is evidence supporting the centrality of this role. However, it is important to stress that the mechanism by which the antioxidant protection occurs and the connection between the antideath and antioxidant actions of Bcl-2 in our system, as well as in mammalian cells, remains unknown.
Acknowledgments
This work was supported by National Institutes of Health grants DK46828 (to J.S. Valentine), AG12282, (to D.E. Bredesen), NS25554, (to D.E. Bredesen), and a pilot research grant from the University of California at Los Angeles Center on Aging based on a generous gift to the center by Harold and Libby Ziff (to J.S. Valentine and E.B. Gralla).
Footnotes
1. Abbreviations used in this paper: SDC, synthetic dextrose complete medium; SOD, superoxide dismutase; YPD, yeast extract/peptone/dextrose.
Please address all correspondence to E.B. Gralla, Department of Chemistry and Biochemistry, University of California at Los Angeles, Box 156905, Los Angeles, CA 90095-1569. Tel.: (310) 825-2807. Fax: (310) 206-7197. e-mail: egralla@chem.ucla.edu
References
- 1.Ameisen JC. The origin of programmed cell death. Science (Wash DC) 1996;272:1278–1279. doi: 10.1126/science.272.5266.1278. [DOI] [PubMed] [Google Scholar]
- 2.Chang EC, Kosman DJ. Intracellular Mn (II)-associated superoxide scavenging activity protects Cu,Zn superoxide dismutase-deficient Saccharomyces cerevisiaeagainst dioxygen stress. J Biol Chem. 1989;264:12172–12178. [PubMed] [Google Scholar]
- 3.Chen CY, Faller DV. Phosphorylation of Bcl-2 protein and association with p21Ras in Ras-induced apoptosis. J Biol Chem. 1996;271:2376–2379. doi: 10.1074/jbc.271.5.2376. [DOI] [PubMed] [Google Scholar]
- 4.Cornillon S, Foa C, Davoust J, Buonavista N, Gross JD, Golstein P. Programmed cell death in Dictyostelium. . J Cell Sci. 1994;107:2691–2704. doi: 10.1242/jcs.107.10.2691. [DOI] [PubMed] [Google Scholar]
- 5.Ellerby LM, Ellerby HM, Park SM, Holleran AL, Murphy AN, Fiskum G, Kane DJ, Testa MP, Kayalar C, Bredesen DE. Shift of the cellular oxidation-reduction potential in neural cells expressing Bcl-2. J Neurochem. 1996;67:1259–1267. doi: 10.1046/j.1471-4159.1996.67031259.x. [DOI] [PubMed] [Google Scholar]
- 6.Fernandez-Sarabia MJ, Bischoff JR. Bcl-2 associates with the ras-related protein R-ras p23. Nature (Lond) 1993;366:274–275. doi: 10.1038/366274a0. [DOI] [PubMed] [Google Scholar]
- 7.Gibbs JB, Marshall MS. The ras oncogene—an important regulatory element in lower eucaryotic organisms. Microbiol Rev. 1989;53:171–185. doi: 10.1128/mr.53.2.171-185.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gralla EB, Kosman D. Molecular genetics of superoxide dismutases in yeasts and related fungi. Adv Genet. 1992;30:251–319. doi: 10.1016/s0065-2660(08)60322-3. [DOI] [PubMed] [Google Scholar]
- 9.Gralla EB, Valentine JS. Null mutants of Saccharomyces cerevisiaeCu,Zn superoxide dismutase: characterization and spontaneous mutation rates. J Bacteriol. 1991;173:5918–5920. doi: 10.1128/jb.173.18.5918-5920.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greenhalf W, Stephan C, Chandhuri B. Role of mitochondria and C-terminal membrane anchor of Bcl-2 in Bax induced growth arrest and mortality in S. cerevisiae. . FEBS Lett. 1996;380:169–175. doi: 10.1016/0014-5793(96)00044-0. [DOI] [PubMed] [Google Scholar]
- 11.Griffith OW. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinyl pyridine. Anal Biochem. 1980;106:207–212. doi: 10.1016/0003-2697(80)90139-6. [DOI] [PubMed] [Google Scholar]
- 12.Guthrie, C., and G.R. Fink. 1991. Guide to Yeast Genetics and Molecular Biology. Methods in Enzymology (Vol. 194). J.N. Abelson and M.I. Simon, editors. Academic Press, Inc., San Diego, CA. 931 pp. [PubMed]
- 13.Hanada M, Aimé-Sempé C, Sato T, Reed JC. Structure-function analysis of Bcl-2 Protein. J Biol Chem. 1995;270:11962–11969. doi: 10.1074/jbc.270.20.11962. [DOI] [PubMed] [Google Scholar]
- 14.Heikkila, R.E. 1985. Autoxidation of 6-hydroxydopamine. In CRC Handbook of Methods for Oxygen Radical Research. R.A. Greenwald, editor. CRC Press, Boca Raton, FL. 233–235.
- 15.Heikkila RE, Felicitas C. A sensitive assay for superoxide dismutase based on the autoxidation of 6-hydroxydopamine. Anal Biochem. 1976;75:356–362. doi: 10.1016/0003-2697(76)90089-0. [DOI] [PubMed] [Google Scholar]
- 16.Hockenbery DM, Oltvai ZN, Yin X-M, Milliman CL, Korsmeyer SJ. Bcl-2 functions in an antioxidant pathway to prevent apoptosis. Cell. 1993;75:241–251. doi: 10.1016/0092-8674(93)80066-n. [DOI] [PubMed] [Google Scholar]
- 17.Ito H, Fukuda Y, Murata K, Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983;153:163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaiser, C., S. Michaelis, and A. Mitchell. 1994. Methods in Yeast Genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY. 234 pp.
- 19.Kane DJ, Sarafian TA, Anton R, Hahn H, Gralla EB, Valentine JS, Ord T, Bredesen DE. Bcl-2 inhibition of neural death: Decreased generation of reactive oxygen species. Science (Wash DC) 1993;262:1274–1277. doi: 10.1126/science.8235659. [DOI] [PubMed] [Google Scholar]
- 20.Kane DJ, Ord T, Anton R, Bredesen DE. Expression of Bcl-2 inhibits necrotic neural cell death. J Neurosci Res. 1995;40:269–275. doi: 10.1002/jnr.490400216. [DOI] [PubMed] [Google Scholar]
- 21.Lapinskas PJ, Cunningham KW, Liu XF, Fink GR, Culotta VC. Mutations in PMR1suppress oxidative damage in yeast cells lacking superoxide dismutase. Mol Cell Biol. 1995;15:1382–1388. doi: 10.1128/mcb.15.3.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu XF, Culotta VC. The requirement for yeast superoxide dismutase is bypassed through mutations in BSD2, a novel metal homeostasis gene. Mol Cell Biol. 1994;14:7037–7045. doi: 10.1128/mcb.14.11.7037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu XF, Elashvili I, Gralla EB, Valentine JS, Lapinskas P, Culotta VC. Yeast lacking superoxide dismutase—isolation of genetic supresssors. J Biol Chem. 1992;267:18298–18302. [PubMed] [Google Scholar]
- 24.Longo VD, Gralla EB, Valentine JS. Superoxide dismutase activity is essential for stationary phase survival in Saccharomyces cerevisiae: mitochondrial production of toxic oxygen species in vivo. J Biol Chem. 1996;271:12275–12280. doi: 10.1074/jbc.271.21.12275. [DOI] [PubMed] [Google Scholar]
- 25.Luck, H. 1965. Catalase. In Methods of Enzymatic Analysis. H.U. Bergmeyer, editor. Verlag Chemie Press, Berlin. 885–892.
- 26.MIPS. 1996. Saccharomyces genome data base. Martinsried Institute for Protein Sequences. Available via http://speedy.mips.biochem.mpg.de/mips/yeast, or ftp://genome-ftp.stanford.edu/yeast/genome_seq/.
- 27.Morishita T, Mitsuzawa H, Nakafuku M, Nakamura S, Hattori S, Anraku Y. Requirement of Saccharomyces cerevisiaeRas for completion of mitosis. Science (Wash DC) 1995;270:1213–1215. doi: 10.1126/science.270.5239.1213. [DOI] [PubMed] [Google Scholar]
- 28.Naito T, Kusano K, Kobayashi I. Selfish behavior of restriction-modification systems. Science (Wash DC) 1995;267:897–899. doi: 10.1126/science.7846533. [DOI] [PubMed] [Google Scholar]
- 29.Rabizadeh S, Gralla EB, Borchelt DR, Gwinn R, Valentine JS, Sisodia S, Wong P, Lee M, Hahn H, Bredesen DE. Mutations associated with amyotrophic lateral sclerosis convert superoxide dismutase from an antiapoptotic gene to a proapoptotic gene—studies in yeast and neural cells. Proc Natl Acad Sci USA. 1995;92:3024–3028. doi: 10.1073/pnas.92.7.3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sato T, Hanada M, Bodrug S, Irie S, Iwama N, Boise LH, Thompson CB, Golemis E, Fong L, Wang H-G, Reed JC. Interactions among members of the Bcl-2 protein family analyzed with a yeast two-hybrid system. Proc Natl Acad Sci USA. 1994;91:9238–9242. doi: 10.1073/pnas.91.20.9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schwartz LM, Smith SW, Jones ME, Osborne BA. Do all programmed cell deaths occur via apoptosis? . Proc Natl Acad Sci USA. 1993;90:980–984. doi: 10.1073/pnas.90.3.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shimizu S, Eguchi Y, Kosaka H, Kamilke W, Matsuda H, Tsujimoto Y. Prevention of hypoxia-induced cell death by Bcl-2 and Bcl-xL. Nature (Lond) 1995;374:811–813. doi: 10.1038/374811a0. [DOI] [PubMed] [Google Scholar]
- 33.Steinman HM. The Bcl-2 oncoprotein functions as a pro-oxidant. J Biol Chem. 1995;270:3487–3490. [PubMed] [Google Scholar]
- 34.Tamai KT, Gralla EB, Ellerby LM, Valentine JS, Thiele DJ. Yeast and mammalian metallothioneins functionally substitute for yeast copper-zinc superoxide dismutase. Proc Natl Acad Sci USA. 1993;90:8013–8017. doi: 10.1073/pnas.90.17.8013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tatchell K. RAS genes and growth control in Saccharomyces cerevisiae. . J Bacteriol. 1986;166:364–367. doi: 10.1128/jb.166.2.364-367.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem. 1969;27:502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- 37.Tsujimoto Y, Croce CM. Analysis of the structure, transcripts, and protein products of bcl-2, the gene involved in human follicular lymphoma. Proc Natl Acad Sci USA. 1986;83:5214–5218. doi: 10.1073/pnas.83.14.5214. [DOI] [PMC free article] [PubMed] [Google Scholar]