Abstract
Catalytically active endothelial nitric oxide synthase (eNOS) is located on the Golgi complex and in the caveolae of endothelial cells (EC). Mislocalization of eNOS caused by mutation of the N-myristoylation or cysteine palmitoylation sites impairs production of stimulated nitric oxide (NO), suggesting that intracellular targeting is critical for optimal NO production. To investigate the molecular determinants of eNOS targeting in EC, we constructed eNOS–green fluorescent protein (GFP) chimeras to study its localization in living and fixed cells. The full-length eNOS–GFP fusion colocalized with a Golgi marker, mannosidase II, and retained catalytic activity compared to wild-type (WT) eNOS, suggesting that the GFP tag does not interfere with eNOS localization or function. Experiments with different size amino-terminal fusion partners coupled to GFP demonstrated that the first 35 amino acids of eNOS are sufficient to target GFP into the Golgi region of NIH 3T3 cells. Additionally, the unique (Gly-Leu)5 repeat located between the palmitoylation sites (Cys-15 and -26) of eNOS is necessary for its palmitoylation and thus localization, but not for N-myristoylation, membrane association, and NOS activity. The palmitoylation-deficient mutants displayed a more diffuse fluorescence pattern than did WT eNOS–GFP, but still were associated with intracellular membranes. Biochemical studies also showed that the palmitoylation-deficient mutants are associated with membranes as tightly as WT eNOS. Mutation of the N-myristoylation site Gly-2 (abolishing both N-myristoylation and palmitoylation) caused the GFP fusion protein to distribute throughout the cell as GFP alone, consistent with its primarily cytosolic nature in biochemical studies. Therefore, eNOS targets into the Golgi region of NIH 3T3 cells via the first 35 amino acids, including N-myristoylation and palmitoylation sites, and its overall membrane association requires N-myristoylation but not cysteine palmitoylation. These results suggest a novel role for fatty acylation in the specific compartmentalization of eNOS and most likely, for other dually acylated proteins, to the Golgi complex.
Targeting of receptors to the plasma membrane is a necessary event for receptor-dependent signal transduction. Integral membrane proteins destined for the plasma membrane are processed via the classical ER–Golgi pathway and are inserted into the plasma membrane via hydrophobic stretches of amino acids encompassing transmembrane domains. Other posttranslational modifications, such as N-glycosylation and cysteine palmitoylation, impart features to the membrane protein that can determine extracellular versus intracellular domains, and can influence protein stability and turnover. In contrast, the mechanisms by which fatty acylated, peripheral membrane proteins target to and reside in intracellular membranes are less well understood.
The α subunit of heterotrimeric G proteins and endothelial nitric oxide synthase (eNOS)1 are peripheral membrane proteins that target to specific intracellular domains (including the Golgi complex and plasmalemmal caveolae) requiring cotranslational N-myristoylation and posttranslational cysteine palmitoylation (Sessa et al., 1995; García-Cardeña et al., 1996a ; Wedegaertner et al., 1995). For dually acylated G proteins and eNOS, mutation of the myristic acid acceptor site glycine-2 inhibits both N-myristoylation and cysteine palmitoylation and converts the membrane-associated form of the proteins to a cytosolic form (Sessa et al., 1993; Liu et al., 1995), suggesting that fatty acylation reactions are essential for membrane association and targeting. Mutation of the palmitoylation sites (cysteines 3 or 5 for G proteins or cysteines 15 and 26 for eNOS) preserves N-myristoylation but has variable effects on overall protein hydrophobicity and membrane targeting (Wedegaertner et al., 1995; Liu et al., 1995; Robinson and Michel, 1995). For eNOS, inhibition of dual acylation prevents Golgi targeting and mutation of the palmitoylation sites attenuates caveolae targeting (Sessa et al., 1995; García-Cardeña et al., 1996a ). In both circumstances, the mislocalization of eNOS has functional consequences; the stimulated production of nitric oxide (NO) is markedly reduced even though the purified mutant enzymes are catalytically identical to wild-type (WT) eNOS (Sessa et al., 1995; Liu et al., 1996). In confirmation of these cellular studies, mislocalization of eNOS in CA1 region stratum radiatum neurons, via expression of a dominant-negative eNOS or inhibition of N-myristoylation, blocks long-term potentiation (Kantor et al., 1996). These data suggest that fatty acylation and Golgi targeting are critical for NOS translocation into specific post-Golgi domains and that localization is required for efficient NO production.
To elucidate the molecular signals and the role(s) of lipid modifications in targeting of eNOS, we fused the green fluorescent protein (GFP) isolated from the jellyfish Aequorea victoria to the carboxyl terminus of eNOS, and we assessed the regions of NOS necessary for trafficking and overall membrane association. Our results demonstrate that the first 35 amino acids, including the N-myristoylation and palmitoylation sites of eNOS, are responsible for its Golgi localization and membrane association. These results provide a novel role for fatty acylation in the specific compartmentalization of eNOS and most likely other dually acylated proteins to the Golgi complex, and suggest that N-myristoylation and cysteine palmitoylation, in addition to imparting hydrophobic character to proteins, are important constituents of a Golgi targeting signal for certain peripheral membrane proteins.
Materials and Methods
Generation of eNOS–GFP Fusions
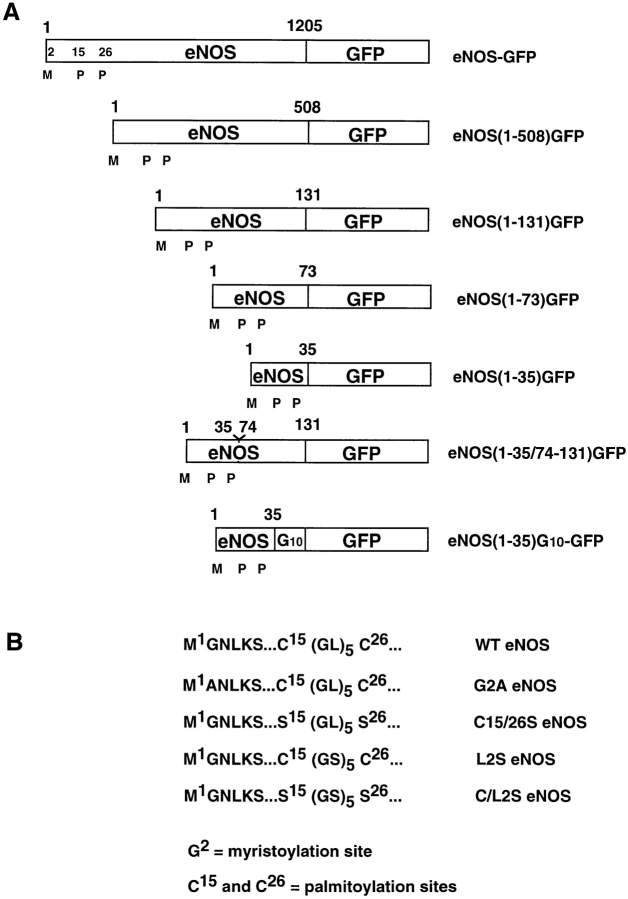
Standard molecular cloning techniques were used to manipulate DNA. The constructs discussed in this paper are summarized in Fig. 2. WT and C15/26S mutant (in which cysteine 15 and/or 26 have been mutated to serine) eNOS cDNAs were constructed and subcloned into the mammalian expression vector pcDNA3 (Liu et al., 1995). The cloned GFP cDNAs (Marshall et al., 1995) originally from TU-65 (Chalfie et al., 1994) and eNOS (WT, C15/26S, G2A) in pcDNA3 were used as templates for PCR. To generate eNOS-GFP pcDNA3 plasmids, the first methionine of GFP was changed into alanine to create an NheI site, and an NheI site was added to the 3′ end of eNOS coding sequence through PCR amplification. The L2S eNOS mutant (the five leucine residues between Cys-15 and -26 mutated into serines) and C/L2S eNOS (the five leucine residues and Cys-15 and -26 mutated into serines) were generated through PCR. The sequences of the ligated PCR fragments cloned into pCDNA3 were verified by DNA sequencing. The fusion proteins were of the predicted molecular masses, as confirmed by Western blotting using GFP or eNOS antisera.
Figure 2.
Schematic illustration of eNOS–GFP fusions (A) and mutants (B). M, myristate; P, palmitate.
Cell Transfection and Fluorescence Microscopy
NIH 3T3 cells were grown in DME containing penicillin (100 IU/ml), streptomycin (100 mg/ml), and 10% (vol/vol) FBS (complete DME), and were transfected for 6 h with indicated plasmids according to the standard calcium phosphate precipitation method.
The localization of eNOS and eNOS-GFP fusions in living and fixed cells was examined by fluorescence microscopy between 48 and 60 h after transfection. In some experiments, eNOS was colocalized with the resident Golgi protein mannosidase II (Man II). Transfected NIH 3T3 cells were fixed in 2% paraformaldehyde in PBS for 10 min at room temperature and subsequently permeabilized with 0.1% (vol/vol) Triton X-100 in PBS/0.1% (wt/vol) BSA for 5 min. Cells were incubated with Man II polyclonal antibody for 1 h, followed by washing and incubation for 45 min with Texas red–labeled second antibodies (Jackson Immunoresearch Laboratories, West Grove, PA). The specificity of the Man II and eNOS antibodies has been demonstrated previously (Moreman and Touster, 1986; Sessa et al., 1995). After washing, slides were mounted with Slowfade (Molecular Probes, Eugene, OR) and the cells were observed with a fluorescence microscope.
Metabolic Labeling and Immunoprecipitation
Transiently transfected NIH 3T3 cells (on T-75 flasks) were preincubated for 30 min with cerulenin (2 μg/ml), an inhibitor of fatty acid synthetase, in serum-free DME containing BSA (10 mg/ml, fatty acid free). Cells were then labeled with 300 μCi/ml of [3H]myristic acid or [3H]palmitic acid (45– 50 Ci/mmol; New England Nuclear, Boston, MA) for 4 h in the same medium. The 3H-fatty acids were dried under N2 and redissolved in DMSO so that the final concentration of DMSO in the labeling medium was 0.1%.
For immunoprecipitation, cells were solubilized by incubation in 1 ml of the modified RIPA buffer for 30 min at 4°C. Lysates were centrifuged at 10,000 g for 5 min to remove insoluble material, and 2 μg of GFP polyclonal antibody (Clontech Laboratories, Palo Alto, CA) was added and incubated for 2 h at 4°C. 15 μl of protein A–Sepharose (Pharmacia Fine Chemicals, Piscataway, NJ) suspension (1:1 in RIPA buffer) was added and incubated for 1 h at 4°C. The beads were pelleted, washed three times with 1 ml RIPA buffer, and then boiled in SDS-PAGE sample buffer for 5 min. Proteins were separated by SDS-PAGE visualized by Coomassie blue staining followed by fluorographic analysis as described previously (Liu et al., 1995). Immunoprecipitation experiments demonstrated that 2 μg of GFP polyclonal antibody quantitatively and specifically precipitated eNOS–GFP fusion proteins from transfected cells (T-75 flask) using the above conditions (data not shown).
Cell Fractionation and NOS Activity Assays
For cell fractionation studies, transfected cells were rinsed with ice-cold PBS, scraped into the same buffer, and centrifuged at 600 g for 5 min. Cell pellets were resuspended in 1 ml of homogenization buffer (50 mM Tris-HCl/0.1 mM EDTA/0.1 mM EGTA/2 μM leupeptin/1 μM pepstatin A/1 μM aprotinin/1 mM PMSF/10 mM NaF/1 mM Na3VO4, pH 7.5) and sonicated at 4°C (15 s, three times, at 50 W of power). Lysates were centrifuged at 100,000 g for 90 min at 4°C to separate the membrane and cytosolic fractions. The relative amount of eNOS–GFP was analyzed by Western blotting with GFP antibodies.
NOS activities of samples were assayed by determining the conversion of [3H]l-arginine into [3H]l-citrulline, the stoichiometric reaction product generated by NOS. In brief, lysates (50-μg proteins) were incubated (total volume = 0.1 ml) in a 50 mM Tris-HCl/0.1 mM EDTA/0.1 mM EGTA buffer, pH 7.5, containing 1 mM NADPH, 3 μM tetrahydrobiopterin, 100 nM calmodulin, 2.5 mM CaCl2, 10 μM l-arginine, and [3H]l-arginine (0.1 μCi, sp act = 55 Ci/mmol) for 20 min at 37°C. After the incubation period, the reaction was quenched by the addition of 1 ml of 20 mM Hepes stop buffer, pH 5.5 (containing 2 mM EDTA and 2 mM EGTA). The reaction mix was then passed over a 1-ml column containing Dowex AG 50WX-8 (Na+ form) resin (preequilibrated in stop buffer), washed with 1 ml of water, and collected directly into a 20-ml liquid scintillation vial containing scintillation cocktail as previously described (Bredt and Snyder, 1990). The amount of [3H]l-citrulline generated per milligram of protein was used as an index of NOS activity.
Results
Fusion of eNOS with GFP Does Not Interfere with the Localization and Catalytic Activity of eNOS
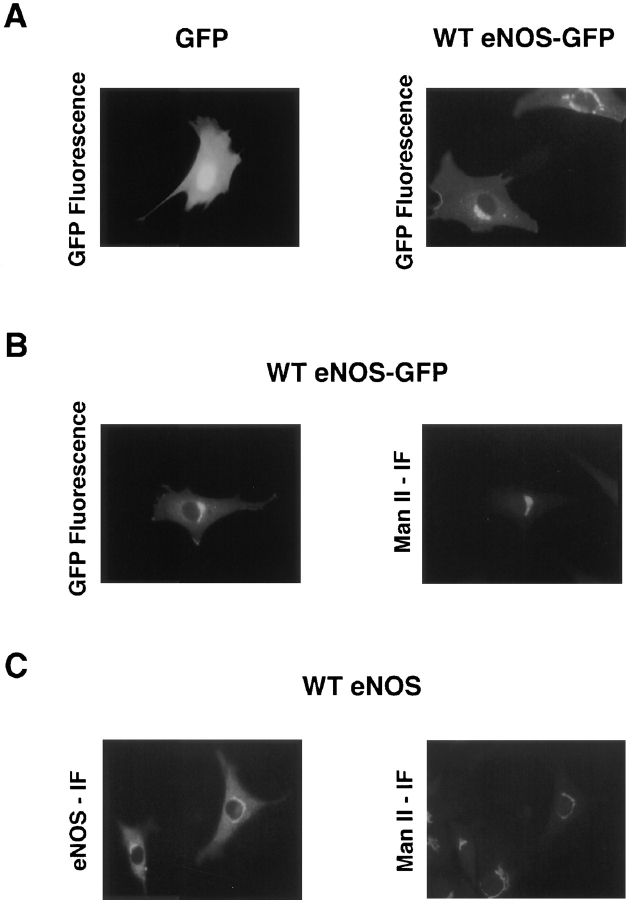
We initially asked whether GFP could be used as a reporter protein to study eNOS localization in living cells. To this end, NIH 3T3 cells were transiently transfected with expression plasmids for GFP alone or with full-length eNOS cloned in frame with GFP (eNOS–GFP), and subcellular localization was determined by fluorescence microscopy. Cells that were transfected with a construct encoding GFP alone displayed diffuse fluorescence throughout the cells (Fig. 1 A, left), occasionally concentrating in the nucleus, which is consistent with previous reports demonstrating that GFP is synthesized as a cytosolic protein and randomly distributes throughout the cytosol (Prasher et al., 1992; Marshall et al., 1995) and, in COS cells, can also accumulate in nuclei (Moriyoshi et al., 1996). When GFP was fused to the carboxyl terminus of eNOS, an intense, perinuclear fluorescent pattern was visualized (Fig. 1 A, right) similar to that of native eNOS in aortic endothelial cells (EC) (Sessa et al., 1995). In addition, there was faint fluorescence diffusely distributed throughout the cell, most likely caused by a cytosolic form of eNOS–GFP or eNOS–GFP attached to cytoplasmic ribosomes (see Fig. 8). These data are in agreement with fractionation studies that show a majority of eNOS in EC (90–95%) tightly associated with intracellular membranes, with the remaining being cytosolic (5–10%; Forstermann et al., 1991; Pollock et al., 1991; Liu et al., 1995). To confirm the identical perinuclear localization of the eNOS–GFP construct to that of nontagged eNOS, NIH 3T3 cells were transfected with eNOS–GFP or WT eNOS, and the proteins were colocalized with the resident intraluminal Golgi protein Man II in fixed cells. Previous data demonstrated that GFP fluorescence was preserved in fixed cells (Chalfie et al., 1994). As seen in Fig. 1 B, eNOS–GFP colocalized with Man II in exactly the same pattern as seen in cells transfected with the WT eNOS cDNA (Fig. 1 C). In some cells, GFP fluorescence also occurred in discrete regions of the plasma membrane, consistent with previous reports that a fraction of eNOS resides in plasmalemmal caveolae and interacts with caveolin-1 (García-Cardeña et al., 1996a ,b). Therefore, tagging of eNOS with GFP does not alter its intracellular localization.
Figure 1.
eNOS–GFP has the same intracellular localization as WT eNOS and colocalizes with Man II. NIH 3T3 cells transfected with GFP, WT eNOS– GFP, or WT eNOS were visualized in live cells by fluorescence microscopy (A), or colocalized with a Golgi marker, Man II, in fixed cells (B and C). IF, immunofluorescence.
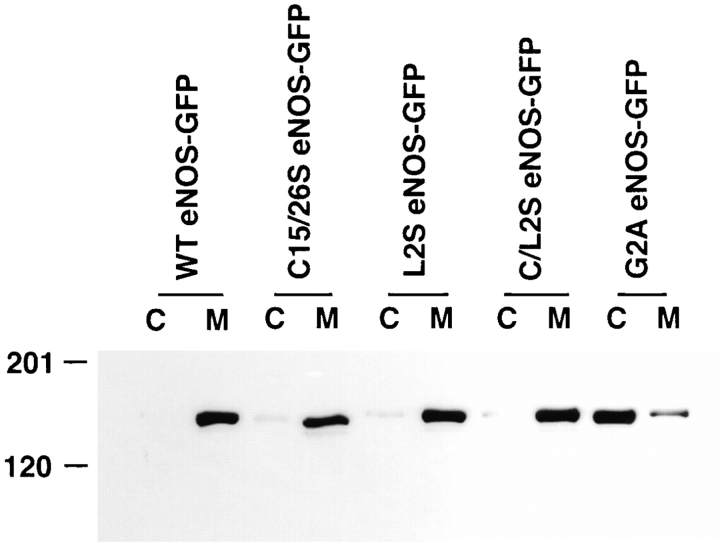
Figure 8.
Inhibition of eNOS palmitoylation does not alter its overall membrane association. NIH 3T3 cells were transfected with WT, C15/26S, L2S, C/L2S, or G2A eNOS–GFP constructs, and lysates were separated into cytosolic (C) and membrane (M) and fractions. The GFP proteins were immunoprecipitated with a GFP polyclonal antibody and analyzed by Western blotting with an eNOS mAb.
To examine if the addition of GFP to the carboxyl terminus of eNOS interfered with the catalytic properties of the enzyme, we performed NOS activity assays in lysates from transfected cells. NOS-specific activities (quantified by the conversion of [3H]l-arginine to [3H]l-citrulline) were 24 ± 3 and 26 ± 4 pmol citrulline/min per mg protein for WT eNOS and WT eNOS–GFP, respectively (n = 2 in duplicate). All NOS activity was completely abrogated by incubation of lysates with the NOS inhibitor nitro-l-arginine methyl ester (1 mM, data not shown). Therefore, the fusion of eNOS with GFP did not interfere with the dimerization of eNOS or NADPH, calcium, and tetrahydrabiopterin-dependent activation of NOS.
The First 35 Amino Acids of eNOS Are Sufficient to Target GFP into the Golgi Region
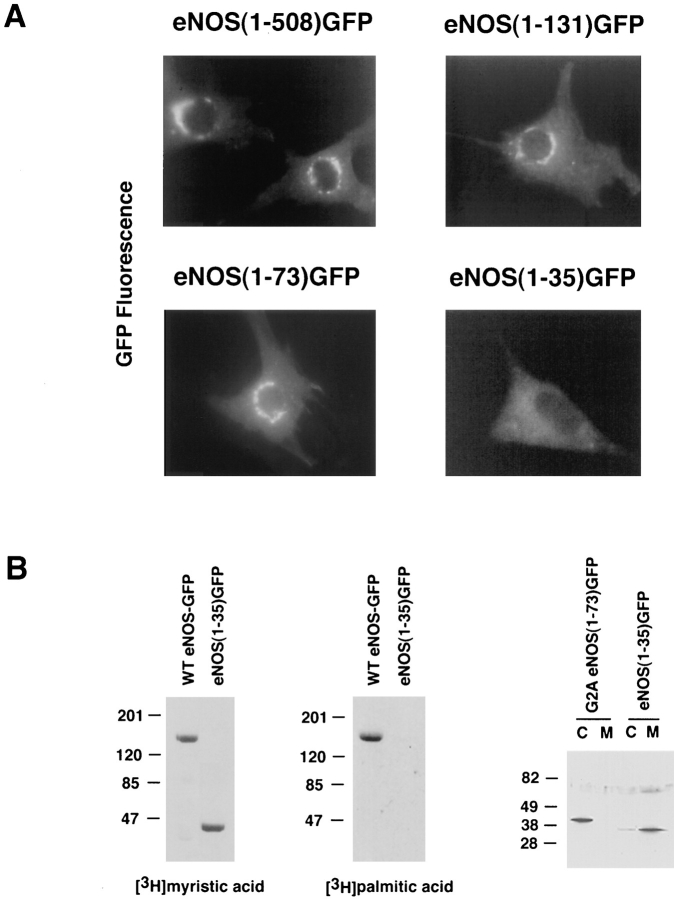
The above observations support the conclusion that the fusion partner rather than GFP determines the subcellular localization of the hybrid protein. Therefore, GFP can be used as a tag to track the targeting signal of eNOS in living cells. To pinpoint the amino acid sequences of eNOS responsible for its localization, constructs encoding varying lengths of the amino terminal regions of eNOS fused to GFP were designed (illustrated in Fig. 2 A). Fig. 3 A shows that fusions of amino acids 1–508, 1–131, or 1–73 of eNOS to GFP are capable of targeting the eNOS–GFP fusion protein into the perinuclear region of living, transfected NIH 3T3 cells; the same pattern exhibited by WT eNOS. This is in contrast to the diffuse fluorescence pattern seen with eNOS (1–35) GFP. The diffuse distribution of this construct suggested that either amino acids 36–73 were important for targeting or that the relatively large GFP protein (238 amino acids) may be interfering with the ability of the first 35 amino acids to act as a targeting signal. Interestingly, in biosynthetic labeling studies, eNOS (1–35) GFP was N-myristoylated, but not palmitoylated, and was enriched in high speed membrane fractions, whereas G2A eNOS (1–73) GFP (Fig. 3 B) and GFP alone (data not shown) were cytosolic in lysates prepared from transfected NIH 3T3 cells. To examine the possibility that the spacing of amino acids between 1–35 and GFP could account for lack of targeting of the chimeric protein, we created two additional constructs, eNOS (1–35/74–131) GFP and eNOS (1–35) G10-GFP. We reasoned that amino acids 74–131 most likely do not contribute significantly to its perinuclear targeting, since eNOS (1–73) GFP and eNOS (1–131) GFP display identical intracellular localization patterns compared to WT eNOS (see Fig. 3 A). As shown in Fig. 4 A, eNOS (1–35/74–131) GFP was localized in the perinuclear region of living cells and colocalized with Man II in fixed cells. Using a heterologous spacer of 10 glycines to separate eNOS from the GFP (eNOS [1–35]G10-GFP) resulted in identical localization into the Golgi region of NIH 3T3 cells (Fig. 4 B). Therefore, the first 35 amino acids of eNOS are responsible for Golgi targeting.
Figure 3.
Targeting of deletion mutants of eNOS-GFP. (A) NIH 3T3 cells were transfected with WT eNOS–GFP or truncated eNOS–GFP constructs, and GFP fluorescence was visualized in live cells by fluorescence microscopy. (B) Fatty acylation of immunoprecipitated eNOS (1–35) GFP and WT eNOS–GFP constructs and relative partitioning of the 1–35 construct between cytosol (C) and membrane (M) fractions (compared to a soluble G2A eNOS [1–73] GFP construct) were determined.
Figure 4.
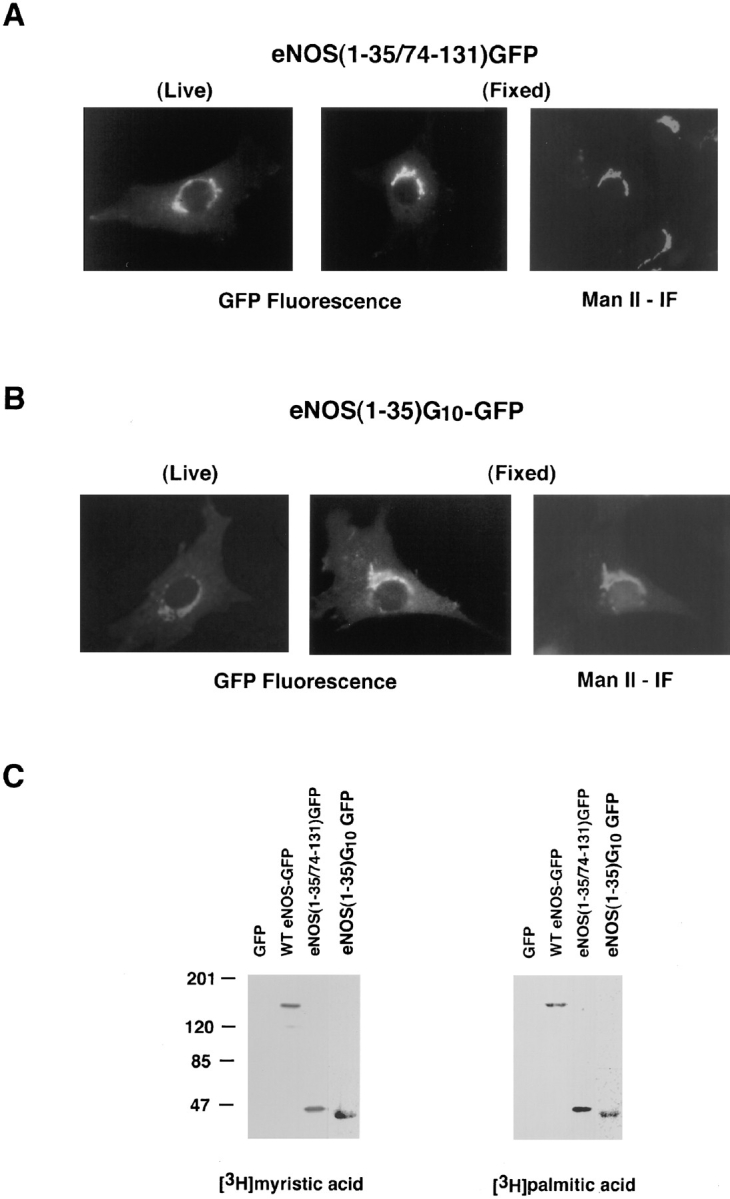
The first 35 amino acids of eNOS constitute a Golgi-targeting signal. (A) eNOS (1–35/74–131) GFP was visualized in live cells (left panel) or colocalized with Man II in fixed cells (center and right panels). (B) eNOS (1–35) G10-GFP was visualized in live cells (left panel) or colocalized with Man II in fixed cells (center and right panels). (C) eNOS (1–35/74– 131) GFP and eNOS (1–35) G10-GFP are myristoylated and palmitoylated. NIH 3T3 cells transfected with GFP or eNOS–GFP constructs were labeled with [3H]myristic acid or [3H]palmitic acid. The GFP proteins were immunoprecipitated with GFP antibodies and analyzed by fluorography. The bands are appropriate sizes from the coding regions.
N-Myristoylation and Cysteine Palmitoylation Are Necessary for eNOS Golgi Targeting
Next, we examined if eNOS(1–35/74–131) GFP and eNOS (1–35)G10-GFP were still N-myristoylated and palmitoylated, since the first 35 amino acid sequence includes the N-myristoylation site (Gly-2) and palmitoylation sites (Cys-15 and -26). When cells transfected with WT eNOS-GFP, eNOS(1–35/ 74–131) GFP, eNOS (1–35)G10-GFP, or GFP alone were metabolically labeled with the appropriate 3H-fatty acid, the full-length and truncated eNOS–GFP fusion proteins were N-myristoylated and palmitoylated, but GFP alone was not (Fig. 4 C). This is in contrast to the eNOS (1–35) GFP that was N-myristoylated but not palmitoylated (Fig. 3 B), suggesting that the spacing between the eNOS and GFP was critical for recognition by palmitoyl transferase.
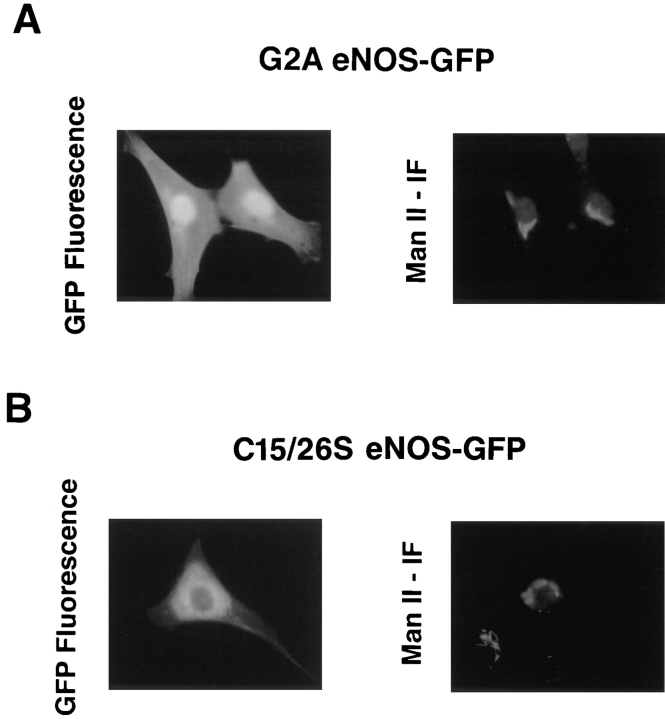
To examine the importance of fatty acylation to targeting, G2A eNOS–GFP (neither N-myristoylated nor palmitoylated) and C15/26S eNOS–GFP (N-myristoylated but not palmitoylated) were constructed and transfected into NIH 3T3 cells. As shown in Fig. 5 A, acylation-defective G2A eNOS–GFP distributed throughout the cells as did GFP alone, while palmitoylation-deficient mutant C15/26S eNOS–GFP (Fig. 5 B) was still retained in the perinuclear region, but in a more diffuse pattern compared to WT eNOS–GFP (Fig. 1). The G2A eNOS construct did not colocalize with Man II (Sessa et al., 1995). For the palmitoylation-deficient protein, only a small fraction of the GFP fusion colocalized with the Golgi marker. Labeling of C15/26S eNOS–GFP-transfected cells with an antibody to calnexin, a resident glycoprotein chaperone of the ER, resulted in an immunofluorescent pattern distinct from the GFP fluorescence (data not shown). Therefore, N-myristoylation and cysteine palmitoylation are critical determinants of Golgi targeting and/or retention.
Figure 5.
Lipid modifications influence the subcellular targeting of eNOS–GFP constructs. NIH 3T3 cells were transfected with G2A eNOS–GFP (nonacylated, A) or C15/26S eNOS–GFP (myristoylated but not palmitoylated, B) constructs, and were colocalized in fixed cells with the resident Golgi protein Man II.
The (Gly-Leu)5 Repeat between the eNOS Palmitoylation Sites Is Important for Its Palmitoylation and Localization but Not for Its Overall Membrane Association
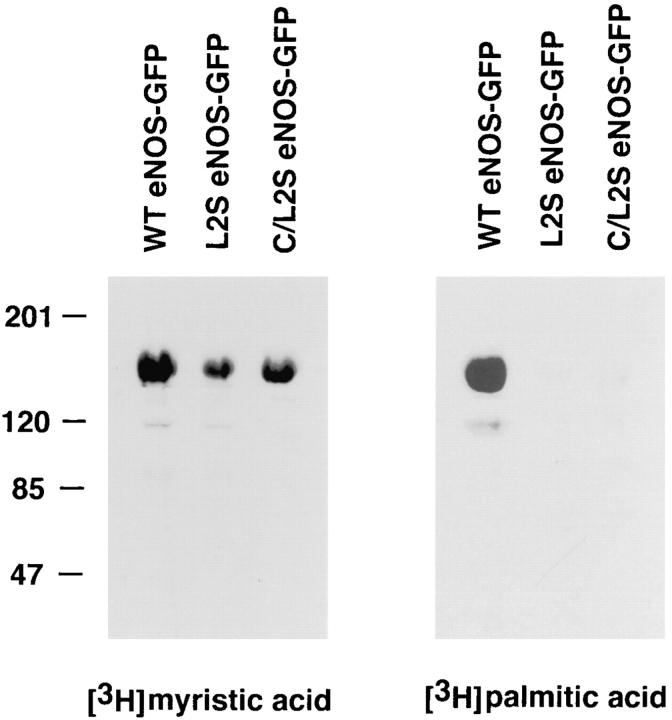
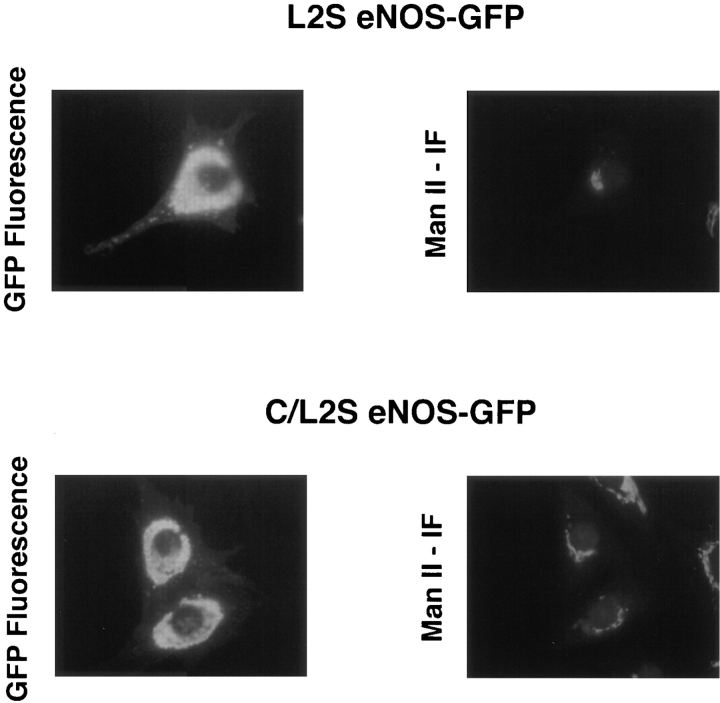
The unique pentameric dipeptide repeat (Gly-Leu)5 located between the C15 and C26 palmitoylation sites of eNOS has been speculated to be important for NOS acylation and intracellular targeting (Liu et al., 1995; Robinson and Michel, 1995; García-Cardeña et al., 1996a ). To see if this repeat influences eNOS fatty acylation and localization, we mutated the five leucine residues to serine in the context of WT eNOS–GFP (named L2S eNOS–GFP) or in the context of the palmitoylation mutant C15/26S eNOS– GFP (named C/L2S eNOS–GFP), respectively (see Fig. 2 B). Transfection of these constructs followed by metabolic labeling with the appropriate 3H-fatty acid showed that mutation of the leucine residues did not influence N-myristoylation but abolished palmitoylation (Fig. 6). Similar amounts of eNOS protein were present in all lanes, as determined by Coomassie blue staining of the gels, before autoradiography (data not shown). Analysis of the cellular localization of these constructs demonstrated that L2S and C/L2S eNOS-GFP were mislocalized (Fig. 7) in a fashion similar to the palmitoylation mutant C15/26S eNOS–GFP (see Fig. 5 B), with only a small fraction of the palmitoylation-deficient proteins colocalizing with Man II and the rest associated with other intracellular organelles.
Figure 6.
Mutation of the leucines between the palmitoylation sites abolishes eNOS palmitoylation. NIH 3T3 cells were transfected with eNOS–GFP constructs, and the cells were labeled with [3H]myristic acid or [3H]palmitic acid. The GFP proteins were immunoprecipitated with GFP antibodies and analyzed by fluorography.
Figure 7.
The glycine-leucine repeat is necessary for eNOS intracellular localization. NIH 3T3 cells were transfected with L2S or C/L2S eNOS–GFP constructs, and proteins were colocalized with Man II. Note that the L2S and C/L2S proteins appear to be mislocalized in a similar manner, suggesting that palmitoylation is required for proper Golgi localization.
We previously showed that mutation of the palmitoylation sites of eNOS does not affect its overall membrane association with respect to removal of the enzyme from membranes by high salt or high pH and its relative distribution between Triton X-114 detergent and aqueous phases, suggesting that hydrophobic factor(s), besides palmitoylation, most likely contribute to its membrane association. To examine the importance of the glycine-leucine repeat in the overall membrane association, NIH 3T3 cells transfected with WT, L2S, or C/L2S eNOS–GFP were fractionated into high speed membranes and cytosolic fractions, and eNOS–GFP fusion proteins were analyzed by Western blotting. As shown in Fig. 8, the palmitoylation-deficient mutants, L2S eNOS–GFP and C/L2S eNOS–GFP, were still associated with the high speed cellular membrane as tightly as WT or C15/26S eNOS–GFP, in agreement with the GFP fluorescence data. NOS activity in cell lysates demonstrated that these two mutants were catalytically active (31 and 27 pmol citruline/min per mg protein for L2S eNOS–GFP and L/C2S eNOS–GFP, respectively). Therefore, the (Gly-Leu)5 repeat is not important for overall membrane association of eNOS, but most likely contributes to its recognition by cysteine palmitoyl transferases.
Discussion
The subcellular compartmentalization of eNOS is critical for optimal production of NO. Mutations that disrupt N-myristoylation or cysteine palmitoylation interfere with proper intracellular targeting and attenuate the stimulated production of NO from intact cells without affecting the catalytic properties of the enzyme in vitro (Sessa et al., 1995; Liu et al., 1996). Thus, the present study was undertaken to better understand the sequences and modifications required for targeting this peripheral membrane protein to the Golgi region of EC. We demonstrate that GFP-tagged eNOS is a useful tool to examine the localization of the wild-type protein in living and fixed cells. The first 35 amino acids of eNOS, including the N-myristoylation and cysteine palmitoylation sites, target the fusion partner into the Golgi region of NIH 3T3 cells. Abolition of fatty acylation in the context of the G2A mutant prevents the colocalization of eNOS with the resident Golgi protein Man II, whereas mutation of the palmitoylation sites or the leucines embedded in the pentameric glycine-leucine repeat flanking the palmitoylation sites reveals a more diffuse perinuclear localization of the enzyme. Thus, our data demonstrate for the first time that fatty acylation is important for Golgi targeting and/or retention, and suggest a model for the subcellular trafficking of other dually acylated proteins that localize in both the Golgi and plasma membrane microdomains, including G protein α subunits.
Previously, we and others demonstrated that eNOS resides primarily on the Golgi and in plasmalemmal caveolae of EC. As seen with the dually acylated Src family members Hck, Fyn, and Fgr, mutation of the palmitoylation sites attenuates protein targeting to caveolae (Shenoy-Scaria et al., 1994; Robbins et al., 1995). Moreover, using both silica purification of caveolae from intact lung endothelium and detergent-free purification of caveolae from cultured cells, the majority of immunoreactive eNOS in the plasma membrane is in the caveolae (García-Cardeña et al., 1996a ; Shaul et al., 1996). Based on confocal microscopy of eNOS in cultured EC and isolation of plasma membranes and caveolae from intact lung and EC in culture, ∼10–50% of eNOS resides in caveolae at a given point in time (García-Cardeña et al., 1996a ). In the present study, the eNOS–GFP fluorescence pattern in living or fixed cells confirms eNOS in both Golgi and plasma membrane domains. However, we should point out that all cells transfected with WT eNOS–GFP show the protein in the Golgi region, whereas only a small number of cells (∼5-10%) show GFP fluorescence readily detectable in the plasma membrane at cell borders. This difference may result from the nature of transient transfections, the timing of microscopic evaluation of the cells after transfection, the dynamic nature of protein trafficking, or the inability to visualize cell surface–associated eNOS. Additionally, regulation of eNOS trafficking in and out of the plasma membrane is likely different in NIH 3T3 cells and EC, because a majority (80%) of microvascular EC show eNOS–GFP in both the Golgi and plasmalemmal domains (Liu, J., T.E. Hughes, and W.C. Sessa, unpublished observations). A priori, it seems logical that the partitioning of eNOS into the caveolae is a more complicated event that likely requires additional modifications such as phosphorylation or regulated protein–protein interactions for anterograde trafficking from Golgi to caveolae or vice versa. This is supported by demonstrations that eNOS is modified by serine and tyrosine phosphorylation (Michel et al., 1993; García-Cardeña et al., 1996b ), interacts with caveolin-1 and other proteins (García-Cardeña et al., 1996b ; Feron et al., 1996; Venema et al., 1996), and that cholesterol-binding drugs, hormones, protein phosphatase inhibitors, microtubule-disrupting agents, and GTP hydrolysis all influence the dynamic nature of caveolae and/or the distribution of caveolin-1 (Murata et al., 1995; Smart et al., 1994a ,b, 1995; Schnitzer et al., 1994–1996; Conrad et al., 1995). Thus, the eNOS–GFP constructs will be useful reagents to elucidate the molecular signals required for efficient Golgi-to-caveolae shuttling in EC.
The use of eNOS–GFP chimeras for the dissection of the eNOS localization signal reveals that the intracellular fate of eNOS is inherent in its first 35 amino acids when an appropriate protein spacer is included between eNOS and GFP. Both eNOS (1–35/74–131) GFP and eNOS (1–35) G10-GFP constructs are N-myristoylated, palmitoylated, and properly targeted to the Golgi region of 3T3 cells. Interestingly, the first 35 amino acids of eNOS fused to GFP or mutation of leucine residues flanking the palmitoylation sites in the context of full-length eNOS attenuate cysteine palmitoylation, but do not influence N-myristoylation or the relative membrane association of the fusion protein, clearly demonstrating that intracellular membrane targeting and biochemical membrane association are not synonymous terms. These data also suggest that N-myristoylation of the eNOS fusion in the context of the first 35 amino acids is sufficient for stable membrane association, but not for proper targeting. It is questionable whether N-myristoylation per se is sufficient for membrane association of a myristoylated protein. Based on energetic measurements modeling the interaction of N-myristoyl peptides with phospholipid vesicles, the calculated Gibbs free energy cannot suffice for stable membrane association to occur (Peitzsch and McLaughlin, 1993). However, we cannot rule out the possibility based on the membrane association of the myristoylated eNOS (1–35) GFP fusion protein, where ionic, hydrophobic, and other interactions between the protein and membrane are likely to occur in vivo to enhance the interaction of NH2-terminal myristate with biological membranes. In the full-length construct, mutation of the N-myristoylation site inhibits palmitoylation and randomly distributes eNOS into the cytosol, whereas mutation of the palmitoylation sites or replacement of glycine with serine in the pentameric glycine-leucine repeat does not affect N-myristoylation and membrane association, but results in the distribution of eNOS to other intracellular membranes other than Golgi. At the present time, we do not know the precise intracellular targeting of the palmitoylation mutants. Because different mutations, all which block palmitoylation but not N-myristoylation, result in similar focal fluorescence patterns, it is possible that nonpalmitoylated eNOS cannot stably interact with proteins or lipids of Golgi or plasma membranes, and that it mislocalizes to another membrane compartment, perhaps the ER or ERGIC.
Lipid modifications via N-myristoylation and cysteine palmitoylation are critical features for the intracellular localization and membrane association of G protein α subunits and Src family members (Shenoy-Scaria et al., 1994; Gauen et al., 1996). G protein α subunits localize in distinct Golgi compartments (Stow et al., 1991; Denker et al., 1996) and in plasma membrane caveolae in epithelial cells, transfected COS cells, and EC (Chun et al., 1994; Schnitzer et al., 1995). However, the Src family of kinases appears to have cell-specific localization patterns. For example, Lck and Fyn tyrosine kinases are N-myristoylated and cysteine palmitoylated, but have distinct intracellular localizations in human T lymphocytes (Ley et al., 1994). In T lymphoblasts, Lck was detected at the plasma membrane, while Fyn localized with centrosomal and microtubule bundles radiating from the centrosome in interphase cells or with mitotic spindle and poles in mitotic cells, but was not detected at the plasma membrane. More recently, Fyn was localized in the plasma membrane of transfected HeLa cells. Mutation of lysines 7 and 9 of Fyn (K7/9A Fyn) did not influence N-myristoylation or cysteine palmitoylation, but changed its typical plasma membrane pattern into a cytoplasmic and nuclear localization (Gauen et al., 1996). In 3T3 cells, Fyn is cotranslationally N-myristoylated on cytoplasmic ribosomes, and is rapidly targeted to the plasma membrane, where it can be palmitoylated (van't Hof and Resh, 1997). In addition, the aquisition of palmitate precedes its apparent localization to Triton-insoluble membranes. Using immunofluorescence microscopy techniques, however, Fyn is localized in the perinuclear membranes and plasmalemma of 3T3 cells and COS cells, similar to eNOS in 3T3 cells and EC (Sessa et al., 1995; Garcia-Cardena et al., 1996a; van't Hof and Resh, 1997). Therefore, it is possible that myristoyl-eNOS initially targets to the plasma membrane, where it can be palmitoylated by a plasma membrane cysteine palmitoyl transferase (Dunphy et al., 1996) and partition into caveolae. If so, then eNOS on the Golgi reflects accumulation from the fission and retrograde transport of caveolae (Schnitzer et al., 1996). Also, if this is true, then the rate of fission and retrograde transport of eNOS far exceeds that of plasma membrane targeting, palmitoylation, and entrance into caveolae. An alternative but not exclusive model is that eNOS is cotranslationally N-myristoylated on a cytoplasmic ribosome (Liu and Sessa, 1994) and targets onto the cytoplasmic face of the Golgi via lipid–lipid or lipid–protein interactions, possibly through an unidentified myristoyl protein chaperone, where it is kinetically trapped on Golgi membranes by subsequent palmitoylation (Schmidt and Catterall, 1987; Gutierrez and Magee, 1991). Palmitoylation, per se, does not contribute to overall hydrophobicity or stable membrane association (Liu et al., 1996), but it significantly contributes to proper subcellular targeting, perhaps by facilitating protein–protein or lipid–protein interactions with caveolin-1 (García-Cardeña et al., 1996b ). Once in the Golgi region, eNOS has the capacity to move to detergent-insoluble domains and caveolae of the plasma membrane because palmitoylation mutants of eNOS do not target to plasmalemmal caveolae and reside in a diffuse perinuclear pattern (García-Cardeña et al., 1996a ; Shaul et al., 1996 and this study). In both situations with Fyn and eNOS, it is clear that fatty acylation orients the amino terminus so that ionic interactions between the acylated protein and target lipids and/or proteins can occur.
The pentameric glycine-leucine repeat embedded in the novel dual acylation motif found in eNOS (M1GXXXS . . . C15(GL)5C26), unlike those found in G proteins or Src family members (Mumby et al., 1994; Resh, 1994), was speculated to be important for its membrane association and targeting (Liu et al., 1995). Changing the hydrophobic leucines to serines in the pentameric glycine-leucine repeat attenuates palmitoylation and results in a more diffuse perinuclear pattern of fluorescence. Similar data were observed with any palmitoylation mutant in the context of full-length or truncated GFP constructs (palmitoylation mutants of eNOS [1–73] GFP and [1–131] GFP, data not shown). This suggests that the intervening sequences between two palmitoylation sites (cysteines 15 and 26) will influence recognition by cysteine palmitoyl transferases without influencing other modifications such as N-myristoylation or dimer assembly (assayed indirectly by activity measurements). Palmitoylation appears to be necessary for targeting eNOS and certain Src family members to caveolae (García-Cardeña et al., 1996a ; Shaul et al., 1996; Shenoy-Scaria et al., 1994). The molecular mechanism for this is not known, but is likely ascribed to its involvement in protein–protein and/or protein–lipid interactions. Palmitoylation of Fyn tyrosine kinase has been shown to participate in its interaction with T cell antigen receptor (Gauen et al., 1996). The lipid composition of different membrane compartments are distinct, with higher cellular cholesterol content found in the Golgi and plasma membrane microdomains, including caveolae. Thus, palmitate may enhance and stabilize the binding of eNOS to caveolin, a cholesterol-binding protein (Murata et al., 1995), other Golgi targets, or specific membrane components. The loss of palmitate on eNOS incurred by site-directed mutagenesis of cysteines 15 and 26 or by mutation of the sequences between the palmitoylation sites may dampen its ability to interact with specific proteins and lipids on Golgi and/or caveolae membranes, resulting in a membrane protein properly targeted but no longer confined to the Golgi. A similar mechanism of membrane tethering was recently proposed for dually acylated G proteins α subunits found in Golgi and plasma membrane (Denker et al., 1996). However, palmitoylation, per se, is not sufficient for Golgi targeting of another peripheral membrane protein, GAD65. GAD65 targets into the Golgi of pancreatic β cells or transfected CHO cells, and mutation of the palmitoylation sites inhibits palmitoylation but does not influence Golgi targeting or stable membrane association (Shi et al., 1994; Solimena et al., 1994). Thus, for the targeting of dually acylated proteins, myristate in the context of nearby amino acids confers their ability to target and to be palmitoylated, and in turn, the palmitate helps the proteins to their final destinations to interact with specific effectors.
Overall, we have shown a potential biosynthetic pathway for the targeting of a representative, dually acylated, peripheral membrane protein, eNOS. Identification of the sequences required for Golgi targeting of eNOS will permit the expression of other transgenes into the Golgi region of heterologous cell systems. More importantly, visualization of the eNOS–GFP in living cells will allow us to examine hormone- and shear stress–regulated movement of eNOS between Golgi and caveolae in real time.
Acknowledgments
We thank Drs. Kelley Moreman and Jennifer Pollock for mannosidase II and eNOS antibodies, respectively.
This work is supported by grants from the National Institutes of Health (HL51948 and HL50974, to W.C. Sessa; F32-HL09224, to J. Liu; and EY08362, to T.E. Hughes), and a grant-in-aid from the American Heart Association. W.C. Sessa is an Established Investigator of the American Heart Association.
Footnotes
1. Abbreviations used in this paper: EC, endothelial cells; eNOS, endothelial nitric oxide synthase; GFP, green fluorescent protein; IF, immunofluorescence; Man II, mannosidase II; NO, nitric oxide; NOS, nitric oxide synthase; WT, wild type.
Please address all correspondence to William C. Sessa, Boyer Center for Molecular Medicine, Room 436D, Yale University School of Medicine, 295 Congress Avenue, New Haven, CT 06536-0812. Tel.: (203) 737-2291. Fax: (203) 737-2290.
References
- Bredt DS, Snyder SH. Isolation of nitric oxide synthetase, a calmodulin-requiring enzyme. Proc Natl Acad Sci USA. 1990;87:682–685. doi: 10.1073/pnas.87.2.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalfie M, Tu Y, Euskirchen G, Ward WW, Prasher DC. Green fluorescent protein as a marker for gene expression. Science (Wash DC) 1994;263:802–805. doi: 10.1126/science.8303295. [DOI] [PubMed] [Google Scholar]
- Chun M, Liyanage UK, Lisanti MP, Lodish HF. Signal transduction of a G protein-coupled receptor in caveolae: colocalization of endothelin and its receptor with caveolin. Proc Natl Acad Sci USA. 1994;91:11728–11732. doi: 10.1073/pnas.91.24.11728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad PA, Smart EJ, Ying Y, Anderson RGW, Bloom GS. Caveolin cycles between plasma membrane caveolae and the Golgi complex by microtubule-dependent and microtubule-independent steps. J Cell Biol. 1995;131:1421–1433. doi: 10.1083/jcb.131.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denker SP, McCaffery JM, Palade GE, Insel PA, Farquhar MG. Differential distribution of α subunits and βγ subunits of heterotrimeric G proteins on Golgi membranes of the exocrine pancreas. J Cell Biol. 1996;133:1027–1040. doi: 10.1083/jcb.133.5.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunphy JT, Greentree WK, Manahan CL, Linder ME. G-protein palmitoyltransferase activity is enriched in plasma membranes. J Biol Chem. 1996;271:7154–7159. doi: 10.1074/jbc.271.12.7154. [DOI] [PubMed] [Google Scholar]
- Feron O, Belhassen L, Kobzik L, Smith TW, Kelly RA, Michel T. Endothelial nitric oxide synthase targeting to caveolae. J Biol Chem. 1996;271:22810–22814. doi: 10.1074/jbc.271.37.22810. [DOI] [PubMed] [Google Scholar]
- Forstermann U, Pollock JS, Schmidt HHHW, Heller M, Murad F. Calmodulin-dependent endothelium-derived relaxing factor/nitric oxide synthase activity is present in the particulate and cytosolic fractions of bovine aortic endothelial cells. Proc Natl Acad Sci USA. 1991;88:1788–1792. doi: 10.1073/pnas.88.5.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Cardeña G, Oh P, Liu J, Schnitzer JE, Sessa WC. Targeting of nitric oxide synthase to endothelial caveolae via palmitoylation. Proc Natl Acad Sci USA. 1996a;93:6448–6453. doi: 10.1073/pnas.93.13.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Cardeña G, Fan R, Stern DF, Liu J, Sessa WC. Endothelial nitric oxide synthase is regulated by tyrosine phosphorylation and interacts with caveolin-1. J Biol Chem. 1996b;271:27237–27240. doi: 10.1074/jbc.271.44.27237. [DOI] [PubMed] [Google Scholar]
- Gauen LKT, Linder ME, Shaw AS. Multiple features of the p59fynsrc homology 4 domain define a motif for immune receptor–based activation motif (ITAM) binding and for plasma membrane localization. J Cell Biol. 1996;133:1007–1015. doi: 10.1083/jcb.133.5.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez L, Magee AI. Characterization of an acyltransferase acting on p21N-ras protein in a cell-free system. Biochem Biophys Acta. 1991;1078:147–154. doi: 10.1016/0167-4838(91)99003-b. [DOI] [PubMed] [Google Scholar]
- Htun H, Barsony J, Renyi I, Gould DL, Hager GL. Visualization of glucocorticoid receptor translocation and intranuclear organization in living cells with a green fluorescent protein chimera. Proc Natl Acad Sci USA. 1996;93:4845–4850. doi: 10.1073/pnas.93.10.4845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantor DB, Lanzrein M, Stary SJ, Sandoval GM, Smith WB, Sullivan BM, Davidson N, Schuman EM. A role for endothelial NO synthase in LTP revealed by adenovirus-mediated inhibition and rescue. Science (Wash DC) 1996;274:1744–1748. doi: 10.1126/science.274.5293.1744. [DOI] [PubMed] [Google Scholar]
- Ley SC, Marsh M, Bebbington CR, Proudfoot K, Jordan P. Distinct intracellular localization of Lck and Fyn protein tyrosine kinases in human T lymphocytes. J Cell Biol. 1994;125:639–649. doi: 10.1083/jcb.125.3.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Sessa WC. Identification of covalently bound amino-terminal myristic acid in endothelial nitric oxide synthase. J Biol Chem. 1994;269:11691–11694. [PubMed] [Google Scholar]
- Liu J, García-Cardeña G, Sessa WC. Biosynthesis and palmitoylation of endothelial nitric oxide synthase: mutagenesis of palmitoylation sites, cysteine 15 and/or 26, argue against depalmitoylation induced translocation of the enzyme. Biochemistry. 1995;34:12333–12340. doi: 10.1021/bi00038a029. [DOI] [PubMed] [Google Scholar]
- Liu J, García-Cardeña G, Sessa WC. Palmitoylation of endothelial nitric oxide synthase is necessary for optimal stimulated release of nitric oxide: implications for caveolae localization. Biochemistry. 1996;35:13277–13281. doi: 10.1021/bi961720e. [DOI] [PubMed] [Google Scholar]
- Marshall J, Raymond M, Moss GWJ, Howe JR, Hughes TE. The jellyfish green fluorescent protein: a new tool for studying ion channel expression and function. Neuron. 1995;14:211–215. doi: 10.1016/0896-6273(95)90279-1. [DOI] [PubMed] [Google Scholar]
- Michel T, Li GK, Busconi L. Phosphorylation and subcellular translocation of endothelial nitric oxide synthase. Proc Natl Acad Sci USA. 1993;90:6252–6256. doi: 10.1073/pnas.90.13.6252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreman KW, Touster O. Topology of mannosidase II in rat liver Golgi membranes and release of the catalytic domain by selective proteolysis. J Biol Chem. 1986;261:10945–10951. [PubMed] [Google Scholar]
- Moriyoshi K, Richards LJ, Akazawa C, O'Leary DDM, Nakanishi S. Labeling neural cells using adenoviral gene transfer of membrane targeted GFP. Neuron. 1996;16:255–260. doi: 10.1016/s0896-6273(00)80044-6. [DOI] [PubMed] [Google Scholar]
- Mumby SM, Kleuss C, Gilman AG. Receptor regulation of G-protein palmitoylation. Proc Natl Acad Sci USA. 1994;91:2800–2804. doi: 10.1073/pnas.91.7.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata M, Peranen J, Schreiner R, Wieland F, Kurzchalia TV, Simons K. VIP21/caveolin is a cholesterol-binding protein. Proc Natl Acad Sci USA. 1995;92:10339–10343. doi: 10.1073/pnas.92.22.10339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peitzsch RM, McLaughlin S. Binding of acylated peptides and fatty acids to phospholipid vesicles: pertinence to myristoylated proteins. Biochemistry. 1993;32:10436–10443. doi: 10.1021/bi00090a020. [DOI] [PubMed] [Google Scholar]
- Pollock JS, Forstermann U, Mitchell JA, Warner TD, Schmidt HHHW, Nakane M, Murad F. Purification and characterization of particulate endothelium-derived relaxing factor synthase from cultured and native bovine aortic endothelial cells. Proc Natl Acad Sci USA. 1991;88:10480–10484. doi: 10.1073/pnas.88.23.10480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasher DC, Eckenrode VK, Ward WW, Prendergast FG, Cormier MJ. Primary structure of the Aequorea victoria green-fluorescent protein. Gene (Amst) 1992;111:229–233. doi: 10.1016/0378-1119(92)90691-h. [DOI] [PubMed] [Google Scholar]
- Resh MD. Myristoylation and palmitoylation of Src family members: the fats of the matter. Cell. 1994;76:411–413. doi: 10.1016/0092-8674(94)90104-x. [DOI] [PubMed] [Google Scholar]
- Robbins SM, Quintrell NA, Bishop JM. Myristoylation and differential palmitoylation of the HCK protein-tyrosine kinases govern their attachment to membranes and association with caveolae. Mol Cell Biol. 1995;15:3507–3515. doi: 10.1128/mcb.15.7.3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson LJ, Michel T. Mutagenesis of palmitoylation sites in endothelial nitric oxide synthase identifies a novel motif for dual acylation and subcellular targeting. Proc Natl Acad Sci USA. 1995;92:11776–11780. doi: 10.1073/pnas.92.25.11776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt JW, Catterall WA. Palmitoylation, sulfation, and glycosylation of the alpha subunit of the sodium channel. Role of post-translational modifications in channel assembly. J Biol Chem. 1987;262:13713–13723. [PubMed] [Google Scholar]
- Schnitzer JE, Oh P, Pinney E, Allard J. Filipin-sensitive caveolae-mediated transport in endothelium: reduced transcytosis, scavenger endocytosis, and capillary permeability of select macromolecules. J Cell Biol. 1994;127:1217–1232. doi: 10.1083/jcb.127.5.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnitzer JE, Liu J, Oh P. Endothelial caveolae have the molecular transport machinery for vesicle budding, docking, and fusion including VAMP, NSF, SNAP, annexin, and GTPases. J Biol Chem. 1995;270:14399–14404. doi: 10.1074/jbc.270.24.14399. [DOI] [PubMed] [Google Scholar]
- Schnitzer JE, Oh P, McIntosh DP. Role of GTP hydrolysis in fission of caveolae directly from plasma membranes. Science (Wash DC) 1996;274:239–242. doi: 10.1126/science.274.5285.239. [DOI] [PubMed] [Google Scholar]
- Sessa WC, Barber CM, Lynch KR. Mutation of N-myristoylation site converts endothelial cell nitric oxide synthase from a membrane to a cytosolic protein. Circ Res. 1993;72:921–924. doi: 10.1161/01.res.72.4.921. [DOI] [PubMed] [Google Scholar]
- Sessa WC, García-Cardeña G, Liu J, Keh A, Pollock JS, Bradley J, Thiru S, Braverman IM, Desai KM. The Golgi association of endothelial nitric oxide synthase is necessary for the efficient synthesis of nitric oxide. J Biol Chem. 1995;270:17641–17644. doi: 10.1074/jbc.270.30.17641. [DOI] [PubMed] [Google Scholar]
- Shaul PW, Smart EJ, Robinson LJ, German Z, Yuhanna IS, Ying Y, Anderson RGW, Michel T. Acylation targets endothelia nitric-oxide synthase to plasmalemmal caveolae. J Biol Chem. 1996;271:6518–6522. doi: 10.1074/jbc.271.11.6518. [DOI] [PubMed] [Google Scholar]
- Shenoy-Scaria AM, Dietzen DJ, Kwong J, Link DC, Lublin DM. Cysteine 3 of Src family protein tyrosine kinase determines palmitoylation and localization in caveolae. J Cell Biol. 1994;126:353–363. doi: 10.1083/jcb.126.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Veit B, Baekkeskov S. Amino acid residues 24-31 but not palmitoylation of cysteines 30 and 45 are required for membrane anchoring of glutamic acid decarboxylase, GAD65. J Cell Biol. 1994;124:927–934. doi: 10.1083/jcb.124.6.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart EJ, Ying Y, Conrad PA, Anderson RGW. Caveolin moves from caveolae to the Golgi apparatus in response to cholesterol oxidation. J Cell Biol. 1994a;127:1185–1197. doi: 10.1083/jcb.127.5.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart EJ, Foster DC, Ying Y, Kamen BA, Anderson RGW. Protein kinase C activators inhibit receptor-mediated potocytosis by preventing internalization of caveolae. J Cell Biol. 1994b;124:307–313. doi: 10.1083/jcb.124.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart EJ, Ying Y, Anderson RGW. Hormonal regulation of caveolae internalization. J Cell Biol. 1995;131:929–938. doi: 10.1083/jcb.131.4.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solimena M, Dirkx R, Jr, Radzynski M, Mundigl O, De Camilli P. A signal located within amino acids 1–27 of GAD65 is required for its targeting to the Golgi complex region. J Cell Biol. 1994;126:331–341. doi: 10.1083/jcb.126.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauber R, Gaitanaris GA, Pavlakis GN. Analysis of trafficking of Rev and transdominant Rev proteins in living cells using green fluorescent protein fusions: transdominant Rev blocks the export of Rev from the nucleus to the cytoplasm. Virology. 1995;213:439–449. doi: 10.1006/viro.1995.0016. [DOI] [PubMed] [Google Scholar]
- Stow JL, de Almeida JB, Narula EJ, Holtzman EJ, Ercolani L, Ausiello DA. A heterotrimeric G protein, Gαi-3, on Golgi membranes regulates the secretion of a heparan sulfate proteoglycan in LLC–PK1 epithelial cells. J Cell Biol. 1991;114:1113–1124. doi: 10.1083/jcb.114.6.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van't Hof W, Resh MD. Rapid plasma membrane anchoring of newly synthesized p59fyn: selective requirement for NH2-terminal myristoylation and palmitoylation at cysteine-3. J Cell Biol. 1997;136:1023–1035. doi: 10.1083/jcb.136.5.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venema VJ, Marrero MB, Venema RC. Bradykinin-stimulated protein tyrosine phosphorylation promotes endothelial nitric oxide synthase translocation to the cytoskeleton. Biochem Biophys Res Commun. 1996;226:703–710. doi: 10.1006/bbrc.1996.1417. [DOI] [PubMed] [Google Scholar]
- Wedegaertner PB, Wilson PT, Bourne HR. Lipid modifications of trimeric G proteins. J Biol Chem. 1995;270:503–506. doi: 10.1074/jbc.270.2.503. [DOI] [PubMed] [Google Scholar]