Abstract
Brain myosin V is a member of a widely distributed class of unconventional myosins that may be of central importance to organelle trafficking in all eukaryotic cells. Molecular constituents that target this molecular motor to organelles have not been previously identified. Using a combination of immunopurification, extraction, cross-linking, and coprecipitation assays, we demonstrate that the tail domain of brain myosin V forms a stable complex with the synaptic vesicle membrane proteins, synaptobrevin II and synaptophysin. While myosin V was principally bound to synaptic vesicles during rest, this putative transport complex was promptly disassembled upon the depolarization-induced entry of Ca2+ into intact nerve endings. Coimmunoprecipitation assays further indicate that Ca2+ disrupts the in vitro binding of synaptobrevin II to synaptophysin in the presence but not in the absence of Mg2+. We conclude that hydrophilic forces reversibly couple the myosin V tail to a biochemically defined class of organelles in brain nerve terminals.
Synaptic vesicles are neuronal organelles that sequester, store, and release neurotransmitters. Functionally mature synaptic vesicles are assembled locally, in the nerve terminal, from at least two different types of precursor vesicles (Okada et al., 1995). Once the mature synaptic vesicle becomes docked at the presynaptic membrane, it is prepared (“primed”) to rapidly discharge its shipment of transmitter into the synaptic cleft upon the arrival of an action potential. This secretory response is triggered by the opening of voltage-regulated Ca2+ channels and is the principle mechanism used by neurons to exchange information.
A majority of the synaptic vesicles in a nerve terminal are sequestered into clusters that are not immediately available for exocytosis (Landis et al., 1988; Hirokawa et al., 1989). Thus, the initial burst of Ca2+-triggered exocytosis is presently understood to result from the fusion of predocked vesicles that had matured to a fusion-competent state before the action potential arrived (Südhof, 1995; Rosenmund and Stevens, 1996). The current working model further interprets the existence of vesicle clusters as a potential reserve pool of synaptic vesicles that may be mobilized in a use-dependent manner to replenish the pool of readily releasable vesicles. Several independent lines of experimental evidence support this scheme. Electrophysiological measurements of membrane fusion report that hippocampal synapses recover from complete synaptic fatigue with a time constant of ∼10 s (Stevens and Tsujimoto, 1995; Rosenmund and Stevens, 1996), suggesting that mechanisms exist for actively replenishing the readily releasable pool of synaptic vesicles. The kinetics of synaptic vesicle recycling indicate that recently retrieved vesicle membranes do not completely fulfill the demand for releasable vesicles during periods of intense secretory activity (Ryan et al., 1993; Ryan and Smith, 1995). This analysis implies that a supply of fresh synaptic vesicles must somehow move from a reserve pool to the presynaptic membrane. In fact, movements of synaptic vesicles have now been observed in a variety of different synaptic preparations (Llinas et al., 1989; Koenig et al., 1993; Henkel et al., 1996). These movements are inconsistent with simple diffusion, since they are reported to be both ATP-dependent and vectorial in nature. Integral synaptic vesicle proteins are transported to the terminal by a microtubule-based superfamily of motor proteins, the kinesins (Okada et al., 1995). However, microtubules do not extend into the cortical cytoskeletal matrix of terminals (Landis et al., 1988; Hirokawa et al., 1989), and the kinesins are rapidly degraded upon their arrival in the terminal (Okada et al., 1995). Moreover, recent imaging studies have shown that intracellular particles move along actin bundles in nerve growth cones, rather than microtubules (Evans and Bridgman, 1995). For these reasons, it seems unlikely that the kinesins move synaptic vesicles between their putative storage sites (the vesicle clusters) and the sites of their release (the active zones). Despite intensive study of the molecular events that govern synaptic vesicle recycling, relatively little is known about the mechanisms that control synaptic vesicle movements within the nerve terminal.
Brain myosins V (p190, dilute, myr 6) are presently the leading candidates for a synaptic vesicle motor protein (Langford, 1995). In the nervous system, the mouse dilute (Mercer et al., 1991) and chicken myosin V (p190; Espreafico et al., 1992) proteins are localized within neurons and are more abundant than myr 6, which is concentrated in the dentate gyrus and choroid plexus (Zhao et al., 1996). The myosin superfamily includes at least 11 different molecular motors that possess a conserved motor domain attached to a variety of neck and tail domains with distinguishing structural and biochemical characteristics (Cheney et al., 1993b ; Mooseker and Cheney, 1995). Both the conventional myosins II and unconventional myosins V have been localized within presynaptic terminals (Espreafico et al., 1992; Mochida et al., 1994; see also Miller et al., 1992); form a two-headed structure that is capable of generating mechanochemical force and moving actin filaments; and possess actin-activated Mg2+-ATPase activity. In addition, both forms of myosins have neck domains that contain variable numbers of putative regulatory calmodulin light chain binding cassettes, and a flexible coiled-coil stalk that is followed by a tail domain which ends as a large (∼400 amino acids) COOH-terminal globular structure (Espreafico et al., 1992; Cheney et al., 1993a ). Diversification of the intracellular localization and functions of the myosins seems to depend on the COOH-terminal tail which is variable in sequence and length (Cheney et al., 1993b ). Myosins II and V appear to regulate complementary presynaptic functions: actin dynamics and the transport of membrane-bound organelles, respectively. The heavy chains of myosins IIA and IIB bind to acidic phospholipids and self-assemble into contractile filaments on the subplasmallemal surface (Murakami et al., 1995; Verkhovsky et al., 1995), which may play an important role in growth cone motility (Miller et al., 1992), the cycling of cortical actin assembly (Bernstein and Bamburg, 1989), and the regulation of neurotransmitter release (Mochida et al., 1994). In contrast, myosins V are not filament-forming, and they colocalize with intracellular membranes (Cheney et al., 1993a ). The class V myosins may have a role in regulating the extension of growth cone filapodia during neuronal development (Wang et al., 1996), as well as the movement of axoplasmic organelles (Kuznetsov et al., 1992; Bearer et al., 1993; Langford, 1995). A potential role for brain myosin V in organelle trafficking and transport is indirectly supported by the finding that this motor protein is capable of moving relatively large (800 nm) artificial beads in motility assays (Wolenski et al., 1995). Moreover, functional null mutations have established that the class V myosins are required in yeast for the intracellular transport of post-Golgi secretory vesicles (Johnston et al., 1991; Lillie and Brown, 1992; Govindan et al., 1995).
Myosin motors bound directly to synaptic vesicles may have an important role in regulating the availability of synaptic vesicles for exocytosis. The present study demonstrates that 2,3-butanedione-2-monoxime (BDM),1 a pharmacological inhibitor of endogenous myosin Mg2+-ATPase activity, significantly decreases the Ca2+-dependent release of glutamate from isolated nerve endings (synaptosomes). In addition, our data reveal that a major portion of the myosin V in nerve terminals was bound to synaptic vesicles and that this interaction was regulated by Ca2+ concentrations that are of physiological relevance. Cross-linking experiments indicated that myosin V specifically complexes with the synaptic vesicle proteins synaptobrevin and synaptophysin. Interactions between these two integral membrane proteins may impose an important additional constraint on the regulated pathway of secretion (Calakos and Scheller, 1994; Edelmann et al., 1995). We now present evidence that the cytoplasmic domain of the synaptobrevin–synaptophysin complex may also function as a binding partner for myosin V, forming a multimeric complex that shall be referred to as the “myosin V transport complex.”
Materials and Methods
Subcellular Fractionation
Rat hippocampal mossy fiber synaptosomes (P3) were isolated as described previously (Terrian et al., 1988). Rat cerebrocortical synaptosomes were prepared according to the method of Stahl et al. (1996). Cerebral cortices were homogenized in 10 vol (wt/vol) of ice-cold 0.32 M sucrose. The homogenate was subjected to low speed centrifugation (3,000 g for 2 min) and the resulting supernatant was centrifuged at 14,000 g for 12 min. Pelleted tissue was resuspended in 0.32 M sucrose, buffered with 10 mM Hepes, pH 7.4. This suspension was then loaded onto a discontinuous Ficoll gradient (Stahl et al., 1996). After centrifugation at 62,483 g for 35 min, the synaptosomal fraction was removed from the layer of 9% Ficoll (wt/ vol) and diluted with three volumes of buffer A (20 mM Hepes, pH 7.4, containing 140 mM NaCl, 5 mM KCl, 5 mM NaHCO3, 1.2 mM Na2HPO4, 1 mM MgCl2, and 10 mM glucose). Synaptosomes were stored as pellets on ice and used within 3.5 h. Cytosol-enriched and detergent-soluble and -insoluble fractions were isolated as described previously (Prekeris et al., 1996). The synaptic vesicle fraction was isolated by passing hypotonically lysed synaptosomes through 18- and 27-gauge syringe needles, three times each, before centrifugation at 10,000 g for 5 min. The synaptic vesicle fraction was recovered as a pellet from the resulting supernatant by ultracentrifugation (288,489 g for 20 min).
Glutamate Release
The on-line fluorometric analysis of glutamate release was performed according to the method of McMahon and Nicholls (1991) using a spectrofluorimeter with a stirred, thermostatted cuvette holder (LS50; Perkin-Elmer Corp., Norwalk, CT). Synaptosomal pellets were resuspended in buffer A (0.7 mg of protein/ml) and incubated for 15 min at 37°C before adding NADP+ (1 mM), glutamate dehydrogenase (45.3 U/ml), and either CaCl2 (1.3 mM) or EGTA (1.3 mM). BDM (10 mM) or an equivalent volume of H2O was also added to the incubation medium, where specified. After an additional 7 min of incubation at 37°C, synaptosomes were depolarized by the addition of 45 mM KCl, and fluorescence was continuously monitored at 340 and 460 nm (excitation and emission, respectively). The data were exported to Sigma Plot Scientific Graphing Software (Jandel Scientific, San Rafael, CA) where the computed concentrations of glutamate were averaged at 10-s time points and corrected for Ca2+-independent release. Exogenous glutamate (4 nmol) was added at the end of each assay to calculate the amount of endogenous glutamate that had been released.
Measurement of Synaptosomal Membrane Potential and Cytosolic Free Calcium
Changes in synaptosomal membrane potentials were monitored using the thiocarbocyanine dye, 3,3′-diethylthiadicarbocyanine iodide (DiSC2[5]). Rat cerebrocortical synaptosomes (P2) were resuspended in buffer A (14.0 mg protein/ml) and incubated for 10 min at 37°C before introducing CaCl2 (1.3 mM final concentration). After an additional 5-min incubation, synaptosomes (0.5 mg protein) were transferred to a continuously stirred quartz cuvette containing 1 ml buffer A and 2 μM DiSC2(5). Fluorescence was monitored on a LS50 spectrofluorometer (Perkin-Elmer Corp.) every 2 s at excitation and emission wavelengths of 643 and 680 nm, respectively. BDM (10 mM), or an equivalent volume of H2O (10 μl), was added to the cuvette after 2 min, and KCl (20 mM) was introduced at 7 min. Changes in cytosolic free calcium [Ca2+]c were monitored using the fluorescence ratio method for the Ca2+ indicator Fura-2, as described previously (Gannon and Terrian, 1991). BDM (10 mM) and KCl (35 mM) were introduced at the times indicated.
Immunoisolation of Synaptic Vesicles
Rat cerebral cortices were homogenized (nine passes at 2,000 rpm in a glass–Teflon homogenizer) in buffer B (4 mM Hepes [pH 7.4], containing 0.3 M sucrose, 100 μg/ml PMSF, 5 μg/ml leupeptin, and 20 μg/ml pepstatin), and centrifuged at 871 g for 10 min. A crude synaptosomal pellet was obtained after centrifugation of the postnuclear supernatant at 12,000 g for 15 min. This pellet was washed twice, using 30 ml of buffer B, before resuspending the final pellet in 5 ml of buffer B and 45 ml of ice-cold H2O. After this hypotonic shock, the synaptosomal lysate was rehomogenized (3 passes at 9,000 rpm) and buffered with 10 mM Hepes (pH 7.4), containing 2.5 mM MgCl2 and 1 mM ATP. The buffered lysates were then incubated for 10 min at 37°C in the presence of either 2 mM EGTA or the specified concentration of CaCl2. Additional divalent metal ions during this incubation were used as specified in the figure legends. The crude synaptic vesicle fraction (LP2) was the supernatant obtained by centrifuging the hypotonically lysed synaptosomes at 32,500 g for 20 min. Synaptic vesicles were immunoisolated from this synaptic vesicle fraction according to the method of Jahn and colleagues (Walch-Solimena et al., 1995). Methacrylate microbeads (Eupergit C1Z) that had been precoated with anti– synaptobrevin II or anti-rab3 antibodies were added at a dilution of 1:2,000 (vol/vol) and the lysate was incubated for 1 h at 4°C. Immunoprecipitates were sedimented at 5,000 g for 5 min and washed three times in sucrose-free buffer B.
Cross-linking and Immunoprecipitation of Synaptosomal Proteins
Synaptosomes were resuspended at a protein concentration of 5 mg/ml in buffer A and incubated at 37°C for 10 min. The intact synaptosomes were then incubated in a shaking water bath for 1 h at 37°C in the absence and presence of the chemical cross-linking reagent dithiobis(succinimidyl propionate) (DTSP). DTSP was dissolved in dimethylformamide (final concentrations of 2.5 mM and 2.4%, respectively). Glycine (100 mM) was next added to quench the cross-linker, and the synaptosomal suspension was incubated for an additional 30 min. Synaptosomes were then pelleted and lysed by the addition of buffer C (10 mM Tris-HCl, pH 7.4, 1 mM EGTA, 1 mM EDTA, 100 mM NaCl, 1% Triton X-100, and 0.2 mM PMSF). After removing cellular debris by centrifugation at 15,000 g for 5 min, the synaptosomal lysate was preabsorbed with protein A–agarose beads. This precleared lysate was used for all immunoprecipitations. Anti–synaptobrevin II, anti-synaptophysin, and anti–synapsin I antibodies were all used at a dilution of 1:50 (vol/vol), while the anti–synaptotagmin I antibody was used at a dilution of 1:100 (vol/vol). All antisera were incubated with the synaptosomal lysate overnight at 4°C. Where mAbs were used, anti–mouse IgG was also added to a final dilution of 1:50 (vol/vol). Immunocomplexes were sedimented using protein A–agarose beads and washed three times with buffer C. Where specified, cross-linked adducts were cleaved by reduction for 30 min in 25 mM Tris-HCl (pH 7.4), containing 50 mM DTT and 1% β-mercaptoethanol, and analyzed by SDS-PAGE and immunoblotting.
In coimmunoprecipitation experiments, freshly prepared synaptosomal lysates were forced through 18- and 27-gauge syringe needles (see above) before adding 10 mM Hepes (pH 7.4), 100 mM NaCl, and the specified combination of 2 mM EGTA, 0.1 mM CaCl2, 1 mM ATP, or 1 mM adenosine 5′-O′[3-thiotriphosphate] (ATPγS). After incubating for 10 min at 37°C, either Triton X-100 or CHAPS was added to a final concentration of 1% (vol/vol) and all samples were incubated for an additional 30 min on ice before sedimentation (15,000 g for 5 min). The resulting supernatants were preabsorbed with protein A–agarose beads and immunoprecipitations were carried out using anti–synaptobrevin II or anti-synaptophysin mAbs. Control samples were incubated in the absence of primary antisera. Anti–mouse IgG was added to all samples at either 1 h or 12 h, as specified below. Immunoprecipitates were analyzed by SDS-PAGE and immunoblotting.
Other Methods and Materials
SDS-PAGE was carried out according to Laemmli (1970). Immunoblotting on nitrocellulose membranes was performed as previously described (Terrian and Ways, 1995) using the enhanced chemoluminescence method of detection (Amersham Corp., Buckinghamshire, UK). Treatment effects were evaluated by determining the significance of the difference between means using a paired t test. Curve fits on experimental values were performed using the Sigma Plot Scientific Graphing Software (Jandel Scientific). All reagents used in this study were purchased from the Sigma Chemical Co. (St. Louis, MO), unless otherwise specified. Protein A–agarose was obtained from Life Technologies, Inc. (Gaithersburg, MD). DiSC2(5) and Fura-2AM were purchased from Molecular Probes, Inc. (Eugene, OR). Antibodies directed against GAP-43 (43-kD guanosinetriphosphatase-activating protein) and mice IgG were obtained from Sigma Immunochemicals (St. Louis, MO). Anti–synaptobrevin II and anti-rab3 immunocoated beads were a gift from Dr. Reinhard Jahn (Yale University, New Haven, CT). The following antibodies were generously provided: C 7.2 anti-synaptophysin, and C1 69.1 anti–synaptobrevin II (Dr. Reinhard Jahn, Yale University, New Haven, CT), anti–chicken brain myosin V (Dr. Richard Cheney, University of North Carolina at Chapel Hill, Chapel Hill, NC), anti–myosin IIA and anti–myosin IIB (Dr. Richard Adelstein, National Heart, Lung, and Blood Institute, Bethesda, MD), anti–Na+/K+ ATPase (Dr. Donald Fletcher, East Carolina University, Greenville, NC), anti–synapsin I (Dr. Paul Greengard, The Rockefeller University, New York), and V216 anti–synaptotagmin I (Dr. Thomas Südhof, Southwestern Medical Center, Dallas, TX).
Results
Myosin Mg2+-ATPase Activity Enhances the Sustained Release of Glutamate
Do actin-dependent motors work to enhance the availability of synaptic vesicles for exocytosis? In our first experiments, BDM was used as a broad spectrum inhibitor of myosin Mg2+-ATPase activity to address this fundamental question. BDM (10 mM) significantly diminished the Ca2+-dependent release of glutamate from rat cerebrocortical synaptosomes (Fig. 1), without affecting basal glutamate release (data not shown). When the data were curve fit using a four-parameter logistic function (Logistic.fit; Jandel Scientific), the results indicated that BDM inhibited the K+-evoked release of glutamate by ∼70% after 2 min of continuous depolarization (Fig. 1). Moreover, the extent of inhibition produced by BDM increased over time. BDM inhibits both conventional and unconventional myosin Mg2+-ATPases with low affinity in vitro (50% inhibiting concentration is ∼5 mM), including nonmuscle myosins II and V (Cramer and Mitchison, 1995). This pharmacological agent interacts with myosin subfragment 1 to prevent the release of Pi from the key intermediate myosin–ADP·Pi (Herrmann et al., 1992) and does not affect kinesin Mg2+-ATPase activity or actin assembly at a concentration of 10 mM (Cramer and Mitchison, 1995; Lin et al., 1996). It is also important to note that 10 mM BDM had no effect on the resting membrane potential of intact synaptosomes, monitored with the potential-sensitive dye DiSC2(5) (Fig. 1, inset), or on the level of cytosolic free Ca2+ measured using the Ca2+ indicator Fura-2 (data not shown). At the concentration used in these experiments, BDM by itself had no effect on the fluorescence measured. These data provide the first preliminary evidence of a functional link between actin-dependent motors and glutamate exocytosis, but do not reveal whether a particular myosin is critical for this secretory response.
Figure 1.

Effect of myosin Mg2+-ATPase inhibition on the Ca2+-dependent release of endogenous glutamate from cerebrocortical synaptosomes. Synaptosomes were preincubated for 5 min in the absence (Control) or presence (BDM) of 10 mM BDM before the onset of membrane depolarization. Ca2+-dependent glutamate release was calculated by subtracting the 45 mM K+-evoked release measured in the presence of 1.3 mM EGTA (Ca2+-independent release) from the release measured in the presence of 1.3 mM CaCl2 (total release). Data are the means ± SEM of duplicate determinations in three independent experiments. Curve fits (solid lines) were obtained using a four-parameter logistic function. (Inset) Changes in synaptosomal membrane potential as measured using the potential-sensitive fluorescent dye DiSC2(5). Synaptosomes were stained with 2 μM DiSC2(5) and fluorescence measurements (expressed in arbitrary units) were made as described in Materials and Methods. BDM (10 mM) and KCl (25 mM) were added at the indicated times (arrowheads). Data are the means ± SEM of five independent determinations.
Myosin V Is a Synaptic Vesicle-associated Motor Protein
The simple concept that organelles are most likely to be transported by the motors to which they bind prompted a search for synaptic vesicle-associated myosin proteins. Consistent with the presynaptic localization of myosins II and V in cultured neurons (Cheng et al., 1992; Espreafico et al., 1992; Mochida et al., 1994), hippocampal and cerebrocortical synaptosomes were found to contain both conventional (myosin IIA and IIB) and unconventional (myosin V) classes of myosin proteins (Fig. 2 A and data not shown). In initial experiments, the subcellular distribution of these molecular motors was analyzed using a standard protocol that yields a cytosol-enriched fraction (Sol.), and detergent-soluble (TSF) and -insoluble (TIF) extracts of a heavy membrane fraction that are thought to contain the synaptosomal plasma membrane and cytoskeletal matrix, respectively (Fig. 2 A; Prekeris et al., 1996). Equal amounts of protein from each subcellular fraction were probed for myosin IIA, myosin IIB, and myosin V by immunoblotting. As shown in Fig. 2 A, these myosins were enriched in the cytosol-enriched fraction. This result indicates that the major pool(s) of myosins in nerve terminals is (are) either soluble or colocalized with the small membrane fragments that remained in the cytosol-enriched fraction. Of relevance to the present study, the cytosol-enriched fraction has previously been shown to contain small synaptic vesicles (Huttner et al., 1983).
Figure 2.
Myosin V copurifies with rab3- and synaptobrevin II (syb)–containing synaptic vesicles. (A) Hippocampal mossy fiber synaptosomes (P3) were isolated from homogenates of hippocampal tissue (Hom.) and subfractioned into a cytosol-enriched fraction (Sol.) and 1% Triton X-100–soluble (TSF) and –insoluble (TIF) fractions. Equal amounts of protein were analyzed for myosins IIA, IIB, and V by immunoblotting. (B) Synaptosomal lysate supernatant (LS1) was incubated with immunobeads coupled to anti-synaptobrevin II mAbs as described in Materials and Methods. The immunopurified synaptic vesicles (SV) were washed in homogenization buffer and analyzed by SDS-PAGE and immunoblotting. Equal volumes of the LS1 and SV fractions were analyzed for myosins IIA, IIB, and V, synapsin I, synaptophysin, and GAP-43. In a separate experiment, rab3- and syb-containing organelles were independently isolated from the LS1 fraction using immunobeads. Sample concentrations were adjusted for loading equal amounts of the vesicle membrane protein synaptophysin and analyzed for the indicated protein by immunoblotting.
To identify myosins that interact with synaptic vesicles, organelles were immunopurified from a synaptosomal lysate supernatant (LS1) using methacrylate microbeads that had been precoated with anti–synaptobrevin II (syb) antibodies (Fig. 2 B). This method yields a synaptic vesicle fraction that is virtually free of contamination (Walch-Solimena et al., 1995). As shown in Fig. 2 B (left lanes), synaptobrevin II beads cosedimented with the synaptic vesicle marker proteins synapsin I and synaptophysin. Myosin V was also present in the synaptobrevin bead pellet, while neither of the myosin II isoforms could be detected (Fig. 2 B, lane syb). Myosin IIB self-assembles into a filamentous cytoskeletal structure (Murakami et al., 1995) that was absent in the immunobead pellet, confirming that immunopurified synaptic vesicles were not contaminated by significant amounts of this cytoskeletal protein. Furthermore, the synaptic plasma membrane protein, GAP-43, was abundant in the starting material (LS1), but was excluded from the organelles bound to synaptobrevin beads (Fig. 2).
To confirm that myosin V was genuinely associated with synaptic vesicles, these small organelles were also immunopurified using immunobeads coupled to rab3 mAbs. Both the rab family of small GTP-binding proteins and the synaptobrevin homologs (I, II, and cellubrevin) have been implicated in the trafficking of mature, recycled, secretory vesicles (for review see Ferro-Novick and Novick, 1993; Calakos and Scheller, 1996). The rab3-containing organelles were analyzed for myosins by immunoblotting and compared with the synaptobrevin II–containing organelles that were independently immunoisolated from the same lysates. To load approximately equal amounts of total vesicle protein, the concentration of the immunobead fractions was adjusted so that equal amounts of synaptophysin would be loaded onto each gel (Fig. 2 B, lanes rab3 and syb). This analysis demonstrated that equal amounts of myosin V copurified with both synaptic vesicle fractions, as did the synaptic vesicle marker protein synapsin I (Fig. 2 B, lanes rab3 and syb). Myosins IIA and IIB were not detected in either fraction, indicating that synaptic vesicles may be selectively linked to class V myosins in nerve terminals. These data support the conclusion that the interaction of myosin V with synaptic vesicles was not an artifact of the immunoisolation procedure. Moreover, a direct quantitative comparison of equivalent fractional volumes for the LS1 and immunobead fractions estimated that ∼60% of the total myosin V in the starting material copurified with synaptobrevin II–containing synaptic vesicles (immunoblots not shown). This result suggests that brain myosins V may be specifically targeted to the cytoplasmic face of synaptic vesicles. The anti–chicken brain myosin V antibodies used in these studies were raised against the COOH-terminal tail domain of this protein (Espreafico et al., 1992). Because there is a high degree of amino acid sequence similarity among the known brain myosins V within this domain (Zhao et al., 1996), it is possible that this antisera cross-reacts with the p190/dilute/myr 6 homologs of myosin V.
Hydrophilic Forces Link Synaptic Vesicles to the Tail Domain of Myosin V Heavy Chains
To evaluate how brain myosin V binds to synaptic vesicles, synaptobrevin II–containing vesicles were immunoisolated and incubated under a variety of different conditions before resedimentation of the immunobeads. The effect of these treatments on the association of myosin V with this population of vesicles was then examined by Western blot analysis of the immunobead fractions. Synapsin I and synaptophysin were used as controls in these experiments because these proteins are established synaptic vesicle markers that are known to be peripheral and integral to the vesicle membrane, respectively. Sodium pyrophosphate (15 mM) effectively disrupts nonspecific ionic interactions (Amar-Costesec et al., 1974) and had no effect on the binding of myosin V or synapsin I (Fig. 3 A). After phase separation of the synaptic vesicle proteins using Triton X-114 (Bordier, 1981), all of the myosin V and the majority of synapsin I was found in the aqueous phase (AP) whereas synaptophysin was recovered in the detergent phase (DP) (Fig. 3 A). These data are consistent with the notion that a population of mature vesicles possesses myosin V as a cytoplasmically oriented, peripheral membrane protein. Further analysis provided additional support for this proposal. First, this motor protein was accessible to the treatment of intact vesicles with an exogenous protease (Fig. 3 C). Second, myosin V binding was sensitive to increasing ionic strength. When immunopurified vesicles were exposed to 1 M NaCl, the amounts of soluble (S) myosin V and synapsin I were both increased (Fig. 3 B), suggesting that electrostatic interactions play an important role. However, even this high concentration of NaCl only partially disrupted the interactions between myosin V or synapsin I and immunopurified synaptic vesicles. Myosin V did not appear to associate with vesicle membranes through hydrophobic forces, since even high concentrations of phosphatidylcholine (PC) liposomes did not compete with vesicle membranes for the binding of myosin V (Fig. 3 C). Finally, the Ca2+-dependent protease calpain was used to map the region of myosin V that makes contact with synaptic vesicles. Limited proteolysis of brain myosin V by calpain generates two stable fragments that correspond to the head (∼65 kD) and tail (∼80 kD) domains of this molecular motor (Espindola et al., 1992). The 80-kD tail fragment is generated by cleavage between Arg1140 and Met1141 (Nascimento et al., 1996), immediately adjacent to a putative calpain recognition site that is referred to as the PEST (proline-, glutamate-, serine-, and threonine-rich) sequence. Synaptic vesicles were immunopurified from synaptosomal lysates and exposed to calpain. The immunobeads were then sedimented, washed, and probed for bound myosin V. Immunoblotting with antibodies that were raised against the tail domain of chicken brain myosin V revealed that an ∼80-kD fragment of the native protein remained bound to synaptic vesicles after limited proteolysis by calpain (Fig. 3 C). It shall be concluded that the COOH-terminal tail region (amino acids Met1141–Val1830) of brain myosin V binds to synaptic vesicles through intermolecular electrostatic interactions. These electrostatic interactions may involve an association between the myosin V heavy chain(s) and proteins/acidic phospholipids on the cytoplasmic face of synaptic vesicles. Whether or not the head domain of myosin V also interacts with the synaptic vesicle surface remains to be determined.
Figure 3.
Nature of the interaction between myosin V and synaptobrevin II–containing organelles. Immunopurified vesicles were either washed with sucrose-free buffer B (Control) or extracted with 15 mM sodium pyrophosphate (pH 8.2) or 1% Triton X-114 (A); 1 M NaCl (B); and 1 mM phosphatidylcholine (PC) liposomes or exposed to calpain (5 U) (C). Immunobeads were resedimented and the resulting supernatants (S) and pellets (P) were analyzed for myosin V, synapsin I, and synaptophysin by immunoblotting. Immunobead fractions treated with sodium pyrophosphate and probed for synaptophysin were apparently underloaded in the gel shown. Phase separations performed using Triton X-114 yielded an aqueous phase (AP), detergent phase (DP), and insoluble phase (IP). Myo V tail, the ∼80-kD tail fragment generated by calpain cleavage of brain myosin V.
Nature of the Tail Binding Partner(s) for Myosin V
Tail domains of the unconventional myosins display a striking degree of structural divergence (Cheney et al., 1993b ), and it is postulated that each myosin tail sequence has its own specific intracellular receptor, located at the motor's intended destination (Mooseker and Cheney, 1995). Thus, it is critical to identify the tail binding partners that are targeted to a given myosin and to specify the intracellular locus of force production/signal transduction. Despite the emphasis that has been placed on acquiring this fundamental information, only a single “anchoring site” has tentatively been identified for an unconventional myosin, myosin IXB (Reinhard et al., 1995).
To identify synaptic vesicle proteins interacting directly with myosin V, intact synaptosomes were treated with the cleavable, homobifunctional cross-linking reagent, DTSP. This cross-linker has a maximum spacer arm length of 11 Å and is membrane permeable. After the in situ cross-linking of endogenous synaptosomal proteins, we immunoprecipitated the synaptic vesicle proteins synaptobrevin II (syb), synaptophysin (syp), synapsin I, and synaptotagmin (syt) from 1% Triton X-100 synaptosomal lysates (Fig. 4). To determine whether any of these vesicle proteins were complexed with myosin V in an intact nerve terminal, the DTSP cross-linker was cleaved by chemical reduction, and all immunoprecipitates were analyzed for the presence of myosin V. Controls were treated in an identical manner, except that no primary antibodies were added to these samples. Myosin V coimmunoprecipitated with two different synaptic vesicle membrane proteins, synaptobrevin II and synaptophysin (Fig. 4 A). Consistent with previous reports that synaptobrevin II directly binds to synaptophysin (Calakos and Scheller, 1994; Edelmann et al., 1995), these two proteins also coimmunoprecipitated with each other (Fig. 4 A). Anti–synapsin I and anti–synaptotagmin I antibodies did not coimmunoprecipitate myosin V and did not cross-react with the myosin V–synaptobrevin–synaptophysin adduct (Fig. 4 A). Furthermore, none of the immunoprecipitates reacted with antibodies against GAP-43. The clear implication from these findings is that myosin V directly interacts with the cytosolic domain(s) of synaptobrevin/synaptophysin to form a multimeric complex on the surface of synaptic vesicles.
Figure 4.
Cross-linking of myosin V to the synaptobrevin II (syb)–synaptophysin (syp) protein complex in intact nerve terminals. (A) Intact cerebrocortical synaptosomes were treated with the chemical cross-linker DTSP and lysed with 1% Triton X-100. Immunoprecipitates were isolated from precleared lysates using antibodies that were raised against synapsin I (synapsin), syp, synaptotagmin I (syt), or syb. Controls were not exposed to primary antibodies before the addition of a secondary IgG (none). DTSP was cleaved by chemical reduction before analyzing each sample by SDS-PAGE and immunoblotting. Two adducts containing myosin V were visible. Anti-syt antibodies nonspecifically reacted with the IgG heavy chains (h.c.). (B) Synaptosomes were incubated in the absence or presence of DTSP, as indicated, before the isolation of a crude synaptic vesicle fraction. Equal amounts of protein were then separated on 5% (top) and 10% (bottom) nonreducing gels, and analyzed by immunoblotting. An adduct of M r >669 kD contained myosin V, syb, and syp.
After cross-linking, native myosin V dimers are predicted to have a molecular mass of 640 kD (Cheney et al., 1993a ). When cross-linked with a synaptobrevin–synaptophysin heterodimer, this would form a complex of 696 kD that would not move significantly into the SDS-PAGE systems, which were previously used to study synaptobrevin– synaptophysin interactions (10% and 15% acrylamide resolving gels; Calakos and Scheller, 1994; Edelmann et al., 1995, respectively). This is why the cross-linker was cleaved before SDS-PAGE in the initial experiments and might possibly explain why the myosin V–synaptobrevin–synaptophysin complex was not observed in earlier studies. To examine the latter possibility, synaptosomes were incubated in the absence or presence of DTSP, solubilized with Triton X-100, and resolved by SDS-PAGE on nonreducing gels followed by immunoblotting with anti–synaptobrevin II, anti-synaptophysin, and anti–myosin V antibodies. Synaptobrevin II– and synaptophysin-containing adducts were identified on both a 5% and 10% gel (Fig. 4 B). When separated on the 5% gel (Fig. 4 B, top), a single adduct of >669 kD (thyroglobulin standard) was found to contain myosin V, synaptobrevin II, and synaptophysin. Only the cross-linked sample contained this large protein complex and, in three separate experiments, we failed to identify any additional adducts in which all three of these proteins could be detected in either a 5% or 10% gel (data not shown). Four additional adducts with M r of ∼38, 60, 77, and 100 kD comigrated with synaptobrevin II, while adducts with M r of ∼55 and 70 kD comigrated with synaptophysin (Fig. 4 B, bottom). The formation of these adducts was only observed when the intact synaptosomes had been exposed to cross-linker. The M r 55–60-kD complex contained both synaptobrevin II and synaptophysin (Fig. 4 B, bottom). While the putative target membrane SNAP receptor (t-SNARE) syntaxin appears to be excluded from this synaptobrevin–synaptophysin complex (Edelmann et al., 1995), these cross-linking experiments provided the first preliminary evidence that brain myosin V may interact with this synaptic vesicle membrane protein complex in situ (Fig. 4 B).
The fact that myosin V becomes cross-linked with synaptobrevin II and synaptophysin, but not synaptotagmin or synapsin I (Fig. 4 A), indicates that these interactions are relatively specific. To further characterize the specificity of this interaction, and to determine whether the observed coprecipitation was dependent on chemical cross-linking, additional immunoprecipitation experiments were performed in the absence of cross-linker. Because interactions between synaptic vesicle proteins are known to be influenced by the amount and type of detergent used for membrane solubilization (Bennett et al., 1992), synaptosomal lysates were prepared using nonionic (Triton X-100) and zwitterionic (CHAPS) detergents at a final concentration of 1% (vol/vol). The results of this comparison are presented in Fig. 5. Immunoprecipitations from Triton X-100–solubilized and CHAPS-solubilized samples were performed using mAbs against synaptobrevin II and synaptophysin. Both anti–synaptobrevin II and anti-synaptophysin antibodies were relatively efficient at precipitating synaptobrevin or synaptophysin, respectively (right columns in Figs. 5, A and B, correspond to 10% of the total solubilized starting material used for each immunoprecipitation). Western blot analysis of the immunoprecipitates confirmed that interactions between synaptobrevin II and synaptophysin are not disrupted by either detergent (Bennett et al., 1992). Coprecipitation of these vesicle membrane proteins was apparent, in the absence of cross-linking, for both the Triton X-100– and CHAPS-solubilized synaptosomal fractions. In addition, both anti–synaptobrevin II and anti-synaptophysin antibodies efficiently precipitated myosin V from CHAPS-solubilized and Triton X-100–solubilized preparations (Fig. 5). When solubilized for 2 h at 4°C, anti–synaptobrevin II and anti-synaptophysin immunoprecipitates were markedly enriched in myosin V (Fig. 5). This recovery of myosin V was significantly decreased when the starting material was solubilized overnight (data not shown). None of these three proteins were detected in control samples containing only the secondary IgG (Figs. 5, A and B). Finally, two additional vesicle marker proteins (synapsin I and cysteine string protein) were not precipitated by either the anti–synaptobrevin II or anti-synaptophysin antibodies. These observations support the cross-linking results, and indicate that interactions between myosin V and the synaptobrevin–synaptophysin complex are at least partially preserved in low concentrations of the detergents Triton X-100 and CHAPS. While CHAPS does not appear to effectively disrupt interactions between multiple synaptic vesicle membrane proteins, Triton X-100 solubilization prevents the nonspecific aggregation of these proteins (Bennett et al., 1992). Thus, a significant amount of myosin V may be bound to the synaptobrevin– synaptophysin protein complex in brain nerve endings.
Figure 5.
Immunoprecipitation analysis of detergent-solubilized synaptosomes. Triton X-100– (A) and CHAPS-solubilized (B) samples were subjected to immunoprecipitation (I.P.) as described in Materials and Methods, with mAbs against synaptophysin (Syp) or synaptobrevin (Syb). Control samples were incubated with the secondary IgG alone (A and B; IgG). Immunoprecipitates were analyzed by SDS-PAGE and immunoblotting for the presence of Syp, Syb II, myosin V, synapsin I, and cysteine string protein (CSP). A sample corresponding to 10% of the total material used for all immunoprecipitations is shown in the right column of each panel (10% total).
Ca2+ Cooperates with Mg2+ to Disassemble the Myosin V–Synaptobrevin–Synaptophysin Complex
Does myosin V disengage the synaptic vesicle after this organelle has arrived at its appropriate location on the subplasmallemal surface? The extraction experiments (Fig. 3) indicate that myosin V tightly associates with synaptic vesicles. However, the presence of such a large motor protein (total length of a single myosin V is ∼83 nm; Cheney et al., 1993a ) on the vesicle surface might pose a serious hindrance to the protein–protein interactions that are presently understood to mediate vesicle docking and fusion. To determine whether functional links are established between myosin V and synaptic vesicles, experiments were designed to test the hypothesis that this interaction will be disrupted, in a controlled manner, by physiologically relevant signaling events. First, we examined the in situ effects of membrane depolarization on the binding of myosin V to vesicles. Intact synaptosomes were depolarized in the presence of 1.4 mM CaCl2 for the specified time (Fig. 6), sedimented by centrifugation, and washed twice using a Ca2+-free buffer (buffer B). Crude synaptic vesicle fractions were then isolated from each of these samples, and analyzed by SDS-PAGE and immunoblotting for myosin V. Equal amounts of protein were loaded into each lane of the gels. Fig. 6 (top) shows a representative experiment illustrating the effect of depolarization on the association of myosin V with synaptic vesicles. Sustained depolarization of intact synaptosomes had no significant effect on the amount of synaptophysin that cosedimented with the crude synaptic vesicle fraction (Fig. 6). In contrast, the amount of vesicle-associated myosin V decreased by 47 ± 7% (mean ± SEM of four independent experiments) within 60 s of depolarization (Fig. 6). Additional myosin V was progressively displaced from the vesicle surface during the continued depolarization of intact synaptosomes (Fig. 6). This effect of depolarization was Ca2+ dependent, since myosin V did not dissociate from the LP2 fraction when synaptosomes were depolarized in the absence of external Ca2+ (104 ± 16% of control myosin V remained bound).
Figure 6.
Depolarization dissociated the myosin V–vesicle complex in intact nerve terminals. Intact cerebrocortical synaptosomes were incubated for 6 min in the presence of 1.4 mM CaCl2. The concentration of KCl was increased to 40 mM at 0, 1, 3, and 6 min of incubation, giving the specified time of depolarization. Crude synaptic vesicle fractions were immediately isolated and analyzed for myosin V and synaptophysin by SDS-PAGE and immunoblotting. Equal amounts of protein were loaded onto each gel. Synaptophysin was used as a control to correct for the corresponding amounts of myosin V in each sample and the nondepolarized (0 min) values were normalized to 100%. Data are the means ± SEM of four independent experiments.
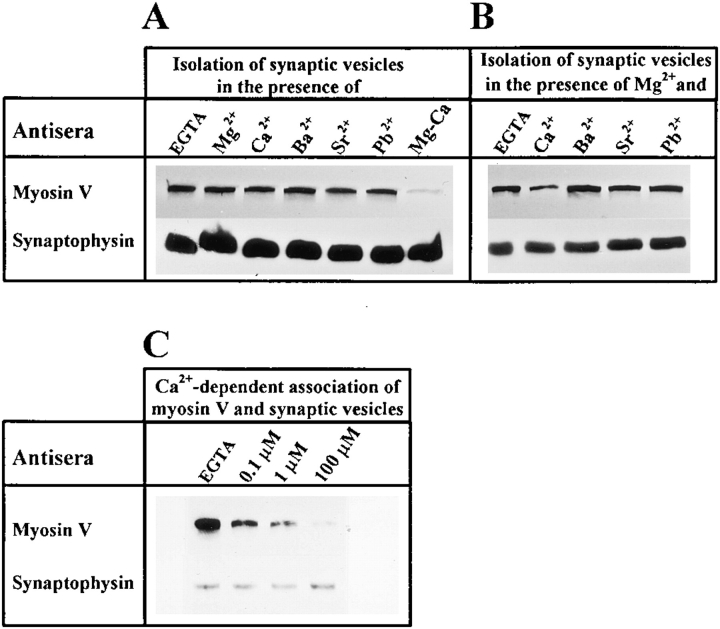
Knowing that the initial effect of membrane depolarization is to open voltage-regulated Ca2+ channels, a series of in vitro binding assays was performed to determine whether Ca2+ directly or indirectly disrupts myosin V binding to synaptic vesicles. Synaptosomes were lysed by osmotic shock, and vesicles were immunoisolated from lysates with anti–synaptobrevin II beads in the presence of 1 mM ATP, 2.5 mM MgCl2, and the specified divalent cation(s). Mg-ATP was added to optimize the recovery of vesicle-bound myosin V by disrupting its interactions with filamentous actin (Nascimento et al., 1996). Changes in the amounts of myosin V that coimmunopurified with the vesicles were determined by SDS-PAGE and immunoblotting. To load equal amounts of total vesicle protein, the concentration of the immunobead fractions was adjusted so that equal amounts of synaptophysin would be loaded onto the gels (Fig. 7). These experiments provided several intriguing findings. First, Ca2+ only altered the pool of vesicle-bound myosin V in the presence of Mg2+ (Fig. 7 A). When Mg2+ was added to synaptosomal lysates, the addition of 100 μM Ca2+ removed 80 ± 4% of the synaptic vesicle-associated myosin V (mean ± SEM of eight independent experiments). Second, this effect was specific for Ca2+ (Fig. 7 B). Divalent cations other than Ca2+ can support neurotransmitter release and these include Sr2+, Ba2+, and Pb2+. However, no dissociation was observed when these divalent metal ions were added to synaptosomal lysates in the absence or presence of Mg2+ (Fig. 7, A and B), indicating that disassembly of the myosin V transport complex specifically requires Ca2+ and Mg2+. Third, the 50% inhibiting concentration value for the Ca2+-induced disruption of myosin V binding was estimated to be 1 μM (Fig. 7 C), a concentration that is within a physiologically relevant domain.
Figure 7.
Ca2+ cooperates with Mg2+ to reverse the binding of myosin V to synaptic vesicles. (A) Synaptic vesicles were immunoisolated from synaptosomal lysates in the presence of 2 mM EGTA or the specified divalent ion(s). All ions were used at a final concentration of 100 μM, except Mg2+ (2.5 mM) and Pb2+ (0.1 μM). The concentration of Pb2+ used in this study corresponds to the value that produces maximal secretory activity (unpublished observation). (B) Synaptic vesicles were immunoisolated from synaptosomal lysates in the presence of 2.5 mM Mg2+ and either 2 mM EGTA or 100 μM Ca2+, Ba2+, Sr2+, or 0.1 μM Pb2+. (C) Synaptic vesicles were immunoisolated from synaptosomal lysates in the presence of 2.5 mM Mg2+ and the specified concentrations of Ca2+. Coimmunopurified proteins were separated by SDS-PAGE. Synaptophysin was used to control for equal loading of all samples and changes in the amount of vesicle-bound myosin V were analyzed by immunoblotting.
Synaptobrevin binding to synaptophysin inhibits assembly of the core fusion complex (Edelmann et al., 1995) and even high concentrations of Ca2+ (500 μM) have not been found to reverse this interaction in vitro (Chapman et al., 1995). Consequently, localized Ca2+ entry may only liberate synaptic vesicles from myosin V without enabling synaptobrevin to interact with the syntaxin–SNAP-25 complex. However, the effect of Ca2+ ions on the interaction between synaptobrevin and synaptophysin had not previously been examined in the presence of Mg2+ and ATP (Chapman et al., 1995). Given the requirement for both Mg2+ and Ca2+ in the dissociation of myosin V from synaptic vesicles (Fig. 7), it was of interest to determine whether synaptobrevin–synaptophysin interactions share a similar sensitivity to this combination of divalent cations. To determine whether Ca2+ is capable of reversing synaptobrevin–synaptophysin interactions in the presence of Mg2+ and ATP, these integral membrane proteins were immunoprecipitated from synaptosomal Triton X-100 extracts using anti-synaptobrevin and anti-synaptophysin mAbs. Immunoprecipitated synaptobrevin and synaptophysin were independently analyzed for the presence of the reciprocal synaptic vesicle binding partner. All coprecipitations shown in Fig. 8 A were performed in the presence of 1 mM ATP and either excess chelator (EGTA) or CaCl2 in the absence (Ca 2+) or presence (Mg–Ca) of MgCl2. Synaptobrevin and synaptophysin were not detected in controls exposed to only IgG (data not shown). In the presence of EGTA, Mg2+, and ATP, synaptobrevin and synaptophysin antibodies coimmunoprecipitated one another (Fig. 8 A, EGTA). Substituting 100 μM Ca2+ for EGTA, in the absence of Mg2+, had no effect on the observed coimmunoprecipitating proteins (Fig. 8 A, Ca 2+). However, synaptobrevin and synaptophysin did not coprecipitate in the presence of Ca2+, Mg2+, and ATP (Fig. 8 A, Mg–Ca).
Figure 8.
Ca2+ cooperates with Mg2+ to reverse synaptobrevin II binding to synaptophysin in an ATP-dependent manner. (A) Rat cerebrocortical synaptosomal lysates were incubated for 10 min at 37°C in the presence of 1 mM ATP and either excess chelator (2 mM EGTA) or 100 μM CaCl2 in the absence (Ca2 +) or presence (Mg–Ca) of 2.5 mM MgCl2. Triton X-100 (1% vol/vol) extracts were precleared with protein A–agarose as described under Materials and Methods and subjected to immunoprecipitation using mAbs directed against synaptobrevin II (syb) or synaptophysin (syp). Immunoprecipitates were washed and analyzed by SDS-PAGE and immunoblotting using both the anti-syb and anti-syp antibodies. Samples were loaded so that the intensities of syb and syp bands were equivalent for each experimental treatment when samples were immunoprecipitated and immunoblotted using the same antibodies. (B) Immunoprecipitations from cerebrocortical lysates were performed according to the procedure described above (A) in the absence or presence of 1 mM ATP or ATPγS, as indicated.
Because ATP was included in incubation media used in the above coimmunoprecipitation experiments, it was possible that a Ca2+-dependent Mg-ATPase might be involved in regulating the interactions of synaptobrevin and synaptophysin. To address this possibility, we repeated the experiments shown in Fig. 8 A in the absence and presence of either ATP or the nonhydrolyzable analogue ATPγS. Fig. 8 B shows that synaptobrevin and synaptophysin both coprecipitated in the absence of exogenous ATP and Ca2+ (lane 1, EGTA). This interaction was disrupted in the presence of Ca2+ and Mg2+, regardless of whether or not ATP was present (Fig. 8 B, lanes 2 and 4). Finally, while synaptobrevin did not coprecipitate with anti-synaptophysin antibodies in the presence of Mg2+, Ca2+, and ATPγS (right, lane 5) this nonhydrolyzable adenine analogue partially reversed the effects of these divalent cations on the immunoprecipitation of synaptophysin with anti-synaptobrevin antibodies (left, lane 5). Collectively, these results indicate that ATP is not essential for the Ca2+/Mg2+- dependent dissociation of the synaptobrevin–synaptophysin complex.
Discussion
Current models of synaptic vesicle biogenesis hold that these organelles are first assembled locally, at synaptic terminals (Kelly, 1991; Okada et al., 1995; Calakos and Scheller, 1996). Once assembled, the mature synaptic vesicle membrane undergoes multiple cycles of exocytosis and retrieval before being targeted for degradation. A fascinating observation that remains to be understood is that these organelles actively move within the terminal during their life cycle, shuttling between the presynaptic plasma membrane and vesicle clusters associated with cytoskeletal elements (Henkel et al., 1996). Detailed descriptions of the molecular interactions that may govern the topogenic site of vesicle fusion, recovery, and clustering have now been proposed (Ferro-Novick and Jahn, 1994; Südhof, 1995; Calakos and Scheller, 1996; Rothman and Wieland, 1996; Takei et al., 1996). However, there is no indication of how synaptic vesicles might actively move between the plasma membrane and the vesicle clusters that fill the remainder of the presynaptic terminal. We have presented direct evidence of a functional link between mature synaptic vesicles and the unconventional motor protein brain myosins V. Identification of the myosins V as synaptic, vesicle-associated motor proteins provides crucial support for the hypothesis that the novel (unconventional) class V myosins may be responsible for “driving” synaptic vesicle movements within terminals.
While a surplus of mature synaptic vesicles is typically docked and primed for fusion, the supply of these vesicles must be actively replenished during bouts of high frequency stimulation (Stevens and Tsujimoto, 1995; Rosenmund and Stevens, 1996). The present analysis of glutamate exocytosis was undertaken with an interest in the possibility that myosin motors may work to forestall synaptic fatigue by replenishing the pool of releasable vesicles during intense presynaptic activity. For this reason, the experimental protocol for measuring release emphasized the events that occur after depletion of the readily releasable and locally recycling vesicle pools (<60 s of continuous depolarization; Ryan et al., 1993), and before the onset of complete synaptic fatigue. In these experiments, BDM was used as a broad spectrum inhibitor of myosin Mg2+-ATPase activity, and found to selectively inhibit the Ca2+-dependent release of glutamate from cerebrocortical synaptosomes. By continuously monitoring K+-evoked release, it could be seen that BDM caused a pronounced inhibition of glutamate exocytosis, and that the extent of this inhibition increased over time. Many details regarding the mechanisms controlling synaptic vesicle movements remain obscure. A fundamental step towards understanding how vesicles may move in synaptic terminals is the recognition that the mechanochemical force that myosins produce substantially enhances the sustained secretion of glutamate from brain nerve terminals.
Identification of the myosin proteins that directly interact with mature synaptic vesicles is another significant prerequisite for understanding how these organelles move in the presynaptic terminal. Nerve terminals contain at least three different classes of myosin proteins: myosins I, II, and V (for review see Mooseker and Cheney, 1995). Myosins Iα and Iβ are both enriched in neuronal growth cones (Wagner et al., 1992; Lewis and Bridgman, 1996). While the intracellular localization of myosin Iβ has remained controversial (Wagner et al., 1992; Ruppert et al., 1993), it has been established that myosin Iα is not associated with membranous organelles (Lewis and Bridgman, 1996). The possibility that myosins IIA or IIB are synaptic vesicle motors now seems doubtful, since neither of these proteins was associated with organelles containing the synaptic vesicle proteins synaptobrevin II or rab3 (Fig. 2 B). However, it must be acknowledged that myosins could move actin-associated organelles in a retrograde direction without binding to the organelle itself. The biochemical properties of brain myosin V are consistent with those of an organelle motor: Ca2+-dependent activation of Mg2+-ATPase activity by very low concentrations of filamentous actin (Nascimento et al., 1996), and the ability to move even large (800 nm) artificial beads along actin filaments (Wolenski et al., 1995) at an average velocity (∼450 nm/s) that is comparable to the vesicle movements previously observed in living nerve terminals (Betz and Henkel, 1994). Moreover, genetic mutations in mouse and yeast have implicated myosins V in membrane trafficking (Johnston et al., 1991; Mercer et al., 1991; Lillie and Brown, 1992; Govindan et al., 1995). This rapidly accumulating body of evidence has inspired several investigators to postulate that class V myosins may be responsible for the actin-based movement of secretory vesicles in both vertebrates and yeast (Johnston et al., 1991; Lillie and Brown, 1992; Cheney et al., 1993a ; Langford, 1995). However, evidence of any physical interaction between a biochemically defined population of organelles and myosin V has remained conspicuously absent. The present study provides the first clear and direct evidence that brain myosin V tightly binds to mature synaptic vesicles in nerve terminals of the mammalian central nervous system.
Synaptic vesicles that contain synaptobrevin and rab3 are tightly bound to a majority of the myosin V in synaptosomes. We have investigated the nature of this interaction and all of our data are consistent with the conclusion that the myosin V tail domain targets this molecular motor to a biochemically defined population of synaptic vesicles. The partnering of this motor protein and organelle seems to involve relatively stable ionic interactions between vesicles and the COOH-terminal M r 80-kD end (Met1141–Val1830) of the myosin V tail. We cannot exclude the possibility that this region of the myosin V tail directly interacts with acidic phospholipids in the outer leaflet of the vesicle membrane. However, coimmunoisolation experiments demonstrated that electrostatic protein–protein interactions are involved in the linkage of myosin V to synaptic vesicles. In cross-linking and coprecipitation experiments, the synaptic vesicle binding partner for myosin V was tentatively identified as the synaptobrevin–synaptophysin complex. Such an organelle-specific targeting signal has not previously been identified for the unconventional myosins. In vitro experiments must be performed to determine whether myosin V binds directly to either of these two vesicle membrane proteins in binary reactions or is specifically targeted to the assembled complex.
The “multiple escort” hypothesis asserts that diverse proteins sequentially interact with synaptobrevin throughout the events leading to synaptic vesicle fusion, retrieval, and recycling (Grote and Kelly, 1996). When bound to synaptophysin, synaptobrevin appears to be unavailable for fusion complex assembly (Edelmann et al., 1995), suggesting that synaptophysin may impose an additional level of constraint in regulated exocytosis. Our findings indicate that a minority of the vesicles that have been detained in the “synaptophysin-bound pool” are complexed with a molecular motor that is capable of moving this cargo along actin filaments in the axonal terminal. We shall refer to the myosin V–synaptobrevin–synaptophysin complex as the “myosin V transport complex,” both as a reference to its putative function and to distinguish it from the other more abundant pools of synaptobrevin-based protein complexes in the synapse. It will now be important to determine how the myosin V transport complex is assembled and whether the formation of this complex is necessary for synaptic vesicle movements. Although the transmembrane domain of synaptobrevin appears to be required for binding to synaptophysin (Edelmann et al., 1995), the domain map for this protein–protein interaction is still incomplete. The NH2 terminus of mammalian synaptobrevins and the proximal portion of the myosin V tail both contain α-helical heptad repeats with relatively high coiled-coil–forming potential, according to the algorithms of Berger et al. (1995) and Lupas (1996). Coiled-coils are formed when two or more right-handed α helices intertwine with each other to produce a left-handed superhelical twist. Analogous with the predicted interactions between synaptobrevins and the t-SNARE proteins, syntaxin and SNAP-25 (Söllner et al., 1993; Chapman et al., 1994; Hayashi et al., 1994), the tail domain of myosin V may also pair with synaptobrevin to form α-helical coiled-coils. Electrostatic interactions between the hydrophilic regions of synaptophysin and myosin V could possibly enhance the stability of these structures. However, the probability that yeast homologs of synaptobrevin form coiled-coils is remote; maximum probabilities for Saccharomyces cervisiae Sec22p, Snc1p, Snc2p, and Bet1p are <10%, according to the Berger et al. (1995) and Lupas (1996) programs. Thus, if eukaryotic cells have conserved a common mechanism for pairing synaptobrevin homologs and t-SNAREs, it may be concluded that the heptad repeats that are characteristic of coiled-coils are not essential for dimerization specificity. Moreover, the phenotypes of dilute and MYO2 mutations (Johnston et al., 1991; Mercer et al., 1991; Lillie and Brown, 1992; Govindan et al., 1995) suggest that class V myosins are capable of performing their function, regardless of whether or not the synaptobrevins can form coiled-coil structures. This observation reinforces the suggestion that the globular end of the myosin V tail (dilute Gly1444–Val1853) might target this motor protein to vesicles (Cheney et al., 1993a ) via the synaptobrevin–synaptophysin complex.
For an organelle motor to operate most efficiently, it must be capable of engaging and disengaging its cargo in an orderly and controlled manner. Activity-dependent cycling of the myosin V transport complex would provide an attractive mechanism for synaptic vesicle movements in the synapse and their controlled release to a cognant docking site. The finding that depolarization uncouples myosin V from synaptic vesicles in the intact synaptosome (Fig. 6) suggests that a mechanism exists for decommissioning the myosin V transport complex, and it provides a fascinating clue concerning how a transport system may operate, by moving vesicles into the site of docking and constraining their interaction with t-SNAREs until the arrival of an action potential. Further in vitro analysis revealed that assembly/disassembly of the myosin V transport complex might be regulated by concentrations of Ca2+ that correspond to the predicted level of Ca2+ present at the sites of exocytotic activity (Llinas et al., 1992). If action potentials initiate vesicle fusion with the plasma membrane and complete disassembly of the myosin V transport complex, it would provide an efficient molecular mechanism for refilling empty docking sites in a use-dependent manner. Because of the high concentration of Mg2+ in the cytoplasm, localized Ca2+ entry may provide a signal that is sufficient to unhitch the vesicle cargo from myosin V, while simultaneously enabling synaptobrevin and synaptotagmin (Chapman et al., 1995) to interact with the syntaxin–SNAP-25 complex. While the molecular nature of this dissociation signal remains unknown, the present results indicate that disassembly of the myosin V transport complex does not require hydrolysis by a Ca2+-dependent Mg2+-ATPase.
During development, myosin V is an essential component of the neuronal growth cone motility system (Wang et al., 1996). In the mature synapse, myosin V may function as an actin-dependent motor that enables vesicles to keep an active itinerary of travel. The notion that myosin V might be a component of the synaptic vesicle transport system is strongly supported by a combination of biochemical, structural, mechanochemical, and genetic evidence (for review see Mooseker and Cheney, 1995). Of central importance to this working hypothesis is the prediction that myosin V remains attached to both its track and cargo during the moment that mechanochemical energy is generated to produce vectorial movement (Langford, 1995). Knowing that myosin V moves along actin filaments in vivo (Evans and Bridgman, 1995), we focused intense effort on obtaining an estimate of the probability that this molecular motor remains associated with actin filaments throughout its duty cycle (i.e., Mg2+-ATPase cycle). In vitro assays indicate that equivalent concentrations of Ca2+ (1–10 μM) stimulate myosin V actin binding, Mg2+-ATPase activity (Nascimento et al., 1996), and vesicle dissociation (Fig. 7 C). If accurate, these measurements would predict that the myosin V transport complex may undergo disassembly during the force-generating phase of the Mg2+-ATPase cycle. However, the functional relationship between myosin V–dependent Mg2+-ATPase activity and the in vitro motility of this protein continues to be debated (Cheney et al., 1993a ; Wolenski et al., 1995). The studies described here will make it possible to address the basic question of whether myosin V remains associated with synaptic vesicles during its duty cycle by providing a molecular description of the protein–protein interactions that may tether myosin V to a biochemically defined population of organelles.
The major finding of the present study is that the tail domain of myosin V interacts with synaptic vesicle membrane proteins in a use-dependent manner. According to our working hypothesis, myosin V may bind to synaptic vesicles that are no longer clustered together via synapsin I–actin interactions. The mobilization of these vesicles is thought to involve the phosphorylation of synapsin I by Ca2+/calmodulin-dependent protein kinase II (Greengard et al., 1993). Determinants of myosin V motor activity in the synapse are unknown but are likely to include factors that control the actin-activated Mg2+-ATPase activity of this molecular motor. After the movement of a synaptic vesicle to the presynaptic membrane, at least two separate mechanisms appear to inhibit the assembly of a 7S prefusion complex. As previously discussed (Calakos and Scheller, 1996), syntaxin does not seem to be available to interact with synaptobrevin while this t-SNARE is bound to the cytosolic protein munc-18. A reciprocal inhibition may be produced by the interaction of synaptophysin and myosin V with synaptobrevin (Edelmann et al., 1995; this study). Disassembly of the myosin V transport complex is associated with depolarization-induced, localized Ca2+ entry and may be a necessary prerequisite for the docking of myosin V–bound synaptic vesicles.
Acknowledgments
We thank C.M. Manring and G.G. Wescott for their expert technical assistance and the other members of our lab for helpful discussions during the course of these studies.
This work was supported by the National Institute of Environmental Health Sciences and the National Science Foundation.
Abbreviations used in this paper
- ATPγS
adenosine 5′-O′[3-thiotriphosphate]
- BDM
2,3-butanedione-2-monoxime
- DiSC2(5)
3,3′-diethylthiadicarbocyanine iodide
- DTSP
dithiobis(succinimidyl propionate)
- LS1
synaptosomal lysate supernatant
- syb
synaptobrevin
- syp
synaptophysin
- syt
synaptotagmin
- t-SNARE
target membrane SNAP receptor
Footnotes
Please address all correspondence to David M. Terrian, Department of Anatomy and Cell Biology, East Carolina University School of Medicine, Greenville, NC 27858-4354. Tel.: (919) 816-3247. Fax: (919) 816-2850.
References
- Amar-Costesec A, Wibo M, Thines-Sempoux D, Beaufay H, Berthet J. Analytical study of microsomes and isolated subcellular membranes from rat liver. J Cell Biol. 1974;62:717–745. doi: 10.1083/jcb.62.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bearer EL, DeGiorgis JA, Bodner RA, Kao AW, Reese TS. Evidence for myosin motors on organelles in squid axoplasm. Proc Natl Acad Sci USA. 1993;90:11252–11256. doi: 10.1073/pnas.90.23.11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett MK, Calakos N, Kreiner T, Scheller RH. Synaptic vesicle membrane proteins interact to form a multimeric complex. J Cell Biol. 1992;116:761–775. doi: 10.1083/jcb.116.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger B, Wilson DB, Wolf E, Tonchev T, Milla M, Kim PS. Predicting coiled coils by use of pairwise residue correlations. Proc Natl Acad Sci USA. 1995;92:8259–8263. doi: 10.1073/pnas.92.18.8259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein BW, Bamburg JR. Cycling of actin assembly in synaptosomes and neurotransmitter release. Neuron. 1989;3:257–265. doi: 10.1016/0896-6273(89)90039-1. [DOI] [PubMed] [Google Scholar]
- Betz WJ, Henkel AW. Okadaic acid disrupts clusters of synaptic vesicles in frog motor nerve terminals. J Cell Biol. 1994;124:843–854. doi: 10.1083/jcb.124.5.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981;256:1604–1607. [PubMed] [Google Scholar]
- Calakos N, Scheller RH. Vesicle-associated membrane protein and synaptophysin are associated on the synaptic vesicle. J Biol Chem. 1994;269:24534–24537. [PubMed] [Google Scholar]
- Calakos N, Scheller RH. Synaptic vesicle biogenesis, docking, and fusion: a molecular description. Physiol Rev. 1996;76:1–29. doi: 10.1152/physrev.1996.76.1.1. [DOI] [PubMed] [Google Scholar]
- Chapman ER, An S, Barton N, Jahn R. SNAP-25, a tSNARE which binds to both syntaxin and synaptobrevin via domains that may form coiled coils. J Biol Chem. 1994;269:27427–27432. [PubMed] [Google Scholar]
- Chapman ER, Hanson PI, An S, Jahn R. Ca2+regulates the interaction between synaptotagmin and syntaxin 1. J Biol Chem. 1995;270:23667–23671. doi: 10.1074/jbc.270.40.23667. [DOI] [PubMed] [Google Scholar]
- Cheney RE, O'Shea MK, Heuser JE, Coelho MV, Wolenski JS, Espreafico EM, Forscher P, Larson RE, Mooseker MS. Brain myosin-V is a two-headed unconventional myosin with motor activity. Cell. 1993a;75:13–23. doi: 10.1016/S0092-8674(05)80080-7. [DOI] [PubMed] [Google Scholar]
- Cheney RE, Riley MA, Mooseker MS. Phylogenic analysis of the myosin superfamily. Cell Motil Cytoskeleton. 1993b;24:215–223. doi: 10.1002/cm.970240402. [DOI] [PubMed] [Google Scholar]
- Cheng TPO, Murakami N, Elzinga M. Localization of myosin IIB at the leading edge of growth cones from rat dorsal root ganglionic cells. FEBS Lett. 1992;311:91–94. doi: 10.1016/0014-5793(92)81374-u. [DOI] [PubMed] [Google Scholar]
- Cramer LP, Mitchison TJ. Myosin is involved in postmitotic cell spreading. J Cell Biol. 1995;131:179–189. doi: 10.1083/jcb.131.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelmann L, Hanson PI, Chapman ER, Jahn R. Synaptobrevin binding to synaptophysin: a potential mechanism for controlling the exocytotic fusion machine. EMBO (Eur Mol Biol Organ) J. 1995;14:224–231. doi: 10.1002/j.1460-2075.1995.tb06995.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espindola FS, Espreafico EM, Coelho MV, Martins AR, Costa FRC, Mooseker MS, Larson RE. Biochemical and immunological characterization of p190–calmodulin complex from vertebrate brain: a novel calmodulin-binding myosin. J Cell Biol. 1992;118:359–368. doi: 10.1083/jcb.118.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espreafico EM, Cheney RE, Matteoli M, Nascimento AAC, De Camilli PV, Larson RE, Mooseker MS. Primary structure and cellular localization of chicken brain myosin-V (p190), an unconventional myosin with calmodulin light chains. J Cell Biol. 1992;119:1541–1557. doi: 10.1083/jcb.119.6.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans LL, Bridgman PC. Particles move along actin filament bundles in nerve growth cones. Proc Natl Acad Sci USA. 1995;92:10954–10958. doi: 10.1073/pnas.92.24.10954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferro-Novick S, Jahn R. Vesicle fusion from yeast to man. Nature (Lond) 1994;370:191–193. doi: 10.1038/370191a0. [DOI] [PubMed] [Google Scholar]
- Ferro-Novick S, Novick P. The role of GTP-binding protein in transport along the exocytotic pathway. Annu Rev Cell Biol. 1993;9:575–599. doi: 10.1146/annurev.cb.09.110193.003043. [DOI] [PubMed] [Google Scholar]
- Gannon RL, Terrian DM. U-50,488H inhibits dynorphin and glutamate release from guinea pig hippocampal mossy fiber terminals. Brain Res. 1991;548:242–247. doi: 10.1016/0006-8993(91)91127-m. [DOI] [PubMed] [Google Scholar]
- Govindan B, Bowser R, Novick P. The role of Myo2, a yeast class V myosin, in vesicular transport. J Cell Biol. 1995;128:1055–1068. doi: 10.1083/jcb.128.6.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greengard P, Valtorta F, Czernik AJ, Benfenati F. Synaptic vesicle phosphoproteins and regulation of synaptic function. Science (Wash DC) 1993;259:780–785. doi: 10.1126/science.8430330. [DOI] [PubMed] [Google Scholar]
- Grote E, Kelly RB. Endocytosis of VAMP is facilitated by a synaptic vesicle targeting signal. J Cell Biol. 1996;132:537–547. doi: 10.1083/jcb.132.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, McMahon H, Yamasaki S, Binz T, Hata Y, Südhof TC, Niemann H. Synaptic vesicle membrane fusion complex: action of clostridial neurotoxins on assembly. EMBO (Eur Mol Biol Organ) J. 1994;13:5051–5061. doi: 10.1002/j.1460-2075.1994.tb06834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkel AW, Simpson LL, Ridge RMAP, Betz WJ. Synaptic vesicle movements monitored by fluorescence recovery after photobleaching in nerve terminals stained with FM1-43. J Neurosci. 1996;16:3960–3967. doi: 10.1523/JNEUROSCI.16-12-03960.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann C, Wray J, Travers F, Barman T. Effect of 2,3-butanedione monoxime on myosin and myofibrillar ATPases. An example of an uncompetitive inhibitor. Biochemistry. 1992;31:12227–12232. doi: 10.1021/bi00163a036. [DOI] [PubMed] [Google Scholar]
- Hirokawa N, Sobue K, Kanda K, Harada A, Yorifuji H. The cytoskeletal architecture of the presynaptic terminal and molecular structure of synapsin 1. J Cell Biol. 1989;108:111–126. doi: 10.1083/jcb.108.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttner WB, Schiebler W, Greengard P, De Camilli P. Synapsin I (protein I), a nerve terminal-specific phosphoprotein. III. Its association with synaptic vesicles studied in a highly purified synaptic vesicle preparation. J Cell Biol. 1983;96:1374–1388. doi: 10.1083/jcb.96.5.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston GC, Prendergast JA, Singer RA. The Saccharomyces cerevisiae MYO2gene encodes an essential myosin for vectorial transport of vesicles. J Cell Biol. 1991;113:539–551. doi: 10.1083/jcb.113.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly RB. Secretory granule and synaptic vesicle formation. Curr Opin Cell Biol. 1991;3:654–660. doi: 10.1016/0955-0674(91)90037-y. [DOI] [PubMed] [Google Scholar]
- Koenig JH, Yamaoka K, Ikeda K. Calcium-induced translocation of synaptic vesicles to the active site. J Neurosci. 1993;13:2313–2322. doi: 10.1523/JNEUROSCI.13-06-02313.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznetsov SA, Langford GM, Weiss DG. Actin-dependent organelle movement in squid axoplasm. Nature (Lond) 1992;356:722–725. doi: 10.1038/356722a0. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (Lond) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Landis DMD, Hall AK, Weinstein LA, Reese TS. The organization of cytoplasm at the presynaptic active zone of a central nervous system synapse. Neuron. 1988;1:201–209. doi: 10.1016/0896-6273(88)90140-7. [DOI] [PubMed] [Google Scholar]
- Langford GM. Actin- and microtubule-dependent organelle motors: interrelationships between the two motility systems. Curr Opin Cell Biol. 1995;7:82–88. doi: 10.1016/0955-0674(95)80048-4. [DOI] [PubMed] [Google Scholar]
- Lewis AK, Bridgman PC. Mammalian myosin Iα is concentrated near the plasma membrane in nerve growth cones. Cell Motil Cytoskeleton. 1996;33:130–150. doi: 10.1002/(SICI)1097-0169(1996)33:2<130::AID-CM5>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Lillie SH, Brown SS. Suppression of a myosin defect by a kinesin-related gene. Nature (Lond) 1992;356:358–361. doi: 10.1038/356358a0. [DOI] [PubMed] [Google Scholar]
- Lin CH, Espreafico EM, Mooseker MS, Forscher P. Myosin drives retrograde F-actin flow in neuronal growth cones. Neuron. 1996;16:769–782. doi: 10.1016/s0896-6273(00)80097-5. [DOI] [PubMed] [Google Scholar]
- Llinas R, Sugimori M, Lin J-W, Leopold PL, Brady ST. ATP-dependent directional movement of rat synaptic vesicles injected into the presynaptic terminal of squid giant synapse. Proc Natl Acad Sci USA. 1989;86:5656–5660. doi: 10.1073/pnas.86.14.5656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinas R, Sugimori M, Silver RB. Microdomains of high calcium concentration in presynaptic terminal. Science (Wash DC) 1992;256:677–679. doi: 10.1126/science.1350109. [DOI] [PubMed] [Google Scholar]
- Lupas A. Coiled coils: new structures and new functions. Trends Biochem Sci. 1996;21:375–382. [PubMed] [Google Scholar]
- McMahon HT, Nicholls DG. Transmitter glutamate release from isolated nerve terminals: evidence for biphasic release and triggering by localized Ca2+ . J Neurochem. 1991;56:86–94. doi: 10.1111/j.1471-4159.1991.tb02566.x. [DOI] [PubMed] [Google Scholar]
- Mercer JA, Seperack PK, Strobel MC, Copelend NG, Jenkins NA. Novel myosin heavy chain encoded by murine dilute coat colour locus. Nature (Lond) 1991;349:709–713. doi: 10.1038/349709a0. [DOI] [PubMed] [Google Scholar]
- Miller M, Bower E, Levitt P, Li D, Chantler PD. Myosin II distribution in neurons is consistent with a role in growth cone motility but not synaptic vesicle mobilization. Neuron. 1992;8:25–44. doi: 10.1016/0896-6273(92)90106-n. [DOI] [PubMed] [Google Scholar]
- Mochida S, Kobayashi H, Matsuda Y, Yuda Y, Muramoto K, Nonomura Y. Myosin II is involved in transmitter release at synapses formed between rat sympathetic neurons in culture. Neuron. 1994;13:1131–1142. doi: 10.1016/0896-6273(94)90051-5. [DOI] [PubMed] [Google Scholar]
- Mooseker MS, Cheney RE. Unconventional myosins. Annu Rev Cell Dev Biol. 1995;11:633–675. doi: 10.1146/annurev.cb.11.110195.003221. [DOI] [PubMed] [Google Scholar]
- Murakami N, Singh SS, Chauhan VPS, Elzinga M. Phospholipid binding, phosphorylation by protein kinase C, and filament assembly of the COOH terminal heavy chain fragments of nonmuscle myosin II isoforms MIIA and MIIB. Biochemistry. 1995;34:16046–16055. doi: 10.1021/bi00049a019. [DOI] [PubMed] [Google Scholar]
- Nascimento AAC, Cheney RE, Tauhata SBF, Larson RE, Mooseker MS. Enzymatic characterization and functional domain mapping of brain myosin-V. J Biol Chem. 1996;271:17561–17569. doi: 10.1074/jbc.271.29.17561. [DOI] [PubMed] [Google Scholar]
- Okada Y, Yamazaki H, Sekine-Aizawa Y, Hirokawa N. The neuron-specific kinesin superfamily protein KIF1A is a unique monomeric motor for anterograde axonal transport of synaptic vesicle precursors. Cell. 1995;81:769–780. doi: 10.1016/0092-8674(95)90538-3. [DOI] [PubMed] [Google Scholar]
- Prekeris R, Mayhew MW, Cooper JB, Terrian DM. Identification and localization of an actin-binding motif that is unique to the epsilon isoform of protein kinase C and participates in the regulation of synaptic function. J Cell Biol. 1996;132:77–90. doi: 10.1083/jcb.132.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhard J, Scheel AA, Diekmann D, Hall A, Ruppert C, Bahler M. A novel type of myosin implicated in signaling by rho family GTPases. EMBO (Eur Mol Biol Organ) J. 1995;14:697–704. doi: 10.1002/j.1460-2075.1995.tb07048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenmund C, Stevens CF. Definition of the readily releasable pool of vesicles at hippocampal synapses. Neuron. 1996;16:1197–1207. doi: 10.1016/s0896-6273(00)80146-4. [DOI] [PubMed] [Google Scholar]
- Rothman JE, Wieland FT. Protein sorting by transport vesicles. Science (Wash DC) 1996;272:227–234. doi: 10.1126/science.272.5259.227. [DOI] [PubMed] [Google Scholar]
- Ruppert C, Kroschewski R, Bahler M. Identification, characterization, and cloning of myr 1, a mammalian myosin-I. J Cell Biol. 1993;120:1393–1403. doi: 10.1083/jcb.120.6.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan TA, Smith SJ. Vesicle pool mobilization during action potential firing at hippocampal synapses. Neuron. 1995;14:983–989. doi: 10.1016/0896-6273(95)90336-4. [DOI] [PubMed] [Google Scholar]
- Ryan TA, Reuter H, Wendland B, Schweizer FE, Tsien RW, Smith SJ. The kinetics of synaptic vesicle recycling measured at single presynaptic boutons. Neuron. 1993;11:713–724. doi: 10.1016/0896-6273(93)90081-2. [DOI] [PubMed] [Google Scholar]
- Söllner T, Bennett MK, Whiteheart SW, Scheller RH, Rothman JE. A protein assembly–disassembly pathway in vitro that may correspond to sequential steps of synaptic vesicle docking, activation, and fusion. Cell. 1993;75:409–418. doi: 10.1016/0092-8674(93)90376-2. [DOI] [PubMed] [Google Scholar]
- Stahl B, Chou JH, Li C, Südhof TC, Jahn R. Rab3 reversibly recruits rabphilin to synaptic vesicles by a mechanism analogous to raf recruitment by ras. EMBO (Eur Mol Biol Organ) J. 1996;15:1799–1809. [PMC free article] [PubMed] [Google Scholar]
- Stevens CF, Tsujimoto T. Estimates for the pool size of releasable quanta at a single central synapse and for the time required to refill the pool. Proc Natl Acad Sci USA. 1995;92:846–849. doi: 10.1073/pnas.92.3.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Südhof TC. The synaptic vesicle cycle: a cascade of protein-protein interactions. Nature (Lond) 1995;375:645–653. doi: 10.1038/375645a0. [DOI] [PubMed] [Google Scholar]
- Takei K, Mundigl O, Daniell L, De Camilli P. The synaptic vesicle cycle: a single vesicle budding step involving clathrin and dynamin. J Cell Biol. 1996;133:1237–1250. doi: 10.1083/jcb.133.6.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrian DM, Ways DK. Persistent enhancement of sustained calcium-dependent glutamate release by phorbol esters: role of calmodulin- independent serine/threonine phosphorylation and actin disassembly. J Neurochem. 1995;64:181–190. doi: 10.1046/j.1471-4159.1995.64010181.x. [DOI] [PubMed] [Google Scholar]
- Terrian DM, Johnston D, Claiborne BJ, Ansah-Yiadom R, Strittmatter WJ, Rea MA. Glutamate and dynorphin release from a subcellular fraction enriched in hippocampal mossy fiber synaptosomes. Brain Res Bull. 1988;21:343–351. doi: 10.1016/0361-9230(88)90146-3. [DOI] [PubMed] [Google Scholar]
- Verkhovsky AB, Svitkina TM, Borisy GG. Myosin II filament assemblies in the active lamella of fibroblasts: their morphogenesis and role in the formation of actin filament bundles. J Cell Biol. 1995;131:989–1002. doi: 10.1083/jcb.131.4.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner MC, Barylko B, Albanesi JP. Tissue distribution and subcellular localization of mammalian myosin I. J Cell Biol. 1992;119:163–170. doi: 10.1083/jcb.119.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walch-Solimena C, Blasi J, Edelmann L, Chapman ER, von Mollard GF, Jahn R. The t-SNAREs syntaxin 1 and SNAP-25 are present on organelles that participate in synaptic vesicle recycling. J Cell Biol. 1995;128:637–645. doi: 10.1083/jcb.128.4.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F-S, Wolenski JS, Cheney RE, Mooseker MS, Jay DG. Function of myosin-V in filopodial extension of neuronal growth cones. Science (Wash DC) 1996;273:660–663. doi: 10.1126/science.273.5275.660. [DOI] [PubMed] [Google Scholar]
- Wolenski JS, Cheney RE, Mooseker MS, Forscher P. In vitro motility of immunoadsorbed brain myosin-V using a Limulusacrosomal process and optical tweezer-based assay. J Cell Sci. 1995;108:1489–1496. doi: 10.1242/jcs.108.4.1489. [DOI] [PubMed] [Google Scholar]
- Zhao L-P, Koslovsky JS, Reinhard J, Bahler M, Witt AE, Provance DW, Jr, Mercer JA. Cloning and characterization of myr 6, an unconventional myosin of the dilute/myosin-V family. Proc Natl Acad Sci USA. 1996;93:10826–10831. doi: 10.1073/pnas.93.20.10826. [DOI] [PMC free article] [PubMed] [Google Scholar]