Abstract
The Polo-like kinase, Plk, has multiple roles in regulating mitosis. In particular, Plk1 has been postulated to function as a trigger kinase that phosphorylates and activates Cdc25C prior to the activation of cyclin B–Cdc2 and thereby initiates its activation. However, the upstream regulation of Plk1 activation remains unclear. Here we have studied the interplay between Plk1 and Cdc2 through meiotic and early embryonic cycles in starfish. Distinct kinases, cyclin B–Cdc2, MAPK along with cyclin B– and/or cyclin A–Cdc2 and cyclin A–Cdc2, were unique upstream regulators for Plk1 activation at meiosis I, meiosis II and embryonic M-phase, respectively, indicating that Plk1 is not the trigger kinase at meiotic reinitiation. When Plk1 was required for cyclin B–Cdc2 activation, the action of Plk1 was mediated primarily through suppression of Myt1 rather than through activation of Cdc25. We propose that Plk1 can be activated by either cyclin A– or cyclin B–Cdc2, and its primary target is Myt1.
Keywords: cell cycle/cyclin B–Cdc2/Myt1/oocyte/Plk1
Introduction
In eukaryotic cells, maturation- or M-phase promoting factor (MPF) controls both entry into and exit from M-phase (reviewed in Nigg, 2001). The major component of MPF is an active complex of the catalytic Cdc2 with the regulatory cyclin B. The activity of cyclin B–Cdc2 kinase is regulated not only by the formation of the complex but also by phosphorylation/dephosphorylation of the Cdc2 subunit (reviewed in Coleman and Dunphy, 1994; Lew and Kornbluth, 1996; O’Farrell, 2001). Cyclin B-associated Cdc2 undergoes inhibitory phosphorylation at Thr14 and Tyr15 by the Wee1 family of protein kinases (Wee1, Mik1 and Myt1) during interphase, and at entry into M-phase, the inhibitory phosphates are removed by the activating phosphatase Cdc25C. The initial activation of cyclin B–Cdc2 appears to be triggered by a reversal of the balance between the opposing Wee1 family kinases and Cdc25C phosphatase. Logically, upon entry into M-phase, inactivation of the Wee1 family and/or activation of Cdc25C should occur prior to the initial activation of cyclin B–Cdc2, though thereafter the active cyclin B–Cdc2 can itself phosphorylate both Wee1 and Cdc25C to create positive feedback loops.
Both Wee1 family kinases and Cdc25C can be phosphorylated in vivo in the absence of cyclin B–Cdc2 kinase activity (Izumi and Maller, 1995; Mueller et al., 1995; Okumura et al., 1996; Okumura et al., 2002). Accordingly, a trigger kinase(s) has been postulated to be responsible for inactivation of the Wee1 family and/or activation of Cdc25C prior to cyclin B–Cdc2 activation. In vitro, the kinases p90rsk and calcium/calmodulin-dependent protein kinase (CaM kinase II) can phosphorylate and regulate either Myt1 or Cdc25C (Palmer et al., 1998; Patel et al., 1999), but it remains unclear whether they function as trigger kinases in vivo. Plx1, the Xenopus homolog of the Polo-like kinase 1 (Plk1), has also been identified as a kinase that can phosphorylate and activate Cdc25C in vitro (Kumagai and Dunphy, 1996). Plk1 is conserved among various organisms and is implicated in multiple stages of M-phase progression (reviewed in Glover et al., 1998; Nigg, 1998, 2001). Plk1 has been proposed as a candidate for the trigger kinase that initiates the activation of cyclin B–Cdc2 via activation of Cdc25C (see Lane and Nigg, 1997), but other studies have shown that Plk1 is a component of the positive feedback loop rather than an initial activator of cyclin B–Cdc2 (Abrieu et al., 1998; Karaiskou et al., 1998; Qian et al., 1998a). Thus, further clarification is needed as to whether Plk1 is the actual trigger kinase.
Plk1 protein levels peak at M-phase in the somatic cell cycle, while they remain constant through meiotic and early embryonic cycles (Golsteyn et al., 1995; Hamanaka et al., 1995; Qian et al., 1998a; Pahlavan et al., 2000). In both cases, Plk1 is phosphorylated during the G2/M-phase transition and shows high activity during M-phase. xPlkk1 and Ste20-like kinase have been identified as direct activators of Plk1 in Xenopus oocytes (Qian et al., 1998b) and somatic cells (Ellinger-Ziegelbauer et al., 2000), respectively. In addition, involvement of other kinases, including cyclin B–Cdc2, has been suggested for Plk1 activation (Kelm et al., 2002). If Plk1 functions as the trigger kinase, molecule(s) other than cyclin B–Cdc2 should cause the initial activation of Plk1. Thus, the upstream regulator(s) of Plk1 remains unclear.
Most recently we have demonstrated that the kinase Akt/PKB, a transducer of multiple cellular signals (reviewed in Brazil and Hemmings, 2001), functions as the trigger kinase through direct phosphorylation and inhibition of Myt1 at the meiotic G2/M-phase transition in starfish oocytes (Okumura et al., 2002). This has prompted us to reconsider the putative role of Plk1 as the trigger kinase, and to search for upstream regulators of Plk1, if it functions as the trigger kinase.
Fully grown immature oocytes of the starfish are arrested at prophase of meiosis I and already contain inactive cyclin B–Cdc2 (Okano-Uchida et al., 1998; reviewed in Kishimoto, 1998). The release from the prophase arrest is equivalent to the G2/M-phase transition and is induced by an extracellular hormonal stimulus with no requirement for new protein synthesis. Once meiosis is reinitiated, starfish oocytes proceed completely through meiosis I and II, and in the absence of fertilization, arrest in interphase as haploid eggs. Upon fertilization, these eggs undergo embryonic mitotic cycles. In the present study, using a specific antibody which prevents the activation of Plk1 in starfish oocytes and eggs, we have examined the involvement of Plk1 in the activation of cyclin B–Cdc2 during meiotic and early cleavage cycles. Our results show that distinct kinases, cyclin B–Cdc2, MAP kinase (MAPK) along with Cdc2 (either with cyclin A or cyclin B) and cyclin A–Cdc2, are upstream regulators for Plk1 activation at meiosis I, meiosis II and early embryonic M-phase, respectively, indicating that Plk1 does not function as a trigger kinase at meiosis I. We have also identified Myt1, rather than Cdc25, as the mediator of Plk1-dependent activation of cyclin B–Cdc2.
Results
Isolation of starfish Plk1 and Wee1 cDNAs, and characterization of antibodies
To examine whether and how Plk1 is involved in cyclin B–Cdc2 activation in starfish meiotic and cleavage cycles, we isolated cDNAs of the starfish homologs of Plk1 and Wee1 (DDBJ/EMBL/GenBank accession no. AB084465 for Plk1, and no. AB064523 for Wee1). These encoded 623 amino acids with the predicted molecular weight of 71.0 kDa for Plk1, and 623 amino acids with 69.9 kDa for Wee1 (Supplementary figure 1A and B, available at The EMBO Journal Online). Starfish Plk1 contained the characteristic polo-box (amino acids 414–443) and the amino acid sequence was 51% identical to that of mammalian Plk1s. Starfish Wee1 was 38% identical to sea urchin Wee1 and 28% identical to vertebrate Wee1s.
An affinity-purified anti-Plk1 antibody recognized a single band of 67 kDa in lysates of immature starfish oocytes (Figure 1, lanes 1 and 3). A slightly slower-migrating band was observed in maturing oocytes at metaphase of meiosis I (Figure 1, lane 2), suggesting phosphorylation at M-phase. An affinity-purified anti-Wee1 antibody specifically recognized starfish Wee1. Wee1 protein was undetectable in immature oocytes and during meiosis I (Figure 1, lanes 4 and 5; see Figures 8B and 9C) as in Xenopus oocytes (Murakami and Vande Woude, 1998; Iwabuchi et al., 2000). The levels of Wee1 protein gradually increased after meiosis I and remained almost constant through cleavage cycles. Wee1 underwent maximal phosphorylation at each M-phase of cleavage cycles (Figure 1, lanes 6 and 7; see Figures 8B and 9C).
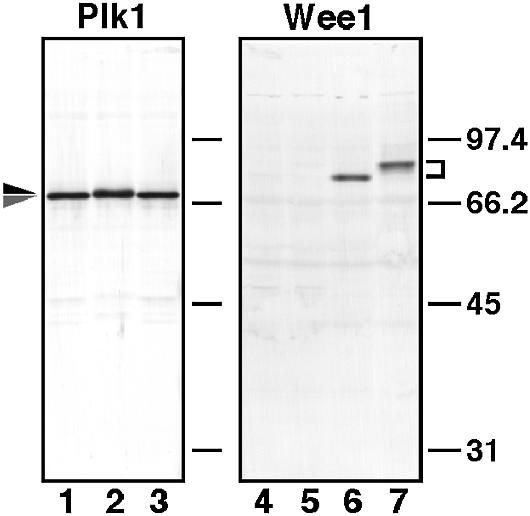
Fig. 1. Specificity of antibodies against starfish Plk1 and Wee1. Lysates from immature oocytes (lanes 1, 3 and 4), oocytes at metaphase of meiosis I (lanes 2 and 5), unfertilized mature eggs (lane 6) and eggs at metaphase of the first cleavage cycle (lane 7) were separated on a 10% SDS–PAGE gel and blotted onto nitrocellulose. Each blot was probed with affinity-purified anti-Plk1 antibody (lanes 1–3) and anti-Wee1 antibody (lanes 4–7). Molecular weight markers are indicated in kDa.
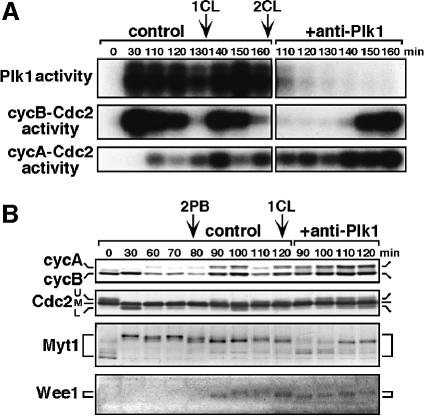
Fig. 8. Prevention of Plk1 reactivation after meiosis II reverses hyperphosphorylation of Myt1 and suppresses cyclin B–Cdc2 activation at entry into M-phase of the first cleavage cycle. Maturing oocytes were fertilized at meiosis I, and then uninjected (control) or injected with anti-Plk1 antibody (+anti-Plk1) at the beginning of the second polar body emission (80 min). (A) Extracts were prepared at the indicated times from groups of five oocytes and eggs, and assayed for Plk1, cyclin B–Cdc2 and cyclin A–Cdc2 kinase activities. (B) Groups of 17 oocytes and eggs were sampled at the indicated times and analyzed by immunoblotting with anti-cyclin A, anti-cyclin B, anti-Cdc2, anti-Myt1 and anti-Wee1 antibodies.
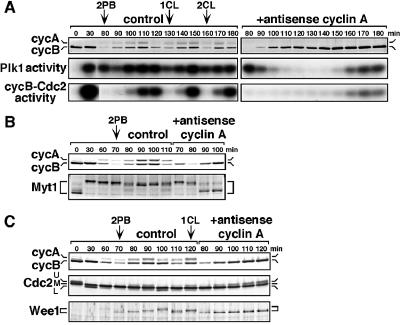
Fig. 9. Inhibition of cyclin A synthesis prevents Plk1 activation, Wee1 phosphorylation and cyclin B–Cdc2 activation at entry into M-phase of the first cleavage cycle. Immature oocytes were uninjected (control) or injected with cyclin A antisense oligonucleotide (+antisense cyclin A), treated with 1-MeAde, and then fertilized at the end of meiosis I. (A) At indicated times, extracts were prepared from groups of eight oocytes and eggs, and immunoblotted with anti-cyclin A and anti-cyclin B antibodies (upper), or assayed for Plk1 (middle) and cyclin B–Cdc2 (lower) kinase activities. (B) Groups of 25 oocytes and eggs were sampled at the indicated times and analyzed by immunoblotting with anti-cyclin A, anti-cyclin B, and anti-Myt1 antibodies. (C) Groups of 16 oocytes and eggs were sampled at the indicated times and analyzed by immunoblotting with anti-cyclin A, anti-cyclin B, anti-Cdc2 and anti-Wee1 antibodies.
Dynamics of Plk1 during meiotic and early cleavage cycles
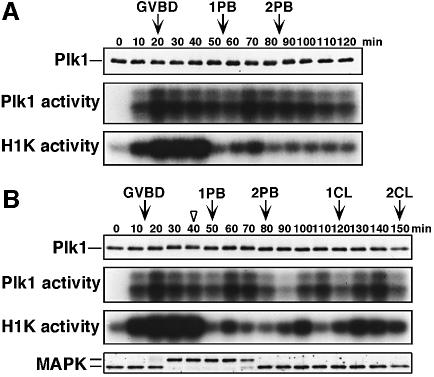
Plk1 protein was present in immature oocytes, and its protein levels remained constant throughout starfish meiotic and cleavage cycles, independent of new protein synthesis (Figure 2, upper and data not shown). Plk1 activity measured in anti-Plk1 immunoprecipitates was very low in immature oocytes and increased before germinal vesicle breakdown (GVBD), almost simultaneously with the activation of cyclin B–Cdc2 but earlier than that of MAPK. Plk1 activity was maintained at significantly elevated levels until and after the completion of meiosis II unless fertilization occurred, though it slightly decreased after GVBD and fluctuated along with the meiotic cycles (Figure 2). When maturing starfish oocytes were inseminated at meiosis I, and proceeded into cleavage cycles after the completion of meiosis II, Plk1 activity was almost lost following the inactivation of MAPK and H1 kinase at the completion of meiosis II, and thereafter fluctuated along with cleavage cycles peaking at each M-phase but with a lag behind H1 kinase activity (Figure 2B).
Fig. 2. Dynamics of Plk1 protein levels and kinase activity during starfish meiotic and cleavage cycles. Extracts were prepared from unfertilized (A) or fertilized (B) oocytes and eggs at 10 min intervals after 1-MeAde addition, and immunoblotted with anti-Plk1 (upper) and anti-MAPK (B, lowest) antibodies. Immunoprecipitates with the anti-Plk1 antibody were assayed for phosphorylation of α-casein (middle). Extracts were also assayed for phosphorylation of histone H1 (lower). Arrows indicate the time of GVBD, the first (1PB) and second (2PB) polar body emission, and the first (1CL) and second (2CL) cleavage. The arrowhead, the time of insemination.
Plk1 activation depends on cyclin B–Cdc2 activation at reinitiation of meiosis I
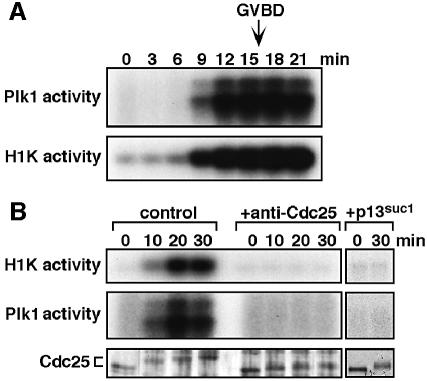
Precise monitoring of the kinetics after hormone addition revealed that the activation of cyclin B–Cdc2 slightly preceded that of Plk1 at meiotic reinitiation (Figure 3A, compare at 6 and 9 min). To examine whether the activation of cyclin B–Cdc2 is required for that of Plk1, we used an anti-starfish Cdc25 antibody, which neutralizes Cdc25 phosphatase activity and hence suppresses cyclin B–Cdc2 activation in vivo (Okumura et al., 1996). Injection of the anti–Cdc25 antibody into immature oocytes completely suppressed the activation of not only cyclin B–Cdc2 but also Plk1 after hormonal stimulus (Figure 3B, +anti-Cdc25). Further, the initial phosphorylation of Cdc25 occurred in the absence of activation both of cyclin B–Cdc2 and Plk1 (Figure 3B, lower). Similar inhibition of Plk1 activation along with the initial phosphorylation of Cdc25 was observed when p13suc1 was used to suppress cyclin B–Cdc2 activation (Figure 3B, +p13suc1). Thus, at reinitiation of meiosis I, Plk1 activation depends on cyclin B–Cdc2 activation, excluding the possibility that Plk1 triggers the activation of Cdc25 prior to cyclin B–Cdc2 activation.
Fig. 3. Suppression of H1 kinase activation prevents Plk1 activation at reinitiation of meiosis I. (A) Extracts were prepared at 3 min intervals after 1-MeAde addition, and Plk1 (upper) and histone H1 kinase (lower) activities were assayed as in Figure 2. (B) Immature oocytes were uninjected (control) or injected with either neutralizing anti-Cdc25 antibody (+anti-Cdc25) or p13suc1 protein (+p13suc1), and then treated with 1-MeAde. Extracts were prepared from groups of 10 oocytes at 10 min intervals, and histone H1 kinase (upper) and Plk1 (middle) activities were determined. Phosphorylation states of Cdc25 were analyzed by immunoblots with the anti-Cdc25 antibody (lower).
Plk1 activity is required neither for reinitiation of nor for exit from meiosis I, but is required for the formation of a bipolar metaphase I spindle
To confirm that Plk1 is not the trigger kinase for cyclin B–Cdc2 activation at meiotic reinitiation, we examined the effect of prevention of Plk1 activation. While Plk1 activity was detectable in anti-Plk1 immunoprecipitates from oocytes undergoing GVBD (see above), it was almost undetectable in those from oocytes undergoing GVBD into which the same anti-Plk1 antibody had been injected prior to hormone addition (Figure 4A), indicating that the anti-Plk1 antibody prevented the activation of Plk1 in vivo. In oocytes lacking Plk1 activation, full activation of cyclin B–Cdc2 and GVBD both occurred normally with only a slight delay (Figure 4B and C) and Cdc25 was fully phosphorylated in metaphase I (see Figure 5C, 45 min). Thus, at reinitiation of meiosis I, Plk1 is neither required for initial activation of cyclin B–Cdc2, nor essential for full activation of both Cdc25 and cyclin B–Cdc2. Further, Plk1 is not required for MAPK activation at meiotic reinitiation (see Figure 5C, 45 min).
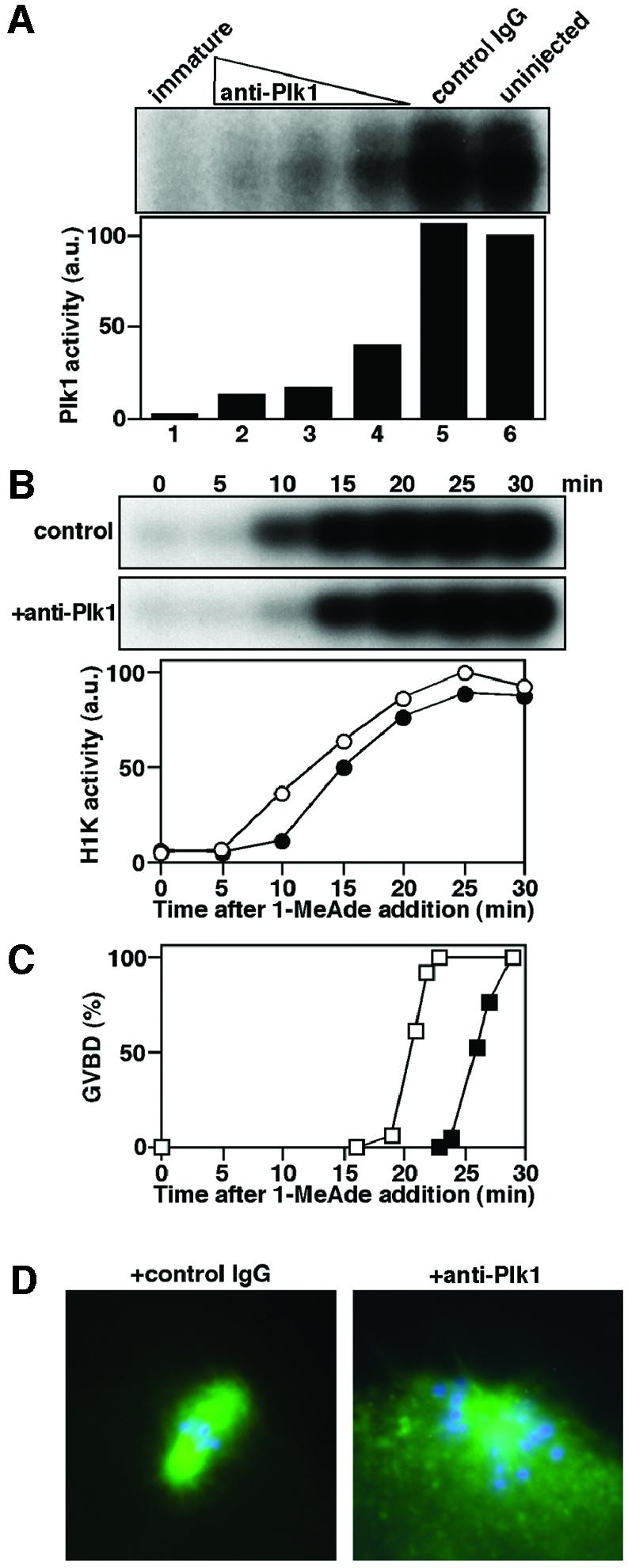
Fig. 4. The anti-Plk1 antibody prevents Plk1 activation and causes abortive spindle formation, but does not suppress cyclin B–Cdc2 activation at reinitiation of meiosis I. (A) Immature oocytes were injected with anti-Plk1 antibody (lanes 2–4, 300, 200 and 100 pg, respectively), control IgG (lane 5, 300 pg) or uninjected (lane 6), and then treated with 1-MeAde. Extracts were prepared from groups of 10 oocytes (after the occurrence of GVBD, lanes 2–6; immature, lane 1). Plk1 activity was detected on an autoradiogram (upper) or quantified in the excised bands by liquid scintillation counting (lower). (B) Extracts were prepared at 5 min intervals after 1-MeAde addition from groups of five oocytes that were uninjected (control, open circle) or injected with anti-Plk1 antibody (+anti-Plk1, closed circle), and histone H1 kinase activity was determined. (C) Time course of GVBD in oocytes that were uninjected (open squares) or injected with anti-Plk1 antibody (closed squares) was monitored during preparation of the extracts in (B). (D) Oocytes that were injected with control IgG (left) or anti-Plk1 antibody (right) were fixed at 20 min after GVBD. Green, immunofluorescence staining with anti-β-tubulin antibody; blue, DAPI staining of DNA.
Fig. 5. Lack of Plk1 activity does not affect cyclin B–Cdc2 inactivation at exit from meiosis I but prevents its reactivation at meiosis II through dephosphorylation of Myt1. Immature oocytes were uninjected (control) or injected with anti-Plk1 antibody (+anti-Plk1), and then treated with 1-MeAde. (A) At the indicated times, extracts were prepared from groups of 10 oocytes and immunoprecipitates with anti-Plk1, anti-cyclin B and anti-cyclin A antibodies were assayed for their associated kinase activities. (B) At the indicated times, groups of 30 oocytes were recovered and immunoblotted with anti-cyclin B and anti-Cdc2 antibodies. Cdc2-U and L represent cyclin B-associated inactive and active forms, respectively. (C) Groups of 40 oocytes that were uninjected (–) or injected (+) with anti-Plk1 antibody were sampled at the indicated times and immunoblotted with anti-Cdc25, anti-Myt1 and anti-MAPK antibodies.
To test another possible role for Plk1 at meiosis I, we examined spindle formation, which is a conserved function for Plk1s in many species (reviewed in Glover et al., 1998; Nigg, 1998). Anti-Plk1 antibody injection resulted in a monopolar spindle with an altered pattern of β-tubulin and a radial distribution of chromosomes around the pole, while in control IgG-injected oocytes, a normal bipolar spindle was formed at metaphase of meiosis I (Figure 4D). These observations demonstrate that in meiosis I of starfish oocytes, Plk1 activity is required for meiotic spindle formation.
We next examined the role of Plk1 on the dynamics of cyclin B–Cdc2 during exit from meiosis I. In the anti-Plk1 antibody-injected oocytes, inactivation of cyclin B–Cdc2 and cyclin B destruction occurred with almost normal kinetics (Figure 5A, middle and 5B, upper). The observed slight delay may reflect that in the activation of cyclin B–Cdc2 (see Figure 4B). Thus, Plk1 activity is dispensable for cyclin B destruction at the end of meiosis I. These observations are in agreement with a report in Drosophila spermatocytes (Carmena et al., 1998), but contrast with other reports that Plk1 is involved in the activation of the cyclin B destruction machinery (reviewed in Glover et al., 1998; Nigg, 1998, 2001).
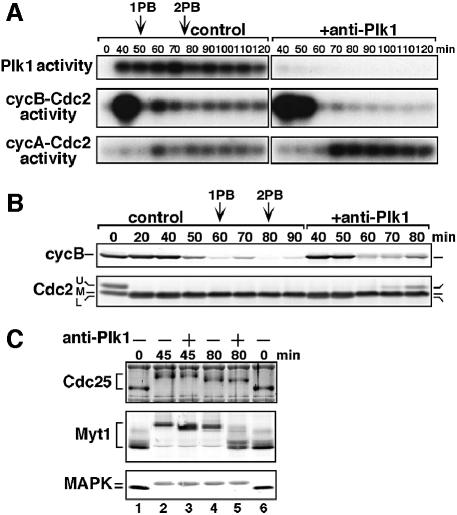
Plk1 is required for activation of cyclin B–Cdc2 at entry into meiosis II through suppression of Myt1
In oocytes lacking Plk1 activity due to antibody injection, cyclin B–Cdc2 failed to be reactivated after exit from meiosis I, despite a normal reaccumulation of cyclin B after its destruction (Figure 5A, middle and 5B, upper; compare 70 min in control with 80 min in +anti-Plk1). Instead, the lack of cyclin B–Cdc2 reactivation appeared to be due to inhibitory phosphorylation of Cdc2, that was not observed at the normal meiosis I to II transition (Figure 5B, lower; compare 60 and 70 min in control with 70 and 80 min in +anti-Plk1). Thus, Plk1 is likely to be required for rapid activation of cyclin B–Cdc2 at entry into meiosis II through preventing inhibitory phosphorylation of Cdc2. This requirement of Plk1 is consistent with the presence of significant levels of Plk1 activity during the meiosis I to II transition (see Figure 2).
In contrast to cyclin B, cyclin A–Cdc2 was activated with normal timing after meiosis I even in the absence of Plk1 activity (Figure 5A, lower), indicating that cyclin A–Cdc2 is activated independently of Plk1 and cyclin B–Cdc2 activities. This may be explained by the fact that cyclin A-associated Cdc2 does not undergo inhibitory phosphorylation during meiotic and cleavage cycles (Okano-Uchida et al., 1998). Thereafter, cyclin A–Cdc2 activity was augmented possibly due to the lack of cyclin A destruction.
To investigate how the absence of Plk1 activity causes inhibitory phosphorylation of cyclin B-associated Cdc2, we examined the state of kinases responsible for the phosphorylation. Since Wee1 protein levels were low at the end of meiosis I, it is unlikely that Wee1 contributes significantly to inhibitory phosphorylation of Cdc2. On the other hand, Myt1 protein, which was present at constant levels through meiotic and cleavage cycles (see Figures 8B and 9B), became severely, though not completely, dephosphorylated after exit from meiosis I in oocytes lacking Plk1 (and cyclin B–Cdc2 but not cyclin A–Cdc2) activity (Figure 5C, 80 min). In contrast, in oocytes in which Plk1 (and cyclin A–Cdc2 but not cyclin B–Cdc2) was active, Myt1 was only slightly dephosphorylated at exit from meiosis I and was still maintained in the highly phosphorylated state (see Figure 7A, +antisense cycB). These observations suggest that elevated Plk1 (but neither cyclin A–Cdc2 nor cyclin B–Cdc2) activity is involved in maintenance of phosphorylated and most possibly suppressed states of Myt1 to cause the rapid activation of cyclin B–Cdc2 at entry into meiosis II.
Fig. 7. Plk1 activity depends on either cyclin A–Cdc2 or cyclin B–Cdc2 after meiosis I but not on cyclin B–Cdc2 at entry into M-phase of the first cleavage cycle. (A) Immature oocytes were uninjected (control) or injected with cyclin A and/or cyclin B antisense oligonucleotides (+antisense), and then treated with 1-MeAde. At the indicated times, extracts were prepared from groups of 10 oocytes and immunoprecipitated with anti-Plk1, anti-cyclin B and anti-cyclin A antibodies for assay of their associated kinase activities, or immunoblotted with anti-cyclin B, anti-cyclin A, anti-Myt1 and anti-MAPK antibodies. (B) Mature eggs at the female pronucleus stage were injected with control IgG (control) or neutralizing anti-Cdc25 antibody (+anti-Cdc25), and then inseminated. At the indicated times, extracts were prepared from groups of 10 eggs and immunoprecipitated with anti-Plk1, anti-cyclin B and anti-cyclin A antibodies for assay of their associated kinase activities, or immunoblotted with anti-Myt1 antibody.
Both MAPK and Cdc2 are required to support Plk1 activity at meiosis II
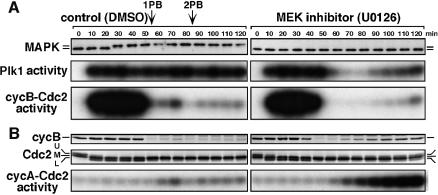
Since the c-Mos-MAPK pathway is required for occurrence of meiosis II in starfish oocytes (Tachibana et al., 2000), we examined whether MAPK contributes to the Plk1 activity during meiotic cycles. In the presence of an MEK inhibitor, U0126, MAPK activation after hormonal stimulus was completely prevented, whereas cyclin B–Cdc2 was activated normally and meiosis I resumed (Figure 6A). In these oocytes lacking MAPK activity, Plk1 activity increased normally but then declined at exit from meiosis I (Figure 6A, middle). Conversely, in the absence of Plk1 activity, MAPK remained active after exit from meiosis I (Figure 5C, 80 min; see also Figure 7A, +antisense cycA & B). These observations suggest that MAPK is involved in supporting the activity of Plk1 after meiosis I.
Fig. 6. Inhibition of MAPK activation causes Plk1 inactivation after exit from meiosis I. Immature oocytes were first preincubated for 10 min in seawater containing DMSO (left) or 50 µM MEK inhibitor, U0126 (right), and then treated with 1-MeAde. (A) Extracts were prepared at 10 min intervals and immunoblotted with anti-MAPK antibody (upper), or immunoprecipitated with anti-Plk1 (middle) and anti-cyclin B (lower) antibodies for kinase assays. (B) The same extracts as in A were immunoblotted with anti-cyclin B (upper) and anti-Cdc2 (middle) antibodies, or assayed for cyclin A–Cdc2 kinase activity (lower).
In the presence of MEK inhibitor, however, Plk1 was reactivated much later following the activation of cyclin A–Cdc2, but before that of cyclin B–Cdc2 (Figure 6A, 90 min). This Plk1 activation is likely to reflect the situation of cleavage cycles (see below, cleavage cycles), since the prevention of the Mos-MAPK pathway causes mitotic cleavage cycles immediately after meiosis I (Tachibana et al., 2000).
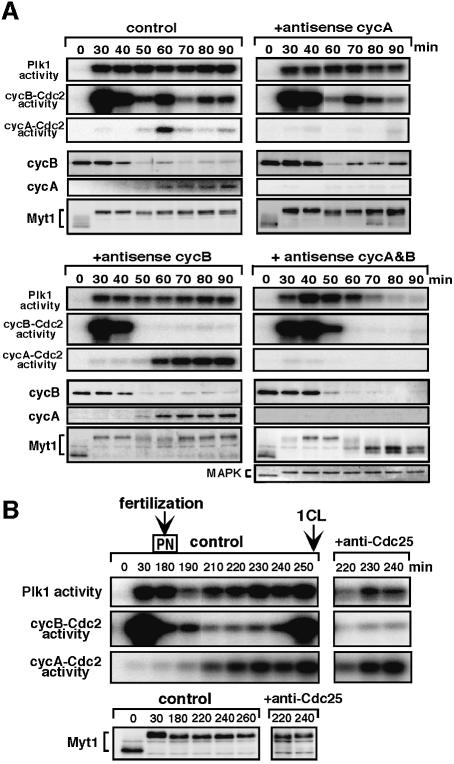
Precise examination of Figure 6A (MEK inhibitor, 50 and 60 min) indicated that the inactivation of cyclin B–Cdc2 preceded that of Plk1 at exit from meiosis I. In addition, protein synthesis was required for MAPK to support Plk1 activity after meiosis I (data not shown). These suggest the dependency of Plk1 activity on cyclin B–Cdc2 not only at meiotic reinitiation but also through meiotic cycles. Then, we prevented the activation of cyclin B–Cdc2 and/or cyclin A–Cdc2 after exit from meiosis I by inhibiting synthesis of cyclin B, cyclin A or both by use of morpholino or phosphorothioated antisense oligonucleotides (Figure 7A). In spite of the fact that MAPK remained active in all cases (Figure 7A, +antisense cycA & B, lowest and data not shown), Plk1 remained active only in the presence of cyclin B–Cdc2 and/or cyclin A–Cdc2 activities, while if both activities were eliminated, Plk1 was inactivated after meiosis I. Thus, Cdc2 (either cyclin B–Cdc2 or cyclin A–Cdc2) activity is required for MAPK to support Plk1 activity at meiosis II. Consistently, activation of MAPK alone in immature starfish oocytes failed to activate Plk1 and cyclin B–Cdc2 (data not shown; Tachibana et al., 2000).
How then does MAPK, along with Cdc2, support Plk1 activity at meiosis II? Under conditions lacking cyclin B–Cdc2 but having cyclin A–Cdc2 activities, Plk1 was active only when MAPK was active (compare Figure 6, MEK inhibitor with Figure 7A, +antisense cycB), indicating a pathway from MAPK to Plk1, but not through Cdc2. Another possible pathway would be from MAPK to the regulators of cyclin B–Cdc2 not via Plk1 and, as a result, would contribute to Plk1 activity. In fact, the phosphorylation states of Cdc25 showed little change in oocytes that were lacking in Plk1 (accordingly, cyclin B–Cdc2) activity but still had MAPK activity (Figure 5C, 80 min), while the absence of MAPK activity caused dephosphorylation of Cdc25 after exit from meiosis I (even in the presence of cyclin A–Cdc2 activity) (Figure 6B; see figure 3C in Tachibana et al., 2000). It is thus most likely that MAPK contributes, but not through Plk1, to the regulation of Cdc25 after meiosis I. Alternatively, immediately downstream of MAPK, p90rsk is suggested to directly phosphorylate and down-regulate Myt1 in Xenopus oocytes (Palmer et al., 1998). This pathway is apparently less likely, since even in the presence of MAPK activity, Myt1 was severely dephosphorylated when Plk1 and Cdc2 lost their activities (Figure 5C, 80 min and Figure 7A, +antisense cycA & B). Nonetheless, considering that Myt1 was still slightly phosphorylated in this case, the pathway from MAPK to Myt1 not via Plk1 is not excluded during the meiosis I to II transition in starfish oocytes as well.
Plk1 activation contributes to cyclin B–Cdc2 activation during cleavage cycles
During cleavage cycles, the H1 kinase activity represents the total of the early activated cyclin A–Cdc2 and the late activated cyclin B–Cdc2 (Okano-Uchida et al., 1998). Considering this, our observation that the activation of H1 kinase preceded that of Plk1 (Figure 2B) which was followed by that of cyclin B–Cdc2 (Figure 9A, control) indicates that first cyclin A–Cdc2, then Plk1 and finally cyclin B–Cdc2, were activated during cleavage cycles. In agreement with this order, when activation of cyclin B–Cdc2 during the first cleavage cycle was prevented by injecting neutralizing anti-Cdc25 antibody into female pronucleus stage eggs, activation of both Plk1 and cyclin A–Cdc2 occurred, although not to full levels (Figure 7B). Thus, in contrast to meiotic cycles, Plk1 activation does not depend on cyclin B–Cdc2 activity at entry into early embryonic M-phase.
Conversely, we examined whether Plk1 is required for cyclin B–Cdc2 activation during the first cleavage cycle. The anti-Plk1 antibody was injected into mature eggs that had been previously inseminated during meiosis I and had just completed meiosis II as judged by emission of the second polar body. In the absence of Plk1 activation, the timing of cyclin B–Cdc2 activation associated with entry into the first cleavage cycle was delayed by more than 30 min (Figure 8A, middle). In eggs lacking Plk1 activity, both accumulation of cyclin B and cyclin A proteins (Figure 8B, upper) and activation of cyclin A–Cdc2 (Figure 8A, lower) occurred normally. However, Cdc2, which might be associated with cyclin B, remained in the phosphorylated and thus inhibited state for a much longer period compared with control eggs (Figure 8B, Cdc2, U-band). This indicated that, in the absence of Plk1 activity in the first cleavage cycle, normal activation of cyclin B–Cdc2 did not occur, even though cyclin A–Cdc2 was activated normally. The delayed activation of cyclin B–Cdc2 may be ascribed to the hyperactivation of cyclin A–Cdc2 (see figure 8 in Okano-Uchida et al., 1998).
To examine why cyclin B–Cdc2 was not activated normally in the absence of Plk1 activity, we next investigated the phosphorylation states of Myt1 and Wee1. In control fertilized eggs, Myt1 showed only a slight dephosphorylation at the completion of meiosis II (Figure 8B, Myt1, 90 min in control). In anti-Plk1 injected eggs, however, Myt1 was transiently dephosphorylated to a level comparable with that in immature oocytes (Figure 8B, Myt1, 90 and 100 min in +anti-Plk1), whereas cyclin A–Cdc2 was activated (Figure 8A, lower). Conversely, Myt1 was phosphorylated even in the absence of cyclin B–Cdc2 activity when Plk1 was activated after fertilization (Figure 7B). In contrast, Wee1 was gradually phosphorylated prior to cyclin B–Cdc2 activation in control fertilized eggs (Figures 8B and 9C, control in Wee1), and the prevention of Plk1 activation did not significantly affect the Wee1 phosphorylation (Figure 8B, +anti-Plk1 in Wee1). These observations suggest that during cleavage cycles Plk1 regulates the phosphorylation states (and most probably, the activity) of Myt1, but not of Wee1, and thus contributes to the normal activation of cyclin B–Cdc2.
Cyclin A–Cdc2 is required for both Plk1 activation and Wee1 phosphorylation during early cleavage cycles
In the presence of the CDK inhibitor roscovitin, which inhibits both cyclin A–Cdc2 and cyclin B–Cdc2 activity, Plk1 activation in the first cleavage cycle was prevented (data not shown). Considering that cyclin B–Cdc2 was not required for Plk1 activation (Figure 7B), it is plausible that cyclin A–Cdc2 contributes to Plk1 activation in the first cleavage cycle. To examine this possibility, we used an antisense cyclin A oligonucleotide, which blocked the synthesis of cyclin A but not cyclin B (Figure 9, upper). The antisense cyclin A oligonucleotide was injected into immature oocytes lacking cyclin A protein. It did not significantly affect either reinitiation of meiosis I as judged by the occurrence of GVBD, or transition from meiosis I to II as judged by the kinetics of cyclin B–Cdc2 kinase activity (data not shown; see also Figure 7A, +antisense cycA). However, in the absence of cyclin A protein (i.e. cyclin A–Cdc2 activity), Plk1 activation after the decrease at the completion of meiosis II did not occur, though Plk1 protein was present (Figure 9A, middle). Under these conditions, cyclin B–Cdc2 also failed to be activated, and inhibitory phosphorylation of Cdc2 continued much longer than in control eggs (Figure 9A, lower and 9C, middle). Although unusually delayed activation of Plk1 and cyclin B–Cdc2 was observed in the absence of cyclin A (Figure 9A, 160 min and later), this may be related to unphysiological over-accumulation of cyclin B protein (Figure 9A, upper).
The defect in cyclin B–Cdc2 activation was more serious in cyclin A-deficient eggs than in Plk1-deficient eggs (compare Figure 9A with Figure 8A), suggesting that in activating cyclin B–Cdc2, cyclin A–Cdc2 might have another role besides Plk1 activation. Myt1 was dephos phorylated after meiosis II in eggs lacking cyclin A protein, to a similar degree as in eggs lacking Plk1 activity (compare Figure 9B with Figure 8B). In contrast, Wee1 phosphorylation, which occurred in eggs lacking Plk1 activity, was not observed in cyclin A-deficient eggs (compare Figure 9C with Figure 8B). Taken together, cyclin A–Cdc2 might contribute to cyclin B–Cdc2 activation during cleavage cycles through two separate pathways, Plk1 activation and Wee1 phosphorylation.
Discussion
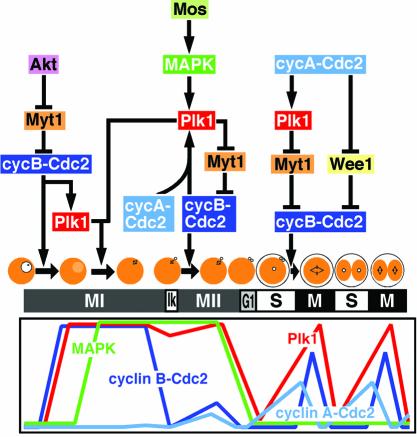
The present study on the interrelations among Plk1, cyclin B–Cdc2, cyclin A–Cdc2 and MAPK through starfish meiotic and early embryonic cycles has revealed that upstream regulators of Plk1 are cyclin B–Cdc2 at reinitiation of meiosis I, MAPK along with Cdc2 at entry into meiosis II and cyclin A–Cdc2 at entry into embryonic M-phase (Figure 10). These imply that Plk1 functions as a trigger kinase for the initial activation of cyclin B–Cdc2 in mitotic cleavage cycles, but not in meiosis I.
Fig. 10. Regulatory pathways for cyclin B–Cdc2 activation during starfish meiotic and early cleavage cycles. At reinitiation of meiosis I (MI), Akt inhibits Myt1 to cause the activation of cyclin B–Cdc2, which induces Plk1 activation. Plk1 is required for spindle formation but not for cyclin B–Cdc2 activation. At entry into meiosis II (Ik and MII), MAPK, along with cyclin A–Cdc2 and/or cyclin B–Cdc2, maintains Plk1 activity, which promotes further activation of cyclin B–Cdc2 through suppression of Myt1. MAPK may also contribute to maintenance of Cdc25 and suppression of Myt1. At entry into M-phase of the first cleavage cycle (M), cyclin A–Cdc2 promotes both Plk1 activation to cause Myt1 suppression and Wee1 phosphorylation, both of which result in cyclin B–Cdc2 activation. Thus, interplay among Plk1, cyclin B–Cdc2, cyclin A–Cdc2 and MAPK is unique at each of three types of cell cycle transition.
Target of Plk1 in triggering cyclin B–Cdc2 activation
The idea that Plk1 is a trigger kinase for Cdc25C activation (reviewed in Lane and Nigg, 1997) arose primarily from two studies: identification of Xenopus egg Plx1, which associates with and phosphorylates Cdc25C in vitro (Kumagai and Dunphy, 1996; Qian et al., 2001), and the inhibitory effect of an anti-Plk1 antibody on entry into M-phase in non-immortalized Hs68 fibroblasts (Lane and Nigg, 1996). This idea implies that Plk1 is initially activated and can phosphorylate/activate Cdc25C in the absence of cyclin B–Cdc2 activity. In a precise sense, however, no previous studies have examined this possibility (see Abrieu et al., 1998; Karaiskou et al., 1998; Qian et al., 1998a, 1999, 2001). Even though Plk1 functions as the trigger kinase for cyclin B–Cdc2 activation, it is still unclear whether the target of Plk1 is Cdc25C.
Unlike the case of meiosis I, our present observations of cleavage cycles agree with the proposed role of Plk1 as the trigger kinase for cyclin B–Cdc2 activation, but there is a substantial difference in the pathway from Plk1 to cyclin B–Cdc2. In contrast to the original proposal that Cdc25C is the direct target of Plk1, our present study revealed that lack of Plk1 activity has no significant effect on the phosphorylation states of either Cdc25 or Wee1, but causes dephosphorylation of Myt1 (Figures 5C, 7A and 8B). These observations suggest that independent of cyclin B–Cdc2 activity, Plk1 might regulate phosphorylation states and presumably activity of Myt1 at entry into M-phase. Consistently, hyperphosphorylation of Myt1 in Xenopus egg cycling extracts was strongly reduced when Plx1 activation was prevented (Abrieu et al., 1998), but no other studies have examined Myt1 in relation to Plk1 (see Karaiskou et al., 1998; Qian et al., 1998a, 1999, 2001). In our preliminary in vitro experiments, however, Plk1 phosphorylated but did not down-regulate Myt1 that was recovered from immature starfish oocytes (data not shown). Nonetheless, it is possible that after meiosis I Plk1 directly down-regulates Myt1, because the phosphorylation state of Myt1 in immature oocytes is exceptionally low (see control in Figures 8B and 9B; Sillge and Nigg, 2003).
Taken together, Myt1, rather than Cdc25C, might be the target of Plk1, when Plk1 functions at the initial step of cyclin B–Cdc2 activation (Figure 10). If Plk1 can affect the activity of cyclin B–Cdc2 via Myt1, then Cdc25C activity might be modified by cyclin B–Cdc2. The direct phosphorylation of Cdc25C by Plk1, if it occurred, might affect the localization of Cdc25C (see Toyoshima-Morimoto et al., 2002).
Interplay between Plk1 and Cdc2
The present observations in vivo are summarized as follows. (1) In meiosis I in which cyclin A is lacking, Plk1 activity is not required for cyclin B–Cdc2 activation and, conversely, Plk1 activation requires cyclin B–Cdc2 activity, indicating the pathway, cyclin B–Cdc2 → Plk1. (2) In meiosis II in which MAPK is essential, Plk1 activity is required not for cyclin A–Cdc2 but for cyclin B–Cdc2 activation and, conversely, Plk1 activity requires either cyclin A–Cdc2 or cyclin B–Cdc2 activity along with MAPK activity, indicating the pathway, MAPK and cyclin A–Cdc2 → Plk1 ←→ cyclin B–Cdc2. (3) In cleavage cycles, Plk1 activity is required not for cyclin A–Cdc2 but for cyclin B–Cdc2 activation and, conversely, Plk1 activation requires cyclin A–Cdc2 but not cyclin B–Cdc2 activity, indicating the pathway, cyclin A–Cdc2 → Plk1 → cyclin B–Cdc2 (Figure 10).
These three pathways may, at first, appear to be somewhat confusing. However, considering that both cyclin A–Cdc2 and cyclin B–Cdc2 are able to cause activation of Plk1, we may assume that Plk1 does not strictly discriminate between cyclin A and cyclin B as its upstream regulator, apart from whether they can contribute equivalently. If so, one possible conclusion would be that Plk1 is always downstream of Cdc2. Consistently, in fission yeast which has only one type of M-phase cyclin, Plo1/Plk does not function as the trigger kinase and rather, its activation depends on Cdc2 (Tanaka et al., 2001). Another conclusion would be that in general (i.e. in the mitotic cycle), cyclin A–Cdc2 triggers the activation of Plk1, which in turn triggers that of cyclin B–Cdc2, while modified patterns occur in the meiotic cycle due to the absence of cyclin A at meiosis I and the presence of MAPK activity at meiosis II (see below). After these triggering steps, positive feedback loops involving Plk1, as reported previously (Abrieu et al., 1998; Karaiskou et al., 1998; Qian et al., 1998a), might function to cause further and rapid activation of cyclin B–Cdc2.
How then does Cdc2 (with its A or B cyclin partner) cause the activation of Plk1? Plk1 is most likely activated by a homolog of xPlkk1 (Qian et al., 1998b) and it has been presumed that Cdc2 acts on a component upstream of the Plkk1–Plk1 pathway, because none of these is substrates for cyclin B–Cdc2 (Qian et al., 2001). In contrast, Kelm et al. (2002) reported recently that Ser340 on Xenopus Plk1 is likely to be phosphorylated by cyclin B–Cdc2, though this site is not conserved in human and starfish Plk1 (Supplementary figure 1A). They also showed that Thr201, which is located within the T-loop and is highly conserved among various Plk1 including starfish, is a major site of activation and phosphorylation, at least in vitro, by PKA or possibly by PDK1. In addition, the pathway from MAPK to Plk1 not via Cdc2 might be present (the present study at meiosis II). Thus, multiple kinases appear to be involved in the activation of Plk1, and the actions of Cdc2 on Plk1 require further study.
Plk1 in the regulation of meiotic and cleavage cycles
Meiosis I. The present observations revealed that during meiosis I, Plk1 is required for bipolar spindle formation, but neither for the activation nor for the inactivation of cyclin B–Cdc2 (Figures 3–5). These are consistent with the original finding that Polo was identified in Drosophila as a gene required for formation of the bipolar spindle (Sunkel and Glover, 1988) and support that a conserved role for Plk1 among many eukaryotic cells (except for budding yeast) is to form the bipolar spindle.
If Plk1 is always downstream of Cdc2 (see above), Plk1 activation in meiosis I is most likely to rely on cyclin B–Cdc2 due to the absence of cyclin A. This implies that in meiosis I, cyclin B–Cdc2 should be initially activated independently of Plk1 and hence Plk1 does not function as the trigger kinase. Consistently, in the case of starfish oocytes, cyclin B–Cdc2 is activated initially through a signaling pathway in which Akt/PKB is the trigger kinase (Okumura et al., 2002). It has been suggested in Xenopus and mouse oocytes also that Plk1 activation requires prior activation of cyclin B–Cdc2 (Karaiskou et al., 1998; Qian et al., 1998a; Pahlavan et al., 2000), though it is still uncertain whether inhibition of cyclin B–Cdc2 activation prevents Plk1 activation at meiotic reinitiation in these oocytes.
Meiosis I to II transition. The present observations indicate that only at meiosis II, Plk1 activity relies on MAPK in addition to Cdc2 (Figures 6 and 7A). Why is MAPK required? What is essential for the successful transition from meiosis I to II is that after exit from meiosis I, cyclin B–Cdc2 is reactivated bypassing inhibitory phosphorylation of Cdc2 (see Iwabuchi et al., 2000; Tachibana et al., 2000). Although cyclin A–Cdc2 is activated at entry into both meiosis II and embryonic M-phase, the activation of cyclin A–Cdc2 alone results in the process of inhibitory phosphorylation of cyclin B-associated Cdc2 even if cyclin B–Cdc2 is finally activated (compare meiosis II with cleavage cycle). This implies that cyclin A alone is not sufficient and MAPK supplements cyclin A for the rapid activation of cyclin B–Cdc2 at entry into meiosis II. The presence of various pathways for such an effect of MAPK was suggested in the present observations: a positive one from MAPK to Plk1, a positive one from MAPK to Cdc25, a possible negative one from MAPK to Myt1, and a negative one from Plk1 to Myt1 (Figures 5, 6, 7A and 10). In addition, at the exit from meiosis I, MAPK might to some extent support cyclin B–Cdc2 activity, which in turn contributes to Plk1 activity at entry into meiosis II (Figure 7A, +antisense A), since cyclin B–Cdc2 was inactivated at exit from meiosis I significantly earlier in the absence of MAPK activity than in its presence (Figure 6A, compare at 50 and 60 min; see also Picard et al., 1996).
Taken together, although the Mos-MAPK pathway governs the meiosis I to II transition, Plk1 is demonstrated to play an indispensable role in mediating the action of MAPK.
M-phase entry in cleavage cycles. In contrast to the meiotic cycles, early embryonic cycles clearly required cyclin A–Cdc2 for Plk1 activation (Figure 9A). Cyclin A–Cdc2 has long been suggested to play an important role at the G2/M-phase transition (for example, Lehner and O’Farrell, 1990; Devault et al., 1992; Pagano et al., 1992), whereas its target remains unclear. Our results provide a possible answer to this question: cyclin A–Cdc2, which is active prior to cyclin B–Cdc2, contributes to both Plk1-dependent hyperphosphorylation of Myt1 and Plk1-independent phosphorylation of Wee1, and thereby to cyclin B–Cdc2 activation (Figure 10).
Materials and methods
Preparation of oocyte and egg extracts
Oocytes of the starfish, Asterina pectinifera, and their extracts were prepared essentially as described previously (Okano-Uchida et al., 1998). See Supplementary data for details.
Isolation and sequencing of starfish Plk1 and Wee1 cDNAs
Starfish homologs of Plk1 and Wee1 cDNAs were isolated by RT–PCR, and 5′ and 3′ RACE. See Supplementary data for details.
Antibodies
The rabbit polyclonal antibodies were raised against starfish Plk1 (amino acids 30–431) and Wee1 (amino acids 301–413) and were affinity purified. See Supplementary data for details.
Microinjection
Microinjection was performed according as described previously (Kishimoto, 1986). Immature oocytes were injected with 0.2 mg/ml of anti-starfish Cdc25 antibody (20 pg/oocyte), 1.5 mg/ml of affinity-purified anti-starfish Plk1 antibody (300 pg/oocyte), 13 mg/ml of p13suc1 protein (1 ng/oocyte), cyclin B morpholino oligo (ATTGTAACCA ATGCGAGTTCTGAGG; final concentration 3 µM) or cyclin A antisense S-oligo (5′-GGACATGTTGAGTGTTC-3′ corresponding to the translation initiation site, nucleotides 101–117; final concentration 3 µM), and then treated with 1-MeAde.
Immunoblotting
Samples were separated on 10% polyacrylamide gels and immunoblotting was performed essentially as described previously (Okano-Uchida et al., 1998). To resolve inhibitory phosphorylation of Cdc2, a modified electrode buffer (75 mM Tris, 450 mM glycine, 0.1% SDS) was used. See Supplementary data for details.
Immunoprecipitation and kinase assays
For histone H1 kinase assays, 1 µl extracts equivalent to half of an oocyte were diluted into 4 µl kinase buffer (80 mM Na-β-glycerophosphate, 20 mM EGTA, 15 mM MgCl2, 1 mM DTT, pH 7.3), then incubated for 15 min at 25°C in a final volume of 10 µl containing 0.3 mg/ml histone H1 (Boehringer), and 0.2 mCi/ml [γ-32P]ATP (Amersham Pharmacia Biotech). For individual kinase assays of cyclin B–Cdc2 or cyclin A–Cdc2, extracts equivalent to one oocyte were incubated with protein A Sepharose CL-4B conjugated with anti-cyclin B or anti-cyclin A antibody for 1 h at 4°C. Beads were washed three times with immunoprecipitation buffer (50 mM Tris–HCl pH 7.5, 150 mM NaCl, 25 mM NaF, 0.5% Nonidet P-40), twice with kinase buffer, and then suspended in 5 µl of kinase buffer. The kinase assay for anti-starfish Plk1 immunoprecipitates was carried out for 45 min at 25°C using dephosphorylated α-casein (Sigma) as a substrate. The reaction was terminated by addition of 10 µl 2× SDS–PAGE sample buffer, followed by boiling for 5 min. Aliquots of 10 µl of samples were run on 12% SDS–PAGE and stained with 0.25% Coomassie Brilliant Blue R-250. The stained gels were autoradiographed with X-ray film overnight at –80°C.
Immunofluorescence
Immunofluorescence staining was performed according to Tachibana et al., 2000. See Supplementary data for details.
Supplementary data
Supplementary data are available at The EMBO Journal Online.
Acknowledgments
Acknowledgements
We thank Drs Keita Ohsumi for discussion and Rindy Jaffe for critical reading of the manuscript. This work was supported by the CREST Research Project of JST, a grant-in-aid from the MEXT, Japan, and a grant from the HFSP to T.K.
References
- Abrieu A., Brassac,T., Galas,S., Fisher,D., Labbe,J.C. and Doree,M. (1998) The Polo-like kinase Plx1 is a component of the MPF amplification loop at the G2/M-phase transition of the cell cycle in Xenopus eggs. J. Cell Sci., 111, 1751–1757. [DOI] [PubMed] [Google Scholar]
- Brazil D.P. and Hemmings,B.A. (2001) Ten years of protein kinase B signalling: a hard Akt to follow. Trends Biochem. Sci., 26, 657–664. [DOI] [PubMed] [Google Scholar]
- Carmena M., Riparbelli,M.G., Minestrini,G., Tavares,A.M., Adams,R., Callaini,G. and Glover,D.M. (1998) Drosophila polo kinase is required for cytokinesis. J. Cell Biol., 143, 659–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman T.R. and Dunphy,W.G. (1994) Cdc2 regulatory factors. Curr. Opin. Cell Biol., 6, 877–882. [DOI] [PubMed] [Google Scholar]
- Devault A., Fesquet,D., Cavadore,J.C., Garrigues,A.M., Labbe,J.C., Lorca,T., Picard,A., Philippe,M. and Doree,M. (1992) Cyclin A potentiates maturation-promoting factor activation in the early Xenopus embryo via inhibition of the tyrosine kinase that phosphorylates cdc2. J. Cell Biol., 118, 1109–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellinger-Ziegelbauer H., Karasuyama,H., Yamada,E., Tsujikawa,K., Todokoro,K. and Nishida,E. (2000) Ste20-like kinase (SLK), a regulatory kinase for polo-like kinase (Plk) during the G2/M transition in somatic cells. Genes Cells, 5, 491–498. [DOI] [PubMed] [Google Scholar]
- Glover D.M., Hagan,I.M. and Tavares,A.A. (1998) Polo-like kinases: a team that plays throughout mitosis. Genes Dev., 12, 3777–3787. [DOI] [PubMed] [Google Scholar]
- Golsteyn R.M., Mundt,K.E., Fry,A.W. and Nigg,E.A. (1995) Cell cycle regulation of the activity and subcellular localization of PLK1, a human protein kinase implicated in mitotic spindle function. J. Cell Biol., 129, 1617–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamanaka R., Smith,M.R., O’Connor,P.M., Maloid,S., Mihalic,K., Spivak,J.L., Longo,D.L. and Ferris,D.K. (1995) Polo-like kinase is a cell cycle-regulated kinase activated during mitosis. J. Biol. Chem., 270, 21086–21091. [DOI] [PubMed] [Google Scholar]
- Iwabuchi M., Ohsumi,K., Yamamoto,T.M., Sawada,W. and Kishimoto,T. (2000) Residual Cdc2 activity remaining at meiosis I exit is essential for meiotic M-M transition in Xenopus oocyte extracts. EMBO J., 19, 4513–4523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi T. and Maller,J.L. (1995) Phosphorylation and activation of the Xenopus Cdc25 phosphatase in the absence of Cdc2 and Cdk2 kinase activity. Mol. Biol. Cell, 6, 215–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaiskou A., Cayla,X., Haccard,O., Jessus,C. and Ozon,R. (1998) MPF amplification in Xenopus oocyte extracts depends on a two-step activation of cdc25 phosphatase. Exp. Cell Res., 244, 491–500. [DOI] [PubMed] [Google Scholar]
- Kelm O., Wind,M., Lehmann,W.D. and Nigg,E.A. (2002) Cell cycle-regulated phosphorylation of the Xenopus Polo-like kinase Plx1. J. Biol. Chem., 277, 25247–25256. [DOI] [PubMed] [Google Scholar]
- Kishimoto T. (1986) Microinjection and cytoplasmic transfer in starfish oocytes. Methods Cell Biol., 27, 379–394. [DOI] [PubMed] [Google Scholar]
- Kishimoto T. (1998) Cell cycle arrest and release in starfish oocytes and eggs. Semin. Cell Dev. Biol., 9, 549–557. [DOI] [PubMed] [Google Scholar]
- Kumagai A. and Dunphy,W.G. (1996) Purification and molecular cloning of Plx1, a Cdc25-regulatory kinase from Xenopus egg extracts. Science, 273, 1377–1380. [DOI] [PubMed] [Google Scholar]
- Lane H.A. and Nigg,E.A. (1996) Antibody microinjection reveals an essential role for human polo-like kinase 1 (Plk1) in the functional maturation of mitotic centrosomes. J. Cell Biol., 135, 1701–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane H.A. and Nigg,E.A. (1997) Cell-cycle control: POLO-like kinases join the outer circle. Trends Cell Biol., 7, 63–68. [DOI] [PubMed] [Google Scholar]
- Lehner C.F. and O’Farrell,P.H. (1990) The roles of Drosophila cyclins A and B in mitotic control. Cell, 61, 535–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew D.J. and Kornbluth,S. (1996) Regulatory roles of cyclin dependent kinase phosphorylation in cell cycle control. Curr. Opin. Cell Biol., 8, 795–804. [DOI] [PubMed] [Google Scholar]
- Mueller P.R., Coleman,T.R. and Dunphy,W.G. (1995) Cell cycle regulation of a Xenopus Wee1-like kinase. Mol. Biol. Cell, 6, 119–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami M.S. and Vande Woude,G.F. (1998) Analysis of the early embryonic cell cycles of Xenopus; regulation of cell cycle length by Xe-wee1 and Mos. Development, 125, 237–248. [DOI] [PubMed] [Google Scholar]
- Nigg E.A. (1998) Polo-like kinases: positive regulators of cell division from start to finish. Curr. Opin. Cell Biol., 10, 776–783. [DOI] [PubMed] [Google Scholar]
- Nigg E.A. (2001) Mitotic kinases as regulators of cell division and its checkpoints. Nat. Rev. Mol. Cell Biol., 2, 21–32. [DOI] [PubMed] [Google Scholar]
- O’Farrell P.H. (2001) Triggering the all-or-nothing switch into mitosis. Trends Cell Biol., 11, 512–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okano-Uchida T., Sekiai,T., Lee,K., Okumura,E., Tachibana,K. and Kishimoto,T. (1998) In vivo regulation of cyclin A/Cdc2 and cyclin B/Cdc2 through meiotic and early cleavage cycles in starfish. Dev. Biol., 197, 39–53. [DOI] [PubMed] [Google Scholar]
- Okumura E., Sekiai,T., Hisanaga,S., Tachibana,K. and Kishimoto,T. (1996) Initial triggering of M-phase in starfish oocytes: a possible novel component of maturation-promoting factor besides cdc2 kinase. J. Cell Biol., 132, 125–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okumura E., Fukuhara,T., Yoshida,H., Hanada Si,S., Kozutsumi,R., Mori,M., Tachibana,K. and Kishimoto,T. (2002) Akt inhibits Myt1 in the signalling pathway that leads to meiotic G2/M-phase transition. Nat. Cell Biol., 4, 111–116. [DOI] [PubMed] [Google Scholar]
- Pagano M., Pepperkok,R., Verde,F., Ansorge,W. and Draetta,G. (1992) Cyclin A is required at two points in the human cell cycle. EMBO J., 11, 961–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahlavan G., Polanski,Z., Kalab,P., Golsteyn,R., Nigg,E.A. and Maro,B. (2000) Characterization of polo-like kinase 1 during meiotic maturation of the mouse oocyte. Dev. Biol., 220, 392–400. [DOI] [PubMed] [Google Scholar]
- Palmer A., Gavin,A.C. and Nebreda,A.R. (1998) A link between MAP kinase and p34cdc2/cyclin B during oocyte maturation: p90rsk phosphorylates and inactivates the p34cdc2 inhibitory kinase Myt1 [published erratum appears in EMBO J. (1999) 18, 1092]. EMBO J., 17, 5037–5047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel R., Holt,M., Philipova,R., Moss,S., Schulman,H., Hidaka,H. and Whitaker,M. (1999) Calcium/calmodulin-dependent phosphorylation and activation of human Cdc25-C at the G2/M phase transition in HeLa cells. J. Biol. Chem., 274, 7958–7968. [DOI] [PubMed] [Google Scholar]
- Picard A., Galas,S., Peaucellier,G. and Doree,M. (1996) Newly assembled cyclin B-cdc2 kinase is required to suppress DNA replication between meiosis I and meiosis II in starfish oocytes. EMBO J., 15, 3590–3598. [PMC free article] [PubMed] [Google Scholar]
- Qian Y.W., Erikson,E., Li,C. and Maller,J.L. (1998a) Activated polo-like kinase Plx1 is required at multiple points during mitosis in Xenopus laevis. Mol. Cell. Biol., 18, 4262–4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Y.W., Erikson,E. and Maller,J.L. (1998b) Purification and cloning of a protein kinase that phosphorylates and activates the polo-like kinase Plx1. Science, 282, 1701–1704. [DOI] [PubMed] [Google Scholar]
- Qian Y.W., Erikson,E. and Maller,J.L. (1999) Mitotic effects of a constitutively active mutant of the Xenopus polo-like kinase Plx1. Mol. Cell. Biol., 19, 8625–8632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Y.W., Erikson,E., Taieb,F.E. and Maller,J.L. (2001) The polo-like kinase Plx1 is required for activation of the phosphatase Cdc25C and cyclin B–Cdc2 in Xenopus oocytes. Mol. Biol. Cell, 12, 1791–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillge H.H.W. and Nigg,E.A. (2003) Capturing Polo kinase. Science, 299, 1190–1191. [DOI] [PubMed] [Google Scholar]
- Sunkel C.E. and Glover,D.M. (1988) polo, a mitotic mutant of Drosophila displaying abnormal spindle poles. J. Cell Sci., 89, 25–38. [DOI] [PubMed] [Google Scholar]
- Tachibana K., Tanaka,D., Isobe,T. and Kishimoto,T. (2000) c-Mos forces the mitotic cell cycle to undergo meiosis II to produce haploid gametes. Proc. Natl Acad. Sci. USA, 97, 14301–14306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K., Petersen,J., Maclver,F., Mulvihill,D.P., Glover,D.M. and Hagan,I.M. (2001) The role of Plo1 kinase in mitotic commitment and septation in Schizosaccharomyces pombe. EMBO J., 20, 1259–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoshima-Morimoto F., Taniguchi,E. and Nishida,E. (2002) Plk1 promotes nuclear translocation of human Cdc25C during prophase. EMBO Rep., 3, 341–348. [DOI] [PMC free article] [PubMed] [Google Scholar]