Abstract
Foxp3 is essential for the commitment of differentiating thymocytes to the regulatory CD4+ T (T reg) cell lineage. In humans and mice with a genetic Foxp3 deficiency, absence of this critical T reg cell population was suggested to be responsible for the severe autoimmune lesions. Recently, it has been proposed that in addition to T reg cells, Foxp3 is also expressed in thymic epithelial cells where it is involved in regulation of early thymocyte differentiation and is required to prevent autoimmunity. Here, we used genetic tools to demonstrate that the thymic epithelium does not express Foxp3. Furthermore, we formally showed that genetic abatement of Foxp3 in the hematopoietic compartment, i.e. in T cells, is both necessary and sufficient to induce the autoimmune lesions associated with Foxp3 loss. In contrast, deletion of a conditional Foxp3 allele in thymic epithelial cells did not result in detectable changes in thymocyte differentiation or pathology. Therefore, in mice the only known role for Foxp3 remains promotion of T reg cell differentiation within the T cell lineage, whereas there is no role for Foxp3 in thymic epithelial cells.
FOXP3 was discovered through the genetic analysis of patients with the hereditary monogenic disorder immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome. Concurrently, disruption of the murine homologue Foxp3 was found to be the causal mutation underlying the scurfy phenotype. The lesions observed in mouse and man included lymphoadenopathy and splenomegaly, exfoliative dermatitis and eczema, autoimmune thrombocytopenia, type 1 diabetes, and autoimmune thyroiditis (1–4). Despite the identification of the genetic basis of the disorder in humans and mice, its immunological mechanism has long been the subject of a great deal of controversy. Although several studies suggested that the autoimmune lesions in mice are mediated by T cells (5–9), early BM transplantation experiments showed that the transfer of mutant hematopoietic stem cells into lethally irradiated wild-type recipients by itself does not result in pathology (2). In agreement with these results, transplantation of mutant Foxp3sf thymi into nude or SCID recipients resulted in the characteristic disease (10). Collectively, these studies implicated impaired differentiation of T cells in Foxp3-deficient thymic stroma as a cause of pathology. The evidence in favor of the latter possibility remained inconclusive, however, because the aforementioned inability of Foxp3 mutant BM-derived cells to induce disease in Foxp3sf → Foxp3wt recipients can be explained by surviving radio-resistant wild-type host T cells capable of providing protection. Indeed, the presence of as little as 3–5% of wild-type BM is sufficient to rescue the disease in mixed BM chimeras (11). Furthermore, the transfer of disease via thymic transplant into a lymphopenic host can be explained by the presence of pathogenic thymocytes within the transplanted thymus.
More recent studies have provided a radically different explanation for of the pathology associated with Foxp3 deficiency. Foxp3 was suggested to be a “master regulator” of CD4+CD25+ T regulatory (T reg) cell differentiation because these cells fail to differentiate in the absence of Foxp3, and forced expression of Foxp3 facilitates differentiation of peripheral non–T reg cells into T reg cells (12–14). Because transfection of Foxp3 endows the transfected cells expressing high levels of Foxp3 with suppressive properties (13, 14), and in mixed BM chimeras generated upon transferring of Foxp3 − and Foxp3 + BM into Rag2 −/− recipients T reg cells differentiate only from the Foxp3 + BM, a T cell–intrinsic role for Foxp3-mediated regulation of tolerance was proposed. This view was formally established by the observation of indistinguishable disease in mice with the germline- and CD4-Cre–mediated T cell lineage–restricted ablation of a conditional Foxp3Flox allele (15).
However, a recent study by Chang et al. (16, 17) revived the old argument by raising an interesting possibility that Foxp3 is expressed in the thymus not only in differentiating T reg cells but also in the thymic epithelium. It was further proposed that Foxp3 expressed in the thymic epithelium plays an essential role in the regulation of double negative (DN) thymocyte maturation and that its dysregulation in the absence of Foxp3 results in fatal autoimmunity. In agreement with this idea, Foxp3wt Rag −/− recipients reconstituted with Foxp3 − BM did not manifest fatal autoimmunity (16).
To resolve this continuing controversy, we have used a genetic approach to revisit potential Foxp3 expression and its role in the thymic epithelium. We found no evidence for Foxp3 expression or function in the thymic epithelium in suppressing the autoimmune symptoms associated with Foxp3 deficiency and in guiding early thymocyte differentiation. Our results demonstrate that Foxp3 has a solely T cell–intrinsic function required to maintain tolerance and prevent autoimmunity.
RESULTS AND DISCUSSION
No detectable Foxp3 protein expression in the thymic epithelium
Recently, apparent expression of Foxp3 in the majority of thymic epithelial cells was observed in experiments using flow cytometry while a subset of thymic cortical epithelial cells was found positive for Foxp3 by immunofluorescence (16). Because epithelial cells are notorious for a high degree of nonspecific antibody binding in flow cytometric assays and are tightly associated with thymocytes in situ, potentially leading to false positive results in immunofluorescence assays, we sought to reexamine Foxp3 expression in thymic epithelial cells and thymocytes by flow cytometric analysis of genetically marked Foxp3-expressing cells using knock-in mice harboring a Foxp3gfp reporter allele.
Previously, through examination of thymic tissue sections using immunofluorescence, we found Foxp3GFP protein predominantly expressed in the medullary region in the thymus with rare Foxp3+ cells in the cortex (15). The cortical expression of GFP was previously ascribed to expression by the few CD4+CD8+ double positive (DP) thymocytes that are GFP+ by flow cytometry (15). To determine if this expression was due instead to epithelial cell expression of the Foxp3GFP allele, we performed flow cytometric analysis of purified CD45− thymic stroma from Foxp3GFP mice and found no expression of GFP (Fig. 1 A). For in situ analysis of epithelial cells, we crossed the Foxp3GFP allele into the Rag2 −/− mouse line. Consistent with our previous studies, no expression of Foxp3 was observed in thymocytes isolated from Rag2 −/− Foxp3GFP mice (not depicted). This finding allowed us to examine Foxp3 expression in thymic stromal cells using immunofluorescence in the absence of “contaminating” Foxp3-expressing thymocytes by examining GFP expression using anti-GFP antibody (not depicted) and polyclonal affinity-purified rabbit antibody specific for Foxp3 (Fig. 1 B). We found no sign of Foxp3 expression in Rag-deficient stroma above background fluorescence observed for Foxp3 − mice, whereas Foxp3 expression was readily detectable in control Rag-sufficient mice (Fig. 1 B). These data demonstrate that the thymic epithelium did not express detectable levels of Foxp3 protein within the sensitivity limit of these assays.
Figure 1.
No expression of Foxp3 in the thymic epithelium. (A) Expression of GFP in CD45− thymic stroma from wild-type (shaded) and Foxp3GFP mice (line). Data is representative of four independent experiments. (B) Immunohistochemical analysis of Foxp3 expression in the thymus of Foxp3GFP, Foxp3GFPRag2 −/−, and Foxp3 − Rag −/− mice. Thymic sections were stained with the affinity-purified rabbit anti-Foxp3 antibody. Bar, 100 μm.
Disruption of Foxp3 in thymocytes is necessary and sufficient to cause autoimmune syndromes
Although the aforementioned studies failed to detect Foxp3 protein in thymic epithelial cells, it can be argued that our detection of Foxp3 protein is not sufficiently sensitive. Thus, the possibility remained that low level of Foxp3 expression in thymic epithelial cells at a certain stage of T cell differentiation is required for prevention of autoimmunity. We addressed this possibility by ablation of a conditional Foxp3Flox allele using Cre recombinase expressed exclusively in thymic epithelial cells. Because the Foxn1 gene is the highly specialized regulator of thymic epithelial cell differentiation and is not expressed in BM-derived cells, we bred Foxp3Flox mice with mice harboring Cre recombinase knocked into the 3′ untranslated region of the Foxn1 locus (Foxn1Cre; unpublished data). The resulting Foxp3Flox × Foxn1Cre mice were examined for signs of lymphoproliferative autoimmune disease and compared with Foxp3Flox × CD4-Cre mice.
The interpretation of these experiments, however, was critically dependent on the specificity of CD4-Cre– and Foxn1Cre-mediated deletion. In this regard, it was proposed that the autoimmunity previously reported in Foxp3Flox × CD4-Cre mice (15) is due to CD4-Cre–mediated deletion of the Foxp3 allele in a subset of CD4-expressing thymic epithelial cells. To directly address this issue, we first examined CD4 expression on CD45− thymic stromal cells using flow cytometry and failed to find CD4+ thymic epithelial cells (Fig. 2 A). Next, we tested the cell type specificity of recombination mediated by CD4-Cre and Foxn1Cre using the Rosa26-stopFlox-YFP recombination reporter allele as a genetic fate-mapping tool. We found that CD4-Cre induced recombination in the majority of thymocytes, but no recombination was detectable in the CD45− thymic stroma (Fig. 2 A). On the contrary, Foxn1Cre-induced recombination occurred in the majority of CD45−G8.8+ thymic epithelial cells, but not CD45−G8.8− stromal cells or thymocytes (Fig. 2 B). A comparable extent of Foxp3 deletion in purified CD45−G8.8+ thymic epithelial cells from Foxp3Flox × Foxn1Cre was confirmed by genomic PCR (not depicted). When the CD45−G8.8+ population was subdivided according to the expression of UEA-1 or MHC class II, both subsets showed similar levels of Cre-mediated recombination (not depicted). Foxn1Cre was therefore active in the vast majority of thymic epithelial cells, but not fibroblasts or thymocytes. Importantly, Foxp3Flox × Foxn1Cre mice remained as healthy as Foxp3WT × Foxn1Cre littermates and showed no signs of T cell activation (not depicted), tissue pathology, or wasting disease (Fig. 2 C). In contrast, Foxp3Flox × CD4-Cre mice developed lethal autoimmune lesions indistinguishable from those in Foxp3 − mice in full agreement with our previously reported observations (15). Thus, Foxp3 gene ablation in thymic epithelium does not result in autoimmunity.
Figure 2.
Disruption of Foxp3 in the T cell lineage is necessary and sufficient to cause autoimmune syndrome. (A) No expression of CD4 in the CD45− thymic stroma. Left: expression of CD4 in wild-type CD45− thymic stroma. Thymic stromal cells were analyzed after incubation with anti-CD4 antibodies (solid line) or no antibody control (shaded). Right: expression of YFP in CD45− thymic stroma and CD45+ thymocytes from wild-type (shaded) and Rosa-stopflYFP × CD4-Cre (solid line) mice. Data representative of 11 and 3 experiments, respectively. (B) Expression of YFP from wild-type (shaded) and Rosa-stopflYFP × Foxn1Cre (line) mice in CD45−G8.8+ epithelium, CD45−G8.8− stroma, and thymocytes. Data is representative of three experiments. (C) Analysis of gross clinical signs and lung and skin histopathology in Foxp3fl × Foxn1Cre (n = 7; no histological infiltrates at 4 or 9 wk of age, no visible disease at >16 wk of age), Foxp3fl × CD4-Cre (n = 6; average life span 4 wk), Foxp3 − (n = 7; average life span 4 wk), and wild-type or Foxp3fl mice (n = 9; no histological infiltrates or visible disease at >16 wk of age). Bar, 1 cm. For representative histology sections shown, lung and ear skin were taken from 4-wk-old male Foxp3fl, Foxp3fl CD4-Cre, and Foxp3fl Foxn1Cre mice. Arrows indicate inflammatory infiltrate. Bar, 100 μm. (D) Disease onset in neonatal Rag1 −/− mice reconstituted with Foxp3 scurfy nude BM (▪; n = 4) or Foxp3 wild-type nude BM (▴; n = 4), and neonatal Rag1 −/− Foxp3 scurfy mice reconstituted with Foxp3 scurfy nude BM (□; n = 5) or Foxp3 wild-type nude BM (•; n = 6).
These results were further supported by our experiments using transfers of T cell–depleted Foxp3 − and Foxp3wt BM, either separately or mixed at a 1:1 ratio into sublethally irradiated Rag2 −/− recipients. All recipients of Foxp3 − BM died by 6–7 wk of age from severe autoimmune disease, whereas recipients of Foxp3wt BM and mixed BM chimeras remained healthy (not depicted). Although donor BM was depleted of CD4+ and CD8+ T cells using magnetic bead sorting, it is impossible to formally exclude the possibility of a few pathogenic T cells remaining in the preparations of Foxp3 − BM. In addition, our experiments also differed from those previously reported by Chang et al. in using mice harboring a Foxp3 − allele generated through targeted mutagenesis and spontaneous Foxp3sf mutation, respectively. To definitively exclude these possible explanations for the contradictory results from the two sets of BM transfer studies, we crossed the Foxp3sf allele to the Foxn1-deficient nude mice, which are characterized by an early block in thymic epithelium differentiation and thus lack mature T cells. As previously reported, Foxp3sf nude mice were not affected by the autoimmunity (2). Thus, we were able to transfer BM isolated from these disease-free mice into Rag1 −/− recipients without the risk of contamination with mature pathogenic T cells. In these experiments, Foxp3sf nude BM reconstitution of neonatal Rag1 −/− mice and Foxp3sfRag1 −/− mice (serving as a positive control) resulted in identical lethal lymphoproliferative disease (Fig. 2 D). In contrast, control Foxp3wt nude BM transfers into either neonatal Rag1 −/− or Foxp3sfRag1 −/− did not result in disease (Fig. 2 D). The findings above demonstrate that genetic ablation of Foxp3 in the thymic epithelium is neither necessary nor sufficient for the development of autoimmune disease.
Distorted thymopoiesis associated with Foxp3 deficiency is secondary to lymphoproliferative autoimmune syndrome
Lack of autoimmunity in Foxp3wt nude → Foxp3sf Rag1 −/− BM chimeras and in Foxp3flox Foxn1Cre mice did not exclude the previously proposed role for expression of Foxp3 in the thymic epithelium in normal thymopoiesis (16). Major thymic aberrations reported for Foxp3 mutant mice include a decrease in thymic cellularity and in the proportion of CD4+CD8+ DP thymocytes (16). To reexamine this possibility, we analyzed thymopoiesis in Foxp3 −, Foxp3flox CD4-Cre, and Foxp3flox Foxn1Cre mice and found that both Foxp3 − and the Foxp3fl CD4-Cre mice showed reduced total thymic cellularity and a decrease in the percentage of CD4+CD8+ DP thymocytes, whereas Foxp3flox Foxn1Cre mice were identical to their wild-type littermates (Fig. 3, A and B). These results demonstrate that deletion of the Foxp3 gene in thymic epithelial cells does not result in detectable changes in thymic T cell maturation, and that an apparent decrease in DP thymocyte subset size and in total thymocyte numbers is due to loss of Foxp3 in thymocytes rather than in the thymic epithelium and is most likely secondary to the severe autoimmunity and cytokine storm common to the Foxp3sf, Foxp3 −, and Foxp3fl CD4-Cre mice.
Figure 3.
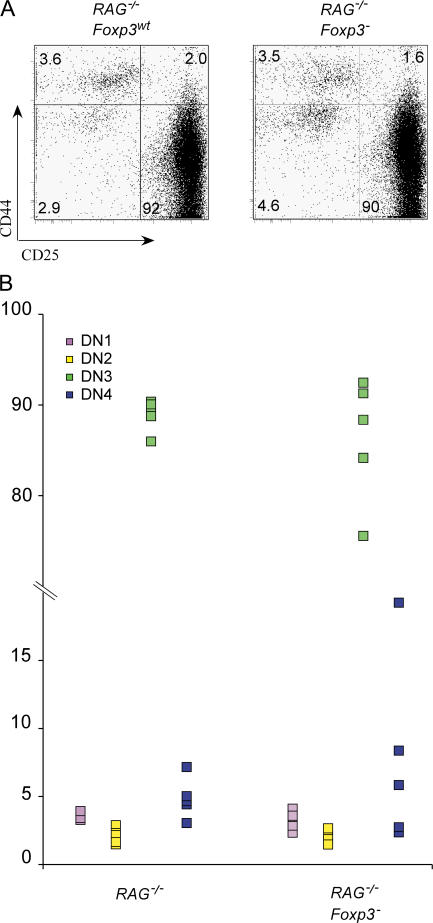
Distorted thymopoiesis is dependent on Foxp3 loss in the T cell lineage. Thymopoiesis was assessed by examining total thymus cellularity and by flow cytometric analysis of thymocyte subsets. (A) Representative CD4 and CD8 thymocyte profiles for wild-type, Foxp3-deficient, Foxp3fl CD4-Cre, and Foxp3fl Foxn1Cre mice. Average thymus cellularity (mean ± SD) is shown below each graph. (B) Percentages of thymocytes in CD4−CD8− DN, CD4+CD8+ DP, CD4+ single positive, and CD8+ single positive subsets. Each data point represents a single mouse. (C) Representative flow cytometric profiles of DN thymocyte subsets and (D) percentages of DN1, DN2, DN3, and DN4 subsets in wild-type, Foxp3-deficient, Foxp3fl CD4-Cre, and Foxp3fl Foxn1Cre mice.
In addition to the aforementioned changes in thymocyte subsets, an expansion of DN1 (CD44+CD25−) cells was reported for both Foxp3sf and Foxp3sf Rag −/− mice (16). To examine this phenomenon, we first analyzed the composition of DN thymocyte subsets in Foxp3 −, Foxp3fl CD4-Cre, and Foxp3fl Foxn1Cre mice. The relative size of the DN1-4 thymocyte subsets was not altered in Foxp3fl Foxn1Cre as compared with control mice; however, we found relative increases in the DN1 subset in Foxp3 − and Foxp3fl CD4-Cre mice (Fig. 3, C and D). To determine whether this is a cell-intrinsic defect in developing thymocytes, we analyzed DN thymocyte subsets in disease-free Foxp3 − Rag2 −/− and control Foxp3wtRag2 −/− littermates. No differences in the relative sizes of DN1, DN2, and DN3 subsets were detected in these mice (Fig. 4, A and B). To exclude the possibility that very few thymic epithelial cells escaping Cre-mediated deletion in Foxp3flox × Foxn1Cre mice are capable of supporting normal thymocyte development, we generated an additional set of BM chimeras by transferring wild-type BM into Foxp3 − Rag2 −/− and Foxp3wtRag2 −/− recipients. The maturation of wild-type thymocytes was not different in the two sets of chimeric mice (Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20062465/DC1). Together with the absence of expression of Foxp3 in DN thymocytes, these experiments show that the changes in DN thymocyte maturation observed in Foxp3 mutant mice are secondary effects of massive peripheral T cell activation.
Figure 4.
Foxp3 deficiency does not affect early thymopoiesis in disease-free Rag-deficient mice. (A) Representative flow cytometric analysis of DN1, DN2, DN3, and DN4 thymocyte subsets and (B) their percentages in Rag2 −/− and Foxp3-deficient Rag2 −/− mice. Each point represents a single mouse (n = 5). No statistically significant differences were observed between two groups of mice.
Collectively, our data demonstrates that the thymic epithelium does not express detectable amounts of Foxp3 protein, and that there are no measurable adverse effects on immunological tolerance attributable to Foxp3 deficiency in thymic stromal cells or in other radiation-resistant nonhematopoietic cells. This conclusion is strongly supported by the lack of autoimmune manifestations and changes in thymocyte maturation upon Foxn1-Cre–mediated ablation of a conditional Foxp3 allele in the thymic epithelium. In contrast, in control experiments, Foxp3 ablation in the T cell lineage resulted in lethal autoimmune pathology typical of germline Foxp3 mutation, as previously reported. In full agreement with these data are the results of the BM transfer experiments, which showed that Foxp3 deficiency in hematopoietic cells is solely responsible for autoimmunity, and that perturbed thymocyte subsets in Foxp3-deficient mice are an indirect consequence of pathology. Thus, in mice the only known role for Foxp3 remains promotion of T reg cell differentiation within the T cell lineage.
MATERIALS AND METHODS
Mice.
Foxp3− (14), Foxp3fl (14), Foxp3GFP (15), CD4-Cre (18), Foxn1ex9cre (unpublished data; Cre was inserted along with an internal ribosome entry site into the 3′ untranslated region of the Foxn1 gene), ROSA-stopfl-YFP (19), Foxp3sf, nude, Rag1−/−, and Rag2−/− mice have all been backcrossed to the B6 background. B6 Foxp3sf mice were backcrossed either to the B6 nude or B6 Rag1−/− backgrounds two generations to produce Foxp3sf nude and Foxp3sf Rag1−/− strains. BM chimeras were constructed using 7 × 106 BM cells/recipient harvested from athymic nude male mice with or without the Foxp3sf mutation and injected i.p. into neonatal (2–3 d) Rag1−/− with or without the Foxp3sf. Experimental mice were age and sex matched and housed in specific pathogen-free conditions. Disease incidence was monitored by frequent visual observation, and postmortem histological analysis of the tissues was performed using hematoxylin and eosin staining (Histology Consultation Services). All mice were used in accordance with guidelines from the Institutional Animal Care Committee of the University of Washington.
Flow cytometry and immunofluorescence.
5–10-wk-old mice were analyzed using the following antibodies: CD45-APC, I-Ab–PE, CD4-PE-Cy7, CD44-PE, CD8-PerCP, CD25-APC, CD8-FITC, CD4-PerCP, and UEA-1 biotin, followed by SAV-PerCP (all from BD Biosciences) and G8.8 supernatant conjugated to Alexa 647. Thymic stroma preparations were enriched from three to six pooled thymi from 5–10-wk-old mice as described previously (20). After enzymatic enrichment, CD45− thymic stromal cells were purified using CD45 microbeads (Miltenyi Biotec) and the AutoMACS system (Miltenyi Biotec) as per the manufacturer's recommendations before flow cytometric analysis (21). Thymic sections were prepared and stained as described previously (22) using rabbit polyclonal IgG anti-Foxp3 antibodies (14), followed by Alexa 546–conjugated goat anti–rabbit IgG. Images were acquired using a Leica SP1/MP confocal microscope.
Online supplemental material.
Fig. S1 shows early thymopoiesis for wild-type BM-derived thymocytes developing in Foxp3-sufficient and Foxp3-deficient hosts assessed by CD44/CD25 flow cytometric profiles of CD4−CD8− thymocytes 4 wk after BM transfer. Fig. S1 is available at http://www.jem.org/cgi/content/full/jem.20062465/DC1.
Acknowledgments
We thank K. Forbush, L. Karpik, and T. Chu for coordinating mouse colonies.
This work was supported by grants from the National Institutes of Health (NIH) and the Juvenile Diabetes Research Foundation. A. Liston is an Abbot Research Fellow-Irvington Fellow, and Z. Chen was supported by an NIH T32 Training Grant. A.Y. Rudensky is a Howard Hughes Medical Institute investigator.
The authors have no conflicting financial interests.
Z. Chen's present address is Dept. of Microbiology and Immunology, University of Miami, Miami, FL 33136.
References
- 1.Wildin, R.S., S. Smyk-Pearson, and A.H. Filipovich. 2002. Clinical and molecular features of the immunodysregulation, polyendocrinopathy, enteropathy, X linked (IPEX) syndrome. J. Med. Genet. 39:537–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Godfrey, V.L., J.E. Wilkinson, E.M. Rinchik, and L.B. Russell. 1991. Fatal lymphoreticular disease in the scurfy (sf) mouse requires T cells that mature in a sf thymic environment: potential model for thymic education. Proc. Natl. Acad. Sci. USA. 88:5528–5532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bennett, C.L., and H.D. Ochs. 2001. IPEX is a unique X-linked syndrome characterized by immune dysfunction, polyendocrinopathy, enteropathy, and a variety of autoimmune phenomena. Curr. Opin. Pediatr. 13:533–538. [DOI] [PubMed] [Google Scholar]
- 4.Ferguson, P.J., S.H. Blanton, F.T. Saulsbury, M.J. McDuffie, V. Lemahieu, J.M. Gastier, U. Francke, S.M. Borowitz, J.L. Sutphen, and T.E. Kelly. 2000. Manifestations and linkage analysis in X-linked autoimmunity-immunodeficiency syndrome. Am. J. Med. Genet. 90:390–397. [DOI] [PubMed] [Google Scholar]
- 5.Godfrey, V.L., J.E. Wilkinson, and L.B. Russell. 1991. X-linked lymphoreticular disease in the scurfy (sf) mutant mouse. Am. J. Pathol. 138:1379–1387. [PMC free article] [PubMed] [Google Scholar]
- 6.Chatila, T.A., F. Blaeser, N. Ho, H.M. Lederman, C. Voulgaropoulos, C. Helms, and A.M. Bowcock. 2000. JM2, encoding a fork head-related protein, is mutated in X-linked autoimmunity-allergic disregulation syndrome. J. Clin. Invest. 106:R75–R81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clark, L.B., M.W. Appleby, M.E. Brunkow, J.E. Wilkinson, S.F. Ziegler, and F. Ramsdell. 1999. Cellular and molecular characterization of the scurfy mouse mutant. J. Immunol. 162:2546–2554. [PubMed] [Google Scholar]
- 8.Blair, P.J., S.J. Bultman, J.C. Haas, B.T. Rouse, J.E. Wilkinson, and V.L. Godfrey. 1994. CD4+CD8− T cells are the effector cells in disease pathogenesis in the scurfy (sf) mouse. J. Immunol. 153:3764–3774. [PubMed] [Google Scholar]
- 9.Zahorsky-Reeves, J.L., and J.E. Wilkinson. 2001. The murine mutation scurfy (sf) results in an antigen-dependent lymphoproliferative disease with altered T cell sensitivity. Eur. J. Immunol. 31:196–204. [DOI] [PubMed] [Google Scholar]
- 10.Godfrey, V.L., B.T. Rouse, and J.E. Wilkinson. 1994. Transplantation of T cell-mediated, lymphoreticular disease from the scurfy (sf) mouse. Am. J. Pathol. 145:281–286. [PMC free article] [PubMed] [Google Scholar]
- 11.Smyk-Pearson, S.K., A.C. Bakke, P.K. Held, and R.S. Wildin. 2003. Rescue of the autoimmune scurfy mouse by partial bone marrow transplantation or by injection with T-enriched splenocytes. Clin. Exp. Immunol. 133:193–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khattri, R., T. Cox, S.A. Yasayko, and F. Ramsdell. 2003. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat. Immunol. 4:337–342. [DOI] [PubMed] [Google Scholar]
- 13.Hori, S., T. Nomura, and S. Sakaguchi. 2003. Control of regulatory T cell development by the transcription factor Foxp3. Science. 299:1057–1061. [DOI] [PubMed] [Google Scholar]
- 14.Fontenot, J.D., M.A. Gavin, and A.Y. Rudensky. 2003. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 4:330–336. [DOI] [PubMed] [Google Scholar]
- 15.Fontenot, J.D., J.P. Rasmussen, L.M. Williams, J.L. Dooley, A.G. Farr, and A.Y. Rudensky. 2005. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 22:329–341. [DOI] [PubMed] [Google Scholar]
- 16.Chang, X., J.X. Gao, Q. Jiang, J. Wen, N. Seifers, L. Su, V.L. Godfrey, T. Zuo, P. Zheng, and Y. Liu. 2005. The Scurfy mutation of FoxP3 in the thymus stroma leads to defective thymopoiesis. J. Exp. Med. 202:1141–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang, X., P. Zheng, and Y. Liu. 2006. FoxP3: a genetic link between immunodeficiency and autoimmune diseases. Autoimmun. Rev. 5:399–402. [DOI] [PubMed] [Google Scholar]
- 18.Wolfer, A., T. Bakker, A. Wilson, M. Nicolas, V. Ioannidis, D.R. Littman, P.P. Lee, C.B. Wilson, W. Held, H.R. MacDonald, and F. Radtke. 2001. Inactivation of Notch 1 in immature thymocytes does not perturb CD4 or CD8T cell development. Nat. Immunol. 2:235–241. [DOI] [PubMed] [Google Scholar]
- 19.Srinivas, S., T. Watanabe, C.S. Lin, C.M. William, Y. Tanabe, T.M. Jessell, and F. Costantini. 2001. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev. Biol. 1:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dooley, J., M. Erickson, and A.G. Farr. 2005. An organized medullary epithelial structure in the normal thymus expresses molecules of respiratory epithelium and resembles the epithelial thymic rudiment of nude mice. J. Immunol. 175:4331–4337. [DOI] [PubMed] [Google Scholar]
- 21.Gray, D.H., A.P. Chidgey, and R.L. Boyd. 2002. Analysis of thymic stromal cell populations using flow cytometry. J. Immunol. Methods. 260:15–28. [DOI] [PubMed] [Google Scholar]
- 22.Lehar, S.M., J. Dooley, A.G. Farr, and M.J. Bevan. 2005. Notch ligands Delta 1 and Jagged1 transmit distinct signals to T-cell precursors. Blood. 105:1440–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]