Abstract
Interleukin (IL) 31Rα (glycoprotein 130–like monocyte receptor and glycoprotein 130–like receptor) heterodimerizes with oncostatin M receptor β to bind IL-31, a cytokine expressed preferentially by CD4+ T helper type 2 (Th2) cells. However, the functions of IL-31–IL-31R signaling in immune regulation remain unknown. Here, we identify a novel role for IL-31R in limiting type 2 inflammation in the lung. After intravenous injection of Schistosoma mansoni eggs, IL-31Rα−/− mice developed severe pulmonary inflammation, characterized by an increase in the area of granulomatous inflammation, increased numbers of resistin-like molecule α+ cells, and enhanced collagen deposition compared to WT counterparts. In vitro, macrophages generated from IL-31Rα−/− mice promoted enhanced ovalbumin-specific CD4+ T cell proliferation and purified naive CD4+ T cells from IL-31Rα−/− mice exhibited enhanced proliferation and expression of Th2 cytokines, identifying a T cell– and macrophage-intrinsic regulatory function for IL-31R signaling. In contrast, the generation of CD4+ T cell–mediated Th1 responses were normal in IL-31Rα−/− mice, suggesting that the regulatory role of IL-31R signaling is limited to type 2 responses. Together, these data implicate IL-31R signaling as a novel negative regulatory pathway that specifically limits type 2 inflammation.
The glycoprotein 130 (gp130) family constitutes the signaling receptors for the IL-6/IL-12 family of cytokines that includes IL-6, -11, -12, -23, and -27, cardiotrophin-1, cardiotrophin-like cytokine, leukemia inhibitory factor, and oncostatin M (1), many of which have important pro- and antiinflammatory functions as well as roles in regulating the development and differentiation of immune cells (2, 3). The most recent addition to this family was the gp130-like monocyte receptor or IL-31Rα, which heterodimerizes with oncostatin M receptor β to form the IL-31R signaling complex and is expressed in the testis, thymus, bone marrow, and skin (4–7). Signaling via IL-31R results in the phosphorylation of STAT1, STAT5, and, most prominently, STAT3 (4–7). The ligand for IL-31R, IL-31, is expressed by activated CD4+ T cells, predominantly of the Th2 subset. Recent studies in both humans and mice have suggested a link between IL-31–IL-31R expression and skin inflammation (7–11) although the functional significance of endogenous IL-31– IL-31R interactions in influencing innate and adaptive immune responses remains unknown. In this paper, we describe the generation of IL-31Rα−/− mice and demonstrate a role for IL-31R signaling in limiting the magnitude of type 2 inflammation in the lung.
RESULTS AND DISCUSSION
Normal development and composition of the immune system in IL-31Rα−/− mice
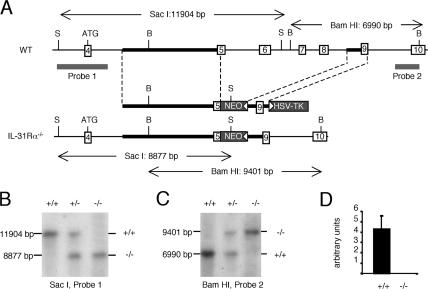
To determine the functions of endogenous IL-31–IL-31R interactions, IL-31Rα−/− mice were generated by homologous recombination. The four exons encoding the ligand-binding cytokine receptor homology domain (exons 5–8) were replaced by a neomycin resistance cassette (Fig. 1 A). Correct homologous recombination was confirmed by Southern blot analysis (Fig. 1, B and C) and the absence of IL-31Rα expression confirmed by Taqman PCR (Fig. 1 D). Comprehensive flow cytometric analysis of thymocytes, splenocytes, lymph node cells, and peripheral blood leukocytes did not indicate any substantial differences between naive WT and IL-31Rα−/− animals in the composition of immune cells in these compartments (Table S1, available at http://www.jem.org/cgi/content/full/jem.20061791/DC1). Importantly, analysis of peripheral blood did not reveal substantial anemia or thrombocytopenia, which has been reported for mice deficient in oncostatin M receptor β (12).
Figure 1.
Generation of IL-31Rα−/− mice. (A) The relevant section of the native IL-31Rα locus (top), the targeting construct (middle), and the correctly targeted locus (bottom) are depicted. Open boxes with numbers indicate exons and gray boxes indicate the selection genes neomycin phosphotransferase (NEO) and thymidine kinase (TK), with white arrowheads depicting their transcriptional orientation. Thick black lines indicate the position of the 5′ and 3′ homology arms. The size of restriction fragments resulting from digestion with SacI (S) and BamHI (B) are indicated, and the location of the two probes used to detect these fragments by Southern blot are shown by thick gray lines. (B and C) Southern blot analysis of SacI digests probed with probe 1 and BamHI digests probed with probe II, respectively. Genotype is indicated as follows: +/+, WT; +/−, heterozygous; −/−, IL-31Rα−/−. (D) Expression of IL-31Rα relative to rpl19 was determined by quantitative Taqman PCR.
Mice deficient in IL-31Rα develop exacerbated type 2 inflammation in the lung after Schistosoma mansoni egg injection
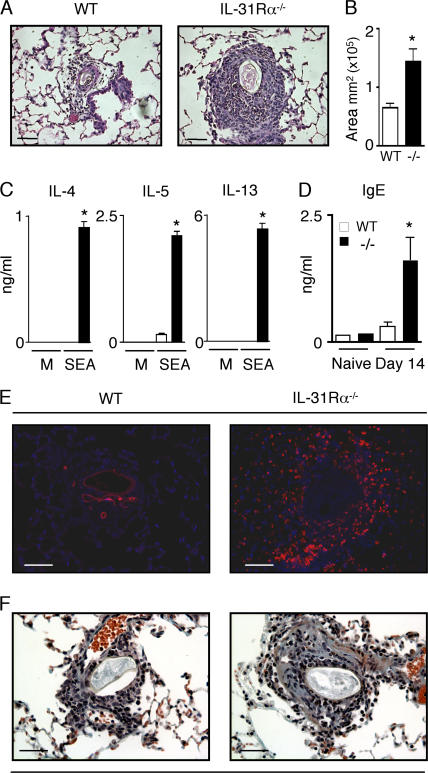
Given the predominant expression of IL-31 in Th2 cells (7), we investigated the potential regulatory functions of IL-31–IL-31R interactions in an in vivo model of type 2 inflammation. Upon intravenous injection, S. mansoni eggs travel through the bloodstream and become lodged in the small blood vessels of the lung where they induce the formation of IL-4– and IL-13–dependent type 2 granulomas (13). After delivery of S. mansoni eggs, WT mice developed characteristic egg-induced granulomas composed of a cuff of epithelioid macrophages surrounded by granulocytes and lymphocytes (Fig. 2 A). In contrast, egg-injected IL-31Rα−/− mice developed more severe inflammation in the lung parenchyma associated with a greater than twofold increase in the mean area of inflammation surrounding each egg as well as increased granulocyte infiltration (Fig. 2, A and B). Draining mediastinal lymph node cells were isolated from egg-injected WT and IL-31Rα−/− mice and restimulated with S. mansoni egg antigen. The S. mansoni egg-induced granuloma model can either use preimmunization with S. mansoni eggs systemically followed by i.v. challenge or direct i.v. primary challenge without prior sensitization. Direct primary challenge i.v. results in relatively low expression of Th2 cytokines but in the development of granulomas in the lung parenchyma (14). Consistent with this, after primary challenge with S. mansoni eggs, lymph node cells isolated from injected WT mice produced detectable levels of IL-5 but no IL-4 or IL-13. In contrast, lymph node cells from IL-31Rα−/− mice exhibited significantly higher levels of IL-4, IL-5, and IL-13 after antigen-specific restimulation (P < 0.05; Fig. 2 C). No cytokines were detected in antigen-stimulated lymph node cells from naive WT or IL-31Rα−/− mice (unpublished data). S. mansoni egg-injected IL-31Rα−/− mice also exhibited significantly increased serum IgE levels compared with WT mice (Fig. 2 D), which is consistent with increased expression of Th2 cytokines.
Figure 2.
Enhanced S. mansoni egg–induced type 2 inflammation in the lungs of IL-31Rα−/− mice. (A and B) Paraffin sections of lungs from egg-injected mice at day 14 after injection were H &E stained and the area of inflammation surrounding WT (open bar) and IL-31Rα−/− (closed bar) S. mansoni egg-induced granulomas was measured using OpenLab software. Mean ± SEM; n = 40 granulomas per group. (C) Draining mediastinal lymph node cells from egg-injected WT and IL-31Rα−/− mice were stimulated with either media or 20 μg/ml S. mansoni egg antigen (SEA) for 48 h. Supernatants were analyzed by ELISA for IL-4, -5, and -13. Mean ± SEM of replicate cultures. (D) Serum levels of IgE from WT and IL-31Rα−/− mice were measured by ELISA. Mean ± SEM; n = 3 mice per group. (E) Immunofluorescence staining for RELM-α (red) and counterstain for DAPI (blue). (F) Collagen deposition in the lungs of WT and IL-31Rα−/− mice was detected by Masson's trichrome staining; blue staining demarks collagen. Results are representative of three independent experiments of three mice per group. Asterisk indicates statistically significant as determined by two-tailed Student's t test (P < 0.05). Bars, 60 μm.
Alternatively activated macrophages (AAMs) are a dominant feature of S. mansoni egg-induced granulomas (15). Differentiation of AAMs is dependent on Th2 cytokines, and this macrophage population expresses arginase and resistin-like molecule α (RELM-α) and has been implicated in granuloma formation, tissue remodeling, and fibrosis (16–18). Consistent with exacerbated expression of Th2 cytokines and increases in AAMs, the frequency of RELM-α+ cells was increased in the lung granulomas of egg-injected IL-31Rα−/− mice (Fig. 2 E). RELM-α and arginase expression have been correlated with increases in collagen deposition and fibrosis (19–21), and, accordingly, elevated collagen deposition was observed in the lungs of injected IL-31Rα−/− mice compared with WT counterparts (Fig. 2 F). In total, the increased granuloma sizes, exacerbated Th2 cytokine production, high serum IgE levels, elevated frequency of RELM-α+ cells, and enhanced collagen deposition in the lung parenchyma are indicative of dysregulated acute type 2 inflammation in the absence of IL-31R signaling.
IL-31R signaling influences the proliferation and cytokine production of naive CD4+ T cells after polyclonal stimulation
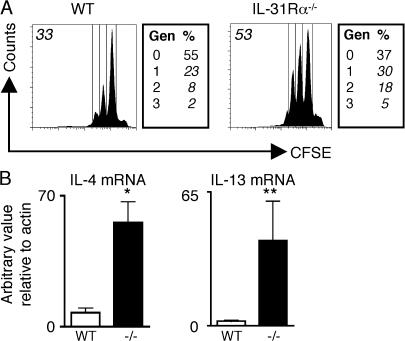
S. mansoni egg-induced granuloma formation is dependent on CD4+ Th2 cytokine responses, and a previous study has reported that IL-31 and IL-31R are up-regulated by activated human T cells (7). To test if IL-31R signaling directly influenced CD4+ T cell function and could contribute to the dysregulated type 2 inflammation in the lungs of IL-31Rα−/− mice, purified naive CD44lowCD62Lhigh CD4+ T cells from WT or IL-31Rα−/− mice were CFSE labeled and stimulated under neutral conditions with plate-bound anti-CD3/anti-CD28. 4 d after stimulation, IL-31Rα−/− CD4+ T cells exhibited enhanced proliferation compared with WT counterparts (Fig. 3 A). Upon activation, purified naive CD4+ T cells from IL-31Rα−/− mice also expressed significantly higher levels of IL-4 messenger RNA (mRNA; P < 0.05) and consistently elevated levels of IL-13 mRNA (Fig. 3 B). However, under Th2-polarizing conditions, similar proliferation and expression of IL-4 and IL-13 was observed between WT and IL-31Rα−/− CD4+ T cells, suggesting that IL-31–IL-31R interactions do not regulate differentiated Th2 cells (Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20061791/DC1). Rather, these data demonstrate that IL-31R signaling can directly influence the proliferation and expression of Th2 effector cytokines by naive CD4+ T cells.
Figure 3.
Naive IL-31Rα−/− CD4+ T cells exhibit enhanced proliferation and expression of Th2 effector cytokines. (A) Purified naive CD4+ T cells from WT or IL-31Rα−/− mice were CFSE labeled and stimulated with plate-bound anti-CD3/anti-CD28 for 4 d under neutral conditions. Boxes to the right indicate the percentage of events in each proliferating generation (Gen). Numbers in italics in upper left indicate percentage of CFSE-dim cells. (B) cDNA derived from stimulated cells was assayed by real-time PCR for IL-4 or IL-13 mRNA levels relative to actin. Results expressed as the mean ± SEM for three independent experiments. *, P < 0.05; **, P < 0.1.
IL-31Rα−/− macrophages exhibit enhanced accessory cell function
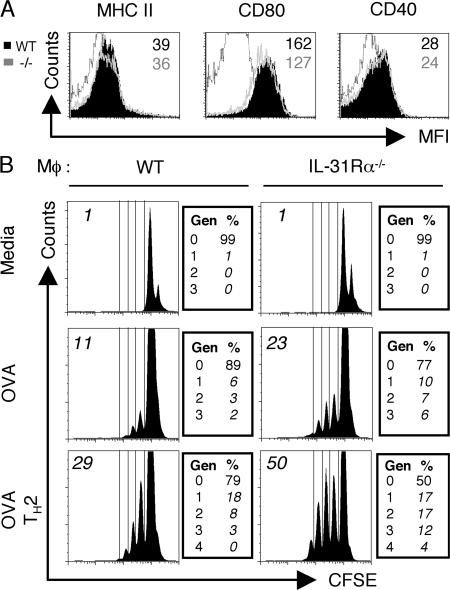
Macrophage infiltration is a characteristic of S. mansoni egg-induced granulomas and the alternative activation of macrophages is necessary for survival of mouse hosts during S. mansoni infection (15). It has been previously reported that resting and activated human monocytes express IL-31Rα (5–7) and we have observed the induction of IL-31Rα expression on stimulated mouse macrophages (unpublished data). To address a role for IL-31R signaling in the regulation of macrophage function, WT and IL-31Rα−/− macrophages were pulsed overnight with OVA protein in the presence or absence of recombinant IL-4 and co-cultured with CFSE-labeled OTII transgenic OVA-specific T cells. Flow cytometric analysis of WT and IL-31Rα−/− bone marrow–derived macrophages revealed no considerable differences in surface expression of MHC class II, CD80, or CD40 (Fig. 4 A). However, under both neutral and Th2-polarizing conditions, T cells co-cultured with IL-31Rα−/− macrophages proliferated to a greater extent than those cultured with WT macrophages (Fig. 4 B). When macrophages were stimulated with LPS and co-cultured with OVA-specific CD4+ T cells under Th1-polarizing conditions there were no differences in CD4+ T cell proliferation (Fig. S2, available at http://www.jem.org/cgi/content/full/jem.20061791/DC1). These data support a role for IL-31R signaling in limiting innate responses by negatively regulating the T cell stimulatory function of macrophages.
Figure 4.
IL-31Rα−/− macrophages exhibit enhanced accessory cell function. (A) Bone marrow–derived macrophages from either WT (filled black) or IL-31Rα−/− (gray line) mice were characterized for surface molecule expression of MHC II, CD80, or CD40. Numbers refer to mean fluorescence intensity (black, WT; gray, IL-31Rα−/−). Black line is isotype control. (B) WT or IL-31Rα−/− macrophages were pulsed overnight with OVA protein in the presence or absence of IL-4 when indicated. CFSE-labeled OTII T cells were added 24 h after OVA pulse and co-cultured with macrophages for 4 d under neutral or Th2-polarizing conditions. Histograms are gated on the live CD4+ population; boxes to the right indicate the percentage of events in each proliferating generation (Gen). Numbers in italics in top left indicate percentage of CFSE-dim cells. Data are representative of six independent experiments.
IL-31R signaling specifically limits type 2 immune responses
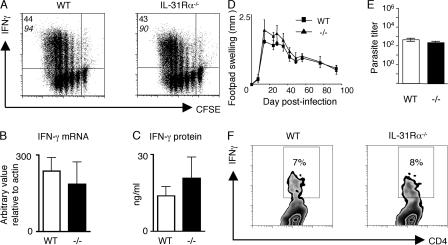
Whereas IL-31 is made predominantly by CD4+ Th2 cells, IL-31 mRNA expression is modestly induced by CD4+ T cells stimulated under both neutral and Th1 conditions (7). We therefore investigated whether IL-31–IL-31R signaling could negatively regulate type 1 immune responses in vitro or in vivo. Purified CD4+ T cells from naive WT and IL-31Rα−/− mice stimulated polyclonally under Th1 polarizing conditions exhibited no differences in their ability to proliferate or acquire expression of IFN-γ as measured by intracellular cytokine staining, real-time PCR, or ELISA (Fig. 5, A–C), indicating that IL-31R signaling does not influence Th1 cell differentiation in vitro. Furthermore, after infection with the intracellular parasite Leishmania major, immunity to which is critically dependent on IFN-γ production by CD4+ T cells (22), WT and IL-31Rα−/− mice exhibited equivalent footpad swelling, with peak lesions observed at 4 wk after infection and control of parasite replication and resolution of cutaneous inflammation occurring by week 12 (Fig. 5, D and E). Consistent with the in vitro T cell differentiation data (Fig. 5 A), the frequency of L. major–specific IFN-γ–producing CD4+ T cells in the draining popliteal lymph node was similar in infected WT and IL-31Rα−/− mice, indicating normal Th1 cell polarization in IL-31Rα−/− mice (Fig. 5 F). IL-31Rα−/− mice also displayed equivalent type 1 cytokine-dependent resistance after infection with Listeria monocytogenes (unpublished data). These results suggest that IFN-γ responses are not influenced by IL-31R signaling and support a role for IL-31–IL-31R interactions as a distinct negative feedback pathway operating during type 2 inflammation.
Figure 5.
Normal Th1 cell differentiation in IL-31Rα−/− mice. (A) Purified CD4+ T cells from WT and IL-31Rα−/− mice were CFSE labeled and stimulated with plate-bound anti-CD3/anti-CD28 under Th1-polarizing conditions. Cells were assayed for proliferation and cytokine production by flow cytometry. The top number indicates the percentage of proliferating CD4+ T cells producing IFN-γ and the bottom number in italics is the percentage of CFSE-dim cells. (B) cDNA from purified naive CD4+ T cells cultured under Th1-polarizing conditions was subjected to real-time PCR analysis for IFN-γ expression relative to actin. (C) Supernatants from the cultures in A were assayed by ELISA for IFN-γ levels. (D) WT and IL-31Rα−/− mice were infected with L. major promastigotes in the hind footpad. Lesion size was determined by measuring swelling of the infected footpad and subtracting that of the uninfected contralateral footpad. (E) Parasite load in the footpad was quantified by limiting dilution analysis at 12 wk after infection. Values represent the mean ± SEM for three mice per group. (F) Cells from the draining lymph nodes of L. major– infected WT or IL-31Rα−/− mice were restimulated with soluble L. major antigen and analyzed by flow cytometry. Plots are gated on CD4+ cells and the number in the box indicates the percentage of CD4+ T cells producing IFN-γ.
We have identified IL-31–IL-31R signaling as a novel negative regulator of type 2 inflammation in the lung that can directly influence the function of antigen-presenting cells and CD4+ T cells. A recent study demonstrated IL-31 transgenic mice develop spontaneous dermatitis characterized by alopecia and cutaneous inflammation, suggesting IL-31 may have a role in promoting inflammation (7). The demonstration of a negative regulatory role for IL-31–IL-31R signaling in type 2 inflammation presented in this paper provides a mechanistic explanation for the observations made in the IL-31 transgenic mouse model. Rather than promoting Th2 cytokine-mediated disease, the overexpression of IL-31 in transgenic mice may actively suppress Th2 responses with consequential augmentation of Th1 cytokine-mediated inflammation in the skin. Although no analysis of cytokine expression in the skin of IL-31 transgenic mice was performed in that study (7), the elevated type 2 inflammation observed in the lungs of IL-31Rα−/− mice is consistent with this interpretation of the results.
In the present study, we identify a regulatory function for IL-31–IL-31R interactions in an acute model of type 2 inflammation in the lung; although whether this pathway influences pathologic consequences of chronic type 2 inflammation such as fibrosis and tissue remodeling will require further investigation. Notwithstanding this, a negative regulatory function for IL-31R is supported by in vitro studies that identified STAT3, a transcription factor associated with suppression of DC, T cell, NK, and neutrophil functions (23), as the dominant STAT pathway activated after IL-31R ligation (4–7). The IL-27–IL-27R pathway is another gp130 family member that can negatively regulate inflammatory responses in multiple disease settings (24–27). However, the IL-31–IL-31R pathway is unique in at least two aspects. First, IL-31 is expressed by differentiated CD4+ Th2 cells, whereas IL-27 is expressed by antigen-presenting cells. Second, unlike IL-27–IL-27R interactions, IL-31R–mediated suppression appears to be specific for Th2 cytokine responses as deficiency in IL-31Rα does not influence Th1 cell differentiation in vitro or the magnitude of the Th1 cytokine response to intracellular pathogens. This specificity in function highlights the potential of manipulating the IL-31–IL-31R signaling pathway in the treatment of inflammatory diseases characterized by overexpression of Th2 cytokines at mucosal sites.
MATERIALS AND METHODS
Mice.
WT C57BL/6 mice and OTII transgenic mice were ordered from the Jackson Laboratory. Mice were bred in a specific pathogen–free environment at the University of Pennsylvania. All experiments were performed following the guidelines of the University of Pennsylvania Institutional Animal Care and Use Committee. IL-31Rα−/− mice were generated by constructing a targeting vector that deleted the cytokine receptor homology domain of IL-31Rα (encoded by exons 5–8 of the mouse transcript) from the following DNA fragments: a 5′ homology arm of 5,289 bp was a PCR-amplified fragment from C57/BL6 genomic DNA using primers 5′-TTAATTAAATCTACATGTGTGCGGAGGC-3′ and 5′-GCGGCCGCATGTTCTCTGGCTTAGTCGGCAGG-3′; a PGK-neo resistance cassette; a 3′ homology arm of 1,108 bp short arm defined by primers 5′-GGCGCGCCAGGGGTGGTGGTGGATGGAT-3′ and 5′-GGCGCGCCGTCTACAGGGTTAACCTA-3′ (Fig. 1 A); and an HSV-driven thymidine kinase selection cassette for negative selection. This construct was electroporated into C57BL/6 embryonic stem (ES) cells, and homologous recombination occurred in 4 out of 200 clones after selection with G418 and ganciclovir. To verify correct targeting of the locus, genomic DNA from ES cells and animals was analyzed by Southern blot. Digestion with SacI followed by hybridization of membranes with probe 1 (a 2,400-bp genomic DNA fragment obtained by PCR with oligos 5′-GAGCTCCCGGGATCACGTCC-3′ [sense] and 5′-AGGCCTCCTCTGGAGCTGGG-3′ [antisense]) yielded an 11,904-bp fragment for the WT allele and an 8,877-bp fragment for the correctly targeted mutant allele. Similarly, digestion of genomic DNA with BamHI followed by hybridization of membranes with probe 2 (a 1,028-bp genomic DNA fragment obtained by PCR with oligos 5′-ATCGATAGGTTCAGTGGTA-3′ [sense] and 5′-AAGTACTGTATGTGGTAGCC-3′ [antisense]) yielded a 6,990-bp fragment for the WT allele and a 9,401-bp fragment for the correctly targeted mutant allele. Two ES cell clones were injected into blastocysts, and animals that transmitted the mutant allele in their germline were obtained. For genotyping, a PCR-based method with a common antisense primer (5′-GGCAGTAACTGAAGTAACAG-3′) in combination with WT- (5′-CAAAGTCACAATGTAGCTGG-3′) and knockout-specific (5′-CGCCTTCTTGACGAGTTCTT-3′) sense primers was used. This primer-triplet amplifies a 492-bp fragment for the WT allele and an 808-bp fragment for the mutant allele. PCR was performed in a thermocycler (model PE9700; PerkinElmer) using the following conditions: 1 cycle of 94°C, 4'; 35 cycles of 94°C, 30", 60°C, 30", and 72°C, 60"; and 1 cycle of 72°C, 7'. IL-31Rα−/− mice were born at Mendelian frequencies and were equivalent in size, weight, and fertility to littermate controls. No abnormalities in organ development or inflammation were found upon histopathological examination, and analysis of clinical chemistry and hematology parameters did not reveal differences between WT and IL-31Rα−/− mice.
FACS analysis of blood cell subsets.
Spleens, thymi, and lymph nodes were isolated from 6–8-wk-old mice and single cell suspensions were prepared. Peripheral blood was obtained by cardiac puncture and treated with EDTA to prevent coagulation, and erythrocytes were lysed using ACK lysing buffer (Biosource International). All cells were incubated for 30 min on ice in Hanks' balanced salt solution supplemented with 2% heat-inactivated bovine calf serum. Cells were stained with fluorochrome-conjugated antibodies against CD4, CD8, CD11b, CD11c, CD25, CD62L, B220, F4/80, and NK1.1 and analyzed using a FACSCalibur flow cytometry system (BD Biosciences).
S. mansoni granuloma model.
WT C57BL/6 or IL-31Rα−/− mice were injected i.v. with 5,000 S. mansoni eggs. At day 14 after primary injection, animals were killed. Lungs were inflated with 4% paraformaldehyde, embedded in paraffin, cut in 5-μm sections, and stained with hematoxylin and eosin (H&E) or Masson's trichrome. H&E sections were analyzed using Openlab software (Improvision) to assess the area of egg-induced inflammation by subtracting the area of the egg from the total area of the granuloma for each granuloma in a section. Paraffin sections were analyzed for expression of RELM-α by immunofluorescence via incubation with an anti–RELM-α antibody (1:1,000; PeproTech) followed by addition of a Cy3-conjugated anti-rat secondary antibody (1:600; The Jackson Laboratory). Serum was assayed by ELISA for IgE using OptIEA IgE kit (BD Biosciences). Single cell suspensions of draining cranial mediastinal lymph nodes from egg-injected mice or peripheral lymph nodes from naive mice were plated at 1.5 million/ml in complete media (DMEM [Life Technologies] supplemented with 10% heat-inactivated FBS, 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mM l-glutamine, and 50 μM 2-mercaptoethanol) and stimulated for 48 h with either media alone or 20 μg/ml of soluble S. mansoni egg antigen (SEA). Supernatants were assayed for IL-4, -5, and -13 using standard sandwich ELISA protocols.
T cell proliferation assays.
Naive CD4+ T cells were isolated via negative depletion with a naive CD4+ T cell column from R&D Systems (purity >90% CD4+, CD44low, and CD62Lhigh), labeled with 5 μg/ml CFSE (Invitrogen), and stimulated with 1 μg/ml of plate-bound anti-CD3/anti-CD28 (eBioscience) under neutral or Th2- (40 ng/ml IL-4 [R&D Systems], 10 μg/ml anti–IL-12 [C17.8], and 50 μg/ml anti–IFN-γ [XMG-6]) polarizing conditions. At day 4 cells were stimulated with 50 ng/ml PMA (Sigma-Aldrich), 500 ng/ml ionomycin (Sigma-Aldrich), and 10 μg/ml brefeldin A (Sigma-Aldrich) for 4 h. After stimulation, cells were surface stained for CD4, fixed, acquired on a FACSCalibur cytometer, and analyzed using FloJo software (Tree Star) for proliferation. Purified CD4+ T cells for in vitro Th1 cell differentiation assays were obtained by subjecting whole spleen and lymph node suspensions to negative selection via incubation with hybridoma supernatants (αB220, αFCR, αCD8, and αMHCII) followed by magnetic bead purification (QIAGEN), CFSE labeled, and stimulated with 1 μg/ml anti-CD3/anti-CD28 in the presence of 1 ng/ml rIL-12 and 10 μg/ml anti–IL-4 (11B11). On day 4, cells were harvested and subjected to flow cytometric analysis.
Real-time PCR.
RNA was purified from cells in culture per instructions using an RNeasy kit (QIAGEN). cDNA was generated per standard protocol with Superscript reverse transcriptase (Invitrogen) and used as input for real-time PCR. Taqman PCR reactions were run at standard conditions using a sequence detector (ABI 7500; Applied Biosystems). Taqman Assays on Demand (Applied Biosystems) were used to measure β-actin, IL-4, IL-13, and IFN-γ message levels.
Macrophage: T cell co-culture.
Macrophages were derived in vitro as follows. Bone marrow from C57BL/6 or IL-31Rα−/− mice was plated on 10-cm petri dishes in DMEM supplemented with 100 μg/ml penicillin/streptomycin, 10% FCS, 1% sodium pyruvate, 25 mM Hepes, and 30% l-cell–conditioned media. Media was changed at 3 d and cells were harvested at day 7. Bone marrow–derived macrophages from WT or IL-31Rα−/− mice were plated at 5 × 105 cells/well and pulsed overnight with 500μg/ml OVA protein (Worthington) in the presence or absence of 40 ng/ml IL-4 (R&D Systems). Purified OTII T cells were obtained by passing whole spleen and lymph node suspensions over T cell enrichment columns (R&D Systems). OTII T cells were CFSE labeled and co-cultured with macrophages at a 4:1 ratio at which time anti–IFN-γ and IL-4 were added for Th2-polarizing conditions in the IL-4–primed macrophage cultures. At day 4, cells were stimulated with PMA, ionomycin, and brefeldin A for 4 h and analyzed by flow cytometry. For Th1-polarizing conditions, macrophages were primed with 10 ng/ml LPS and OVA. OT II T cells were CFSE-labeled and added at a 4:1 ratio with 1 ng/ml IL-12 and 10 μg/ml anti–IL-4 (11B11).
L. major infection and parasite titers.
L. major parasites (MHOM/IL/80/ Friedlin) were grown in Grace's insect culture medium (Life Technologies) supplemented with 20% heat-inactivated FBS (HyClone Laboratories), 2 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin (Sigma-Aldrich). Soluble Leishmanial antigen was prepared as previously described (28). Mice were injected in the hind left footpad with 5 × 106 parasites. Footpad swelling was measured weekly using digital calipers (Mitutoyo), and lesion size was determined by subtracting the size of the uninfected contralateral footpad from the size of the infected footpad. To quantify parasites in the footpad, single-cell suspensions of lesions were prepared and plated in twofold serial dilutions (initial dilution of 1:100) in Grace's insect culture medium. Each sample was plated in quadruplicate, and the mean of the negative log parasite titer was determined after 7 d of culture at 26°C.
Statistics.
Statistical analysis was performed using a two-tailed Student's t test (*, P < 0.05).
Online supplemental material.
The immune cell composition of the thymus, spleen, lymph nodes, and peripheral blood of WT or IL-31Rα−/− mice is shown in Table S1. Naive WT and IL-31Rα−/− T cell proliferation and cytokine production under Th2-polarizing conditions is shown in Fig. S1. Fig. S2 shows the proliferation of OVA-specific TCR transgenic T cells isolated from OTII mice co-cultured with LPS-primed, OVA-pulsed WT or IL-31Rα−/− macrophages. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20061791/DC1.
Acknowledgments
We would like to acknowledge Justin Taylor and Leanne Johnson for technical assistance and the members of the Department of Pathobiology for helpful discussions.
This work was supported by National Institutes of Health (NIH) A161570 (D. Artis), NIH T32 Training Grant AI 007532-08 (J. Perrigoue), the Pilot Feasibility Program of the National Institute of Diabetes and Digestive and Kidney Diseases grant DK50306 (D. Artis), Irvington Institute for Immunological Research Postdoctoral Fellowship (C. Zaph), and the Crohn's and Colitis Foundation of America's William and Shelby Modell Family Foundation Research Award (D. Artis).
The authors have no conflicting financial interests.
J.G. Perrigoue and J. Li contributed equally to this paper.
References
- 1.Boulay, J.L., J.J. O'Shea, and W.E. Paul. 2003. Molecular phylogeny within type I cytokines and their cognate receptors. Immunity. 19:159– 163. [DOI] [PubMed] [Google Scholar]
- 2.Hofmann, S.R., R. Ettinger, Y.J. Zhou, M. Gadina, P. Lipsky, R. Siegel, F. Candotti, and J.J. O'Shea. 2002. Cytokines and their role in lymphoid development, differentiation and homeostasis. Curr. Opin. Allergy Clin. Immunol. 2:495–506. [DOI] [PubMed] [Google Scholar]
- 3.Hunter, C.A. 2005. New IL-12-family members: IL-23 and IL-27, cytokines with divergent functions. Nat. Rev. Immunol. 5:521–531. [DOI] [PubMed] [Google Scholar]
- 4.Dreuw, A., S. Radtke, S. Pflanz, B.E. Lippok, P.C. Heinrich, and H.M. Hermanns. 2004. Characterization of the signaling capacities of the novel gp130-like cytokine receptor. J. Biol. Chem. 279:36112–36120. [DOI] [PubMed] [Google Scholar]
- 5.Diveu, C., E. Lelievre, D. Perret, A.H. Lak-Hal, J. Froger, C. Guillet, S. Chevalier, F. Rousseau, A. Wesa, L. Preisser, et al. 2003. GPL, a novel cytokine receptor related to GP130 and leukemia inhibitory factor receptor. J. Biol. Chem. 278:49850–49859. [DOI] [PubMed] [Google Scholar]
- 6.Ghilardi, N., J. Li, J.A. Hongo, S. Yi, A. Gurney, and F.J. de Sauvage. 2002. A novel type I cytokine receptor is expressed on monocytes, signals proliferation, and activates STAT-3 and STAT-5. J. Biol. Chem. 277:16831–16836. [DOI] [PubMed] [Google Scholar]
- 7.Dillon, S.R., C. Sprecher, A. Hammond, J. Bilsborough, M. Rosenfeld-Franklin, S.R. Presnell, H.S. Haugen, M. Maurer, B. Harder, J. Johnston, et al. 2004. Interleukin 31, a cytokine produced by activated T cells, induces dermatitis in mice. Nat. Immunol. 5:752–760. [DOI] [PubMed] [Google Scholar]
- 8.Takaoka, A., I. Arai, M. Sugimoto, A. Yamaguchi, M. Tanaka, and S. Nakaike. 2005. Expression of IL-31 gene transcripts in NC/Nga mice with atopic dermatitis. Eur. J. Pharmacol. 516:180–181. [DOI] [PubMed] [Google Scholar]
- 9.Bilsborough, J., D.Y. Leung, M. Maurer, M. Howell, M. Boguniewcz, L. Yao, H. Storey, C. LeCiel, B. Harder, and J.A. Gross. 2006. IL-31 is associated with cutaneous lymphocyte antigen-positive skin homing T cells in patients with atopic dermatitis. J. Allergy Clin. Immunol. 117:418–425. [DOI] [PubMed] [Google Scholar]
- 10.Takaoka, A., I. Arai, M. Sugimoto, Y. Honma, N. Futaki, A. Nakamura, and S. Nakaike. 2006. Involvement of IL-31 on scratching behavior in NC/Nga mice with atopic-like dermatitis. Exp. Dermatol. 15:161–167. [DOI] [PubMed] [Google Scholar]
- 11.Sonkoly, E., A. Muller, A.I. Lauerma, A. Pivarcsi, H. Soto, L. Kemeny, H. Alenius, M.C. Dieu-Nosjean, S. Meller, J. Rieker, et al. 2006. IL-31: a new link between T cells and pruritus in atopic skin inflammation. J. Allergy Clin. Immunol. 117:411–417. [DOI] [PubMed] [Google Scholar]
- 12.Tanaka, M., Y. Hirabayashi, T. Sekiguchi, T. Inoue, M. Katsuki, and A. Miyajima. 2003. Targeted disruption of oncostatin M receptor results in altered hematopoiesis. Blood. 102:3154–3162. [DOI] [PubMed] [Google Scholar]
- 13.Wynn, T.A., and A.W. Cheever. 1995. Cytokine regulation of granuloma formation in schistosomiasis. Curr. Opin. Immunol. 7:505–511. [DOI] [PubMed] [Google Scholar]
- 14.Wynn, T.A., I. Eltoum, I.P. Oswald, A.W. Cheever, and A. Sher. 1994. Endogenous interleukin 12 (IL-12) regulates granuloma formation induced by eggs of Schistosoma mansoni and exogenous IL-12 both inhibits and prophylactically immunizes against egg pathology. J. Exp. Med. 179:1551–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herbert, D.R., C. Holscher, M. Mohrs, B. Arendse, A. Schwegmann, M. Radwanska, M. Leeto, R. Kirsch, P. Hall, H. Mossmann, et al. 2004. Alternative macrophage activation is essential for survival during schistosomiasis and downmodulates T helper 1 responses and immunopathology. Immunity. 20:623–635. [DOI] [PubMed] [Google Scholar]
- 16.Gordon, S. 2003. Alternative activation of macrophages. Nat. Rev. Immunol. 3:23–35. [DOI] [PubMed] [Google Scholar]
- 17.Nair, M.G., D.W. Cochrane, and J.E. Allen. 2003. Macrophages in chronic type 2 inflammation have a novel phenotype characterized by the abundant expression of Ym1 and Fizz1 that can be partly replicated in vitro. Immunol. Lett. 85:173–180. [DOI] [PubMed] [Google Scholar]
- 18.Raes, G., P. De Baetselier, W. Noel, A. Beschin, F. Brombacher, and G. Hassanzadeh Gh. 2002. Differential expression of FIZZ1 and Ym1 in alternatively versus classically activated macrophages. J. Leukoc. Biol. 71:597–602. [PubMed] [Google Scholar]
- 19.Sandler, N.G., M.M. Mentink-Kane, A.W. Cheever, and T.A. Wynn. 2003. Global gene expression profiles during acute pathogen-induced pulmonary inflammation reveal divergent roles for Th1 and Th2 responses in tissue repair. J. Immunol. 171:3655–3667. [DOI] [PubMed] [Google Scholar]
- 20.Liu, T., S.M. Dhanasekaran, H. Jin, B. Hu, S.A. Tomlins, A.M. Chinnaiyan, and S.H. Phan. 2004. FIZZ1 stimulation of myofibroblast differentiation. Am. J. Pathol. 164:1315–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wynn, T.A. 2004. Fibrotic disease and the T(H)1/T(H)2 paradigm. Nat. Rev. Immunol. 4:583–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sacks, D., and N. Noben-Trauth. 2002. The immunology of susceptibility and resistance to Leishmania major in mice. Nat. Rev. Immunol. 2:845–858. [DOI] [PubMed] [Google Scholar]
- 23.Kortylewski, M., M. Kujawski, T. Wang, S. Wei, S. Zhang, S. Pilon-Thomas, G. Niu, H. Kay, J. Mule, W.G. Kerr, et al. 2005. Inhibiting Stat3 signaling in the hematopoietic system elicits multicomponent antitumor immunity. Nat. Med. 11:1314–1321. [DOI] [PubMed] [Google Scholar]
- 24.Miyazaki, Y., H. Inoue, M. Matsumura, K. Matsumoto, T. Nakano, M. Tsuda, S. Hamano, A. Yoshimura, and H. Yoshida. 2005. Exacerbation of experimental allergic asthma by augmented Th2 responses in WSX-1-deficient mice. J. Immunol. 175:2401–2407. [DOI] [PubMed] [Google Scholar]
- 25.Villarino, A., L. Hibbert, L. Lieberman, E. Wilson, T. Mak, H. Yoshida, R.A. Kastelein, C. Saris, and C.A. Hunter. 2003. The IL-27R (WSX-1) is required to suppress T cell hyperactivity during infection. Immunity. 19:645–655. [DOI] [PubMed] [Google Scholar]
- 26.Artis, D., A. Villarino, M. Silverman, W. He, E.M. Thornton, S. Mu, S. Summer, T.M. Covey, E. Huang, H. Yoshida, et al. 2004. The IL-27 receptor (WSX-1) is an inhibitor of innate and adaptive elements of type 2 immunity. J. Immunol. 173:5626–5634. [DOI] [PubMed] [Google Scholar]
- 27.Holscher, C., A. Holscher, D. Ruckerl, T. Yoshimoto, H. Yoshida, T. Mak, C. Saris, and S. Ehlers. 2005. The IL-27 receptor chain WSX-1 differentially regulates antibacterial immunity and survival during experimental tuberculosis. J. Immunol. 174:3534–3544. [DOI] [PubMed] [Google Scholar]
- 28.Scott, P., E. Pearce, P. Natovitz, and A. Sher. 1987. Vaccination against cutaneous leishmaniasis in a murine model. II. Immunologic properties of protective and nonprotective subfractions of soluble promastigote extract. J. Immunol. 139:3118–3125. [PubMed] [Google Scholar]