Abstract
The common γ chain cytokines interleukin (IL)-2 and IL-7 are important regulators of T cell homeostasis. Although IL-2 is implicated in the acute phase of the T cell response, IL-7 is important for memory T cell survival. We asked whether regulated responsiveness to these growth factors is determined by temporal expression of the cytokine-specific IL-2 receptor (R) α and IL-7Rα chains. We demonstrate that IL-2Rα is expressed early after priming in T cell receptor–transgenic CD4+ T cells, whereas IL-7Rα expression is lost. In the later stage of the response, IL-7Rα is reexpressed while IL-2Rα expression is silenced. This reciprocal pattern of IL-2Rα/IL-7Rα expression is disturbed when CD4+ T cells are primed in the absence of IL-2 signals. Primed IL-2−/− or CD25−/− (IL-2Rα−/−) CD4+ T cells, despite showing normal induction of activation markers and cell division, fail to reexpress IL-7Rα late in the response. Because the generation of CD4+ memory T cells is dependent on IL-7–IL-7Rα interactions, primed IL-2−/− or CD25−/− CD4+ T cells develop poorly into long-lived memory cells. Retrovirus-mediated expression of IL-7Rα in IL-2−/− T cells restores their capacity for long-term survival. These results identify IL-2 as a factor regulating IL-7Rα expression and, consequently, memory T cell homeostasis in vivo.
The generation of a population of memory cells providing long-term protective immunity is an important outcome of a primary T cell response and the goal of vaccination. Substantial progress has been made in identifying the factors that contribute to the development and survival of CD8+ memory T cells, whereas less is known about CD4+ memory T cells. It has been postulated that brief stimulation of naive CD8+ T cells with antigen is sufficient to initiate the program leading to effector and memory cell development (1, 2). Long-term persistence of the generated memory CD8+ T cells is independent of continued TCR stimulation (3, 4) but depends on the presence of CD4+ helper T cells (5–9). Besides this newly emerging role of CD4+ T cells in CD8+ T cell memory maintenance, the cytokines IL-7 and IL-15, members of the common γ chain (γc) family, play an important role in the homeostasis of CD8+ memory T cells (10–12). Just as for CD8+ T cells, survival of CD4+ memory T cells does not require continued contact with MHC molecules once the antigen has been cleared (13). However, the functional activity of the surviving memory cells seems to be positively influenced by contact with MHC (14). Also, antigen dose and the maturation state of the APC during priming seem to influence the subsequent capacity of T cells to acquire long-term survival potential (15).
Studies using in vitro priming followed by adoptive transfer into immunocompetent recipient mice have shown that proper effector T cell differentiation and exposure to IL-2 are required for the successful long-term survival of the primed CD4+ T cells in vivo (16, 17). However, there are very few studies addressing the problem of CD4+ memory T cell generation using in vivo priming systems. Moreover, most of these studies use lymphopenic animals, wherein the true requirements for memory cell survival may not surface because of the absence of competition with endogenous lymphocytes for survival factors (17). Thus, it has been postulated that γc family cytokines are not required for the generation and maintenance of CD4+ memory T cells in γc-deficient, lymphopenic mice (18). Recent evidence to the contrary suggests that IL-7 is an important survival factor for resting CD4+ memory T cells residing in an intact lymphoid compartment (19–21), whereas IL-2 contributes to the generation of memory cells during priming (17). The phenotypes of mice deficient in IL-2, IL-7, or their receptors suggest an important role for these cytokines in the maintenance of T cell homeostasis (22). However, because both cytokines have a broad range of activities at different stages of T cell development, as well as on a variety of T cell subsets, it is difficult to define their specific roles in the generation of memory cells in intact, lymphocyte-sufficient animals. Because IL-2 and IL-7 share the γc for signaling, specificity of responsiveness and function will likely depend on temporally regulated expression of their unique, high affinity α chain receptor component. The factors regulating expression of CD25 are fairly well described: TCR engagement or IL-2 itself induces CD25 during T cell priming (23). However, the expression pattern and factors regulating IL-7Rα expression on mature CD4+ T cells are not well defined. Recent data from in vitro experiments suggest that IL-7 and IL-2 are negative regulators of IL-7Rα expression (24, 25). Given the importance of IL-7 signaling for the maintenance of naive and memory T cell populations, the mechanisms that control responsiveness to this cytokine in vivo are clearly crucial for understanding T cell homeostasis and memory.
In this study, we have followed IL-7Rα and IL-2Rα expression on CD4+ T cells during the priming and memory phases of an immune response. Using both in vitro and in vivo systems, we found that IL-2Rα and IL-7Rα show a reciprocal expression pattern after priming. Surprisingly, early up-regulation of IL-2Rα, allowing IL-2 signaling, promoted the generation of activated T cells reexpressing IL-7Rα. IL-7Rα reexpression correlated with the development of a long-lived memory cell population, consistent with the function of IL-7 to promote long-term survival of CD4+ memory T cells (19–21). Thus, IL-2 and IL-7 serve unique functions in the generation of CD4+ memory T cells.
RESULTS
Reciprocal regulation of IL-2Rα and IL-7Rα expression during a T cell response
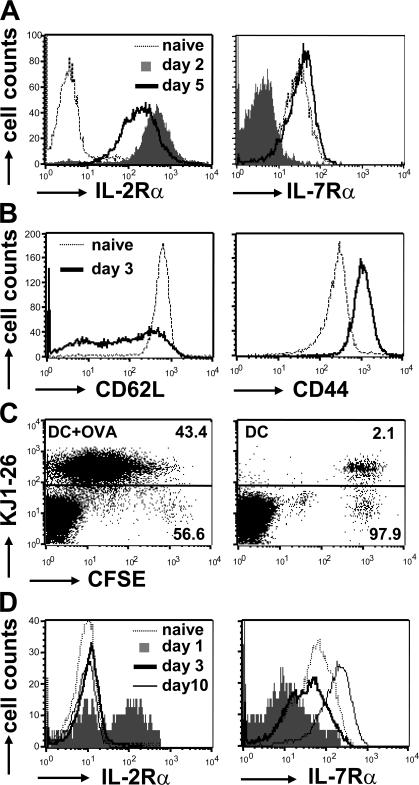
The ability of CD4+ T cells to respond effectively to the cytokines IL-2 and IL-7 depends on the expression of their high affinity IL-2Rα and IL-7Rα receptor chains on the cell surface. To follow expression of IL-2Rα and IL-7Rα during a CD4+ T cell response, naive OVA-specific T cells from DO.11 TCR transgenic mice were primed with 1 μg/ml OVA peptide and APCs in vitro and analyzed for the presence of the receptors by antibody staining and flow cytometry (Fig. 1 A). As expected, naive T cells were negative for IL-2Rα and expressed IL-7Rα. Antigen stimulation of DO.11 T cells led to a strong induction of IL-2Rα by day 2, whereas IL-7Rα was down-regulated in response to TCR triggering. By day 5, however, IL-7Rα expression was restored on activated T cells, whereas IL-2Rα was diminishing (Fig. 1 A).
Figure 1.
Reciprocal regulation of IL-2Rα and IL-7Rα after T cell activation. (A) 2.5 × 105 CD4+ T cells from DO.11 mice were primed in vitro with 1 μg/ml OVA peptide and 2.5 × 106 APCs. Naive T cells and cells harvested from the cultures on days 2 and 5 of priming were stained with KJ1-26, anti-CD4, and antibodies against IL-2Rα and IL-7Rα. Receptor expression levels were analyzed by flow cytometry. Data are representative of two independent experiments. (B) 5 × 106 naive DO.11 T cells were adoptively transferred into BALB/c mice and primed in vivo 24 h later with 3 × 106 OVA-pulsed DCs. Histograms show expression of CD62L and CD44 on naive and primed (day 3) KJ1-26+CD4+ T cells in the spleen. (C) CFSE-labeled DO.11 T cells were primed as in B (left) or with control DCs (no OVA; right). On day 3 after priming, spleen cell suspensions were stained with KJ1-26 and anti-CD4. Dot plots show CFSE dilution versus KJ1-26 staining gated on the CD4+ T cell population. Numbers indicate percentages of KJ1-26+ and KJ1-26− T cells within the total CD4+ T cell population. (D) Spleens of the primed mice from B were harvested on the indicated days, and IL-2Rα and IL-7Rα expression on KJ1-26+CD4+ T cells was determined as in A. Data are representative of three separate experiments.
Because conditions of antigen presentation and cytokine exposure during in vitro priming of T cells do not necessarily mimic the in vivo situation, we developed a system to follow IL-2Rα and IL-7Rα on DO.11 T cells primed in vivo. 5 × 106 naive, carboxyfluorescein diacetate succinimidyl ester (CFSE)–labeled DO.11 T cells were adoptively transferred into unmanipulated BALB/c recipients and primed the next day with 3 × 106 OVA-pulsed bone marrow–derived DCs. Spleens of the mice were harvested on days 1, 3, and 10 after priming, and DO.11 T cells were analyzed for the presence of IL-2Rα and IL-7Rα. To ensure that the DO.11 cells had been properly stimulated, we measured expression of the activation markers CD44 and CD62L and CFSE dilution (Fig. 1, B and C). 3 d after priming with OVA-pulsed DCs, all DO.11 cells showed increased CD44 and reduced CD62L levels and had lost the CFSE dye, whereas no CFSE dilution was observed when control DCs, which did not present OVA, were injected (Fig. 1, B and C). These data demonstrated that the DO.11 cells had been activated and cycled strictly in response to antigen. 1 d after priming with DCs, a substantial fraction of the DO.11 T cells expressed IL-2Rα on the cell surface (Fig. 1 D). In contrast, expression of IL-7Rα was lost early after priming. By day 3, IL-2Rα expression had decreased to the level of naive cells, whereas IL-7Rα was reexpressed. Furthermore, IL-7Rα was further up-regulated by day 10. Thus, priming of DO.11 T cells in vitro and in vivo results in reciprocal expression of IL-2Rα and IL-7Rα, suggesting that regulation of receptor expression determines the temporal responsiveness of T cells to the respective cytokines.
IL-2–IL-2Rα promotes the generation of activated T cells expressing IL-7Rα
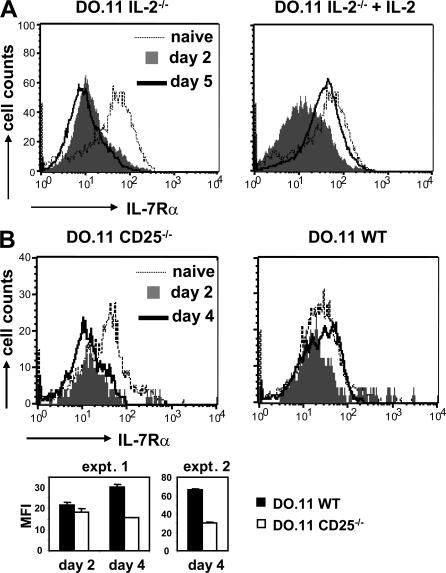
We have previously shown that IL-2 signals are required for the generation of memory cells (17). Because expression of IL-7R appears to be a cardinal property of memory T cells, we postulated that one key function of IL-2 may be to promote sustained expression of the IL-7Rα chain. To address this possibility, IL-2−/− DO.11 T cells were primed in vitro in the absence or presence of recombinant IL-2. Under IL-2–free priming conditions, the T cells show normal cell division and up-regulation of activation markers but fail to survive as well as WT T cells (17). Fig. 2 A demonstrates that IL-2−/− T cells lost IL-7Rα expression early after activation, independent of the presence of IL-2 and similar to WT T cells. However, IL-2−/− T cells failed to reexpress IL-7Rα at the later stages of activation unless IL-2 was added back to the cultures. Thus, IL-2 is required during priming to induce the reexpression of IL-7Rα on late-activated T cells. Analysis of IL-7Rα mRNA by quantitative PCR confirmed the flow cytometry data (unpublished data).
Figure 2.
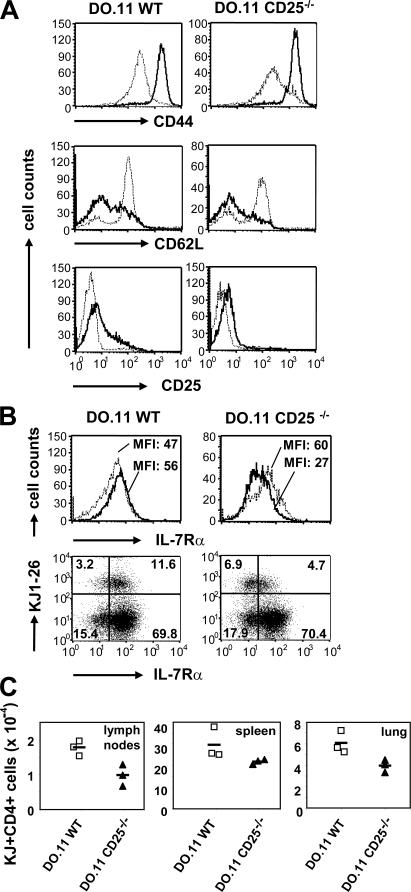
IL-2 regulates IL-7Rα reexpression on activated T cells. (A) 2.5 ×105 IL-2−/− DO.11 T cells were primed in vitro with 1 μg/ml OVA peptide and 2.5 × 106 IL-2−/− APCs in the absence or presence of 10 ng/ml of added recombinant IL-2. IL-2Rα and IL-7Rα expression was examined as described in Fig. 1 A. Results are from one representative experiment out of three. (B) 2 × 106 WT and CD25−/− DO.11 T cells were adoptively transferred into BALB/c recipients and primed in vivo with 106 OVA-pulsed DCs. Histograms show IL-7Rα expression on KJ-26+CD4+ cells in the spleen. (bottom) Average mean fluorescence intensity (MFI) ± SD of IL-7Rα staining on WT and CD25−/− DO.11 T cells from the spleen (two mice per group). Primed WT and CD25−/− DO.11 T cells showed no difference in IL-7Rα expression on day 2 (P = 0.095), but significantly different IL-7Rα levels were measured on day 4 in both expt. 1 (P = 0.007) and expt. 2 (P = 0.0005).
Next, we asked if IL-2 was also required for IL-7Rα expression during in vivo priming of T cells. Initially, we compared IL-7Rα expression on WT and IL-2−/− DO.11 T cells primed in vivo with OVA-pulsed DCs and could not detect any differences between these cell populations. However, it is possible that IL-2 produced by host T cells could compensate for the lack of autocrine IL-2 production by the transferred IL-2−/− DO.11 T cells. Therefore, we analyzed IL-7Rα expression on DO.11 T cells deficient in CD25, the high affinity IL-2Rα. Because CD25−/− T cells are unable to respond to IL-2 present in the environment, they could be primed under exactly the same conditions as WT cells yet would be deprived of IL-2 signals. We found that CD25−/− T cells failed to restore IL-7Rα expression to the level of naive or activated WT cells on day 4 after in vivo priming (Fig. 2 B). Thus, one of the functions of the IL-2–IL-2Rα system during the T cell response is to provide signals for the reexpression of IL-7Rα on late-activated T cells.
IL-7–IL-7Rα is required for memory cell survival
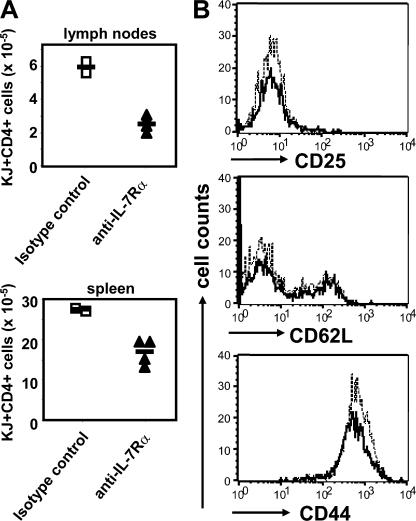
IL-7 is an important survival factor for naive T cells (26). More recently, evidence has been presented that IL-7 is also important for the long-term survival of CD4+ memory T cells (20, 21). Therefore, reexpression of the IL-7Rα at the later stages of T cell activation could be necessary for the long-term survival of activated T cells once the antigen has been cleared. To formally test whether activated DO.11 T cells are dependent on IL-7 for their survival, we primed DO.11 T cells in vitro for 4 d and adoptively transferred 30 × 106 activated T cells into unmanipulated BALB/c mice. Recipient mice were given either a blocking anti–IL-7Rα antibody or an isotype control antibody every other day for 10 d. The number of DO.11 cells surviving in lymph nodes and spleen was determined after 12 d. Treatment with anti–IL-7Rα reduced the number of DO.11 cells present in lymphoid tissues (Fig. 3 A). The recovered cells all showed a resting memory phenotype (Fig. 3 B). Total CD4+ T cell numbers were also reduced, confirming that IL-7 is required for naive T cell survival (unpublished data).
Figure 3.
IL-7–IL-7Rα is required for CD4 memory cell survival. WT DO.11 T cells were primed in vitro as in Fig. 1 A. Primed cells were harvested on day 4, and 30 × 106 cells were adoptively transferred into BALB/c recipients. From day 2 after transfer, 1 mg anti–IL-7Rα or isotype control antibody was injected IP every other day for 10 d. Spleen and lymph nodes of the treated mice were harvested on day 12, and DO.11 T cells were stained with KJ1-26 and anti-CD4 mAbs. (A) Absolute numbers of DO.11 T cells present in lymph nodes and spleen. Each symbol represents an individual mouse. Cell numbers with and without anti–IL-7Rα treatment were significantly different in both lymph nodes (P = 0.001) and spleen (P = 0.009). Horizontal lines represent mean cell numbers. (B) Expression of CD25, CD62L, and CD44 on DO.11 T cells from the spleen of one representative mouse treated with anti-IL-7Rα (bold line) or isotype control (continuous line).
IL-2 during priming is required for both central and effector memory T cells
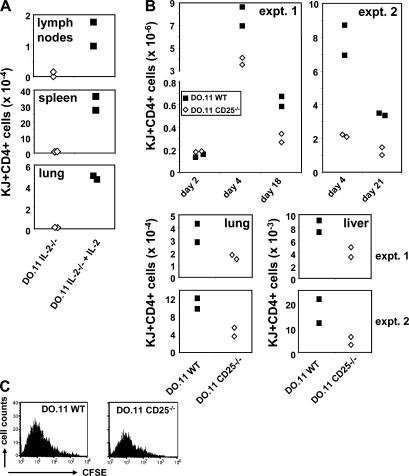
Our previous experiments demonstrating a role of IL-2 in the generation of memory CD4+ T cells have focused on memory cells in lymphoid organs (presumably corresponding to central memory cells) (17). To ask if the same requirement would be seen for memory cells in nonlymphoid peripheral tissues, we primed IL-2−/− DO.11 T cells in vitro for 4 d in the absence or presence of IL-2, adoptively transferred equal numbers of cells to BALB/c mice, and evaluated T cell survival in different organs 4 wk later. In agreement with previous findings (17), DO.11 cells primed in the absence of IL-2 lacked the capacity to survive long-term in the secondary lymphoid organs (Fig. 4 A). We also found that IL-2−/− DO.11 cells failed to survive in nonlymphoid tissues such as the lung, whereas a population of DO.11 was recovered from this organ if the T cells had been primed in the presence of IL-2 (Fig. 4 A). This result shows that the absence of IL-2−/− memory cells in lymphoid organs is not caused by skewed homing of T cells to nonlymphoid organs or preferential development of effector memory cells, which are know to reside in nonlymphoid tissues.
Figure 4.
IL-2 during priming enhances T cell survival in vivo in lymphoid and nonlymphoid tissues. (A) IL-2−/− DO.11 T cells were primed in vitro with or without IL-2, as described in Fig. 2 A, and 10 × 106 primed cells were adoptively transferred to BALB/c recipients. 4 wk later, DO.11 T cell numbers in the lymph nodes, spleen (P = 0.02), and lung (P = 0.0009) of recipient mice were examined as in Fig. 3 A. Data points represent individual mice. (B) 2 × 106 (expt. 1) or 7.5 × 105 (expt. 2) CFSE-labeled naive WT and CD25−/− DO.11 T cells were primed in vivo with 106 (expt. 1) or 3 × 106 (expt. 2) OVA-pulsed DCs, and numbers of KJ1-26+CD4+ cells in the spleen, lung, and liver were determined. The two top panels show WT and CD25−/− DO.11 T cell numbers recovered from the spleens on days 2, 4, and 18–21. Cell numbers did not differ between both groups on day 2 (P = 0.07), but were significantly different on day 4 (expt. 1, P = 0.046; expt. 2, P= 0.023), day 18 (P = 0.029), and day 21 (P = 0.009). Bottom panels show DO.11 T cell numbers present in the lung and liver on day 18 (expt. 1) and day 21 (expt. 2). (C) CFSE dilution of WT and CD25−/− DO.11 cells in the spleen on day 4 after priming. Data shown are from one representative experiment (two mice per group) out of three.
It has been debated what the precise role of IL-2 is during CD4+ T cell responses and whether this cytokine is absolutely required as a T cell growth factor in vivo (27). To evaluate the role of IL-2 during an in vivo T cell response, naive, CFSE-labeled CD25−/− DO.11 T cells were adoptively transferred into BALB/c mice and primed with OVA-pulsed DCs. T cell numbers and cell division were followed over time in different organs. We have found that antigen-presenting DCs do not survive in lymphoid organs beyond 3 d after transfer, because freshly transferred DO.11 T cells cannot be primed after this time (28). Early evaluation of the spleen (day 2) revealed that equal numbers of DO.11 cells had settled there (Fig. 4 B), and no CFSE dilution had taken place by this time (not depicted). On day 4, WT and CD25−/− DO.11 T cells had expanded markedly in the spleen, and both had cycled equivalently (Fig. 4 C). Recovery of CD25−/− cells was significantly less than that of WT cells.
During an in vivo T cell response, the expansion phase is followed by deletion of the activated T cells to restore homeostasis. By days 18–21, ∼60–90% of the primed T cells had been lost (Fig. 4 B). However, a significant population of WT DO.11 cells remained in the spleen and in nonlymphoid tissues, and these cells exhibited a memory phenotype (unpublished data). Approximately 50–60% fewer memory cells were recovered from hosts that had received CD25−/− DO.11 T cells, both in the spleen and in nonlymphoid tissues (lung and liver; Fig. 4 B). These data indicate that IL-2 is not required to drive primed T cells through initial replication but is required for initial survival and for the subsequent development of a memory cell population.
IL-7Rα–reexpressing T cells show an enhanced capacity to survive as memory cells
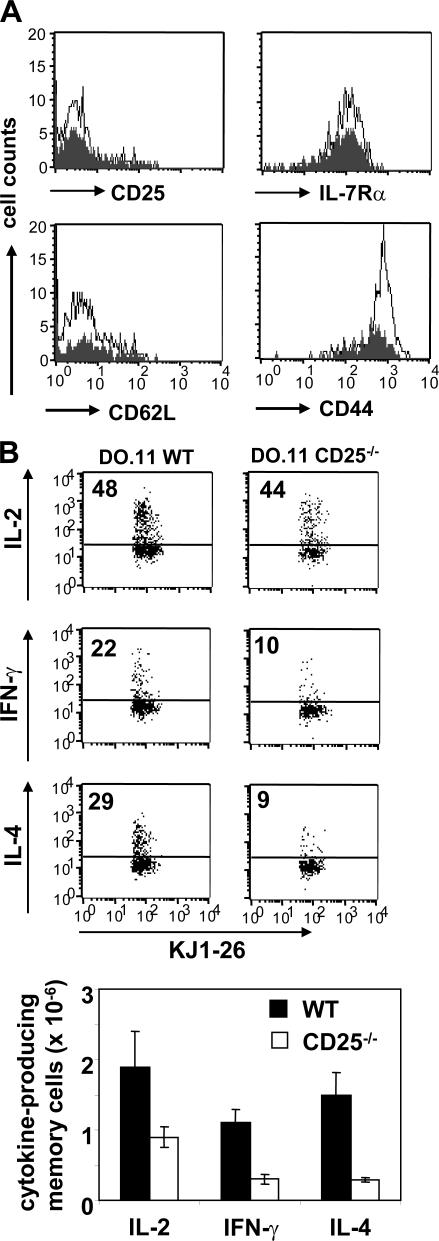
From the previous experiments, it was not clear if the diminished recovery of memory cells from CD25−/− T cells was the consequence of deficient IL-7Rα reexpression or the result of reduced clonal expansion in response to antigenic stimulation. To distinguish between these possibilities, we separated the expansion and memory phases of the response by priming WT and CD25−/− DO.11 T cells in a first recipient, harvesting the primed cells on day 3, and retransferring the recovered cells to a second recipient. Phenotypic analysis of the primed cells showed that at day 3 CD44 and CD62L were up- and down-regulated, respectively, similarly in activated WT and CD25−/− T cells, whereas CD25 was already mostly lost (Fig. 5 A). Confirming the data shown in Fig. 2 B, primed CD25−/− cells showed ∼50% reduction in IL-7Rα expression level compared with WT cells or naive CD25−/− cells (Fig. 5 B). The primed DO.11 WT and CD25−/− cells were subsequently purified from lymph nodes and spleen, equal numbers were transferred to new recipients, and survival followed. In all tissues examined (lymph nodes, spleen, and lung), CD25−/− cells survived poorly compared with WT cells, as predicted from reduced IL-7Rα expression (Fig. 5 C). Next, we asked whether the surviving CD25−/− T cells were bona fide memory cells. The surviving cells, whether WT or CD25−/−, all exhibited a resting, memory phenotype, with high CD44 and low CD62L (Fig. 6 A). Interestingly, the surviving CD25−/− cells all expressed high levels of IL-7Rα, further suggesting that cells expressing high levels of IL-7Rα are preferentially retained. However, the recall responses of the surviving CD25−/− memory T cells, as measured by effector cytokine production upon antigenic challenge, were markedly reduced when the T cells were primed in the absence of IL-2 signals (Fig. 6 B). Thus, it is possible that IL-2, analogous to what recently has been shown for CD8+ memory cells (29), is required for programming secondary responses in CD4+ memory T cells.
Figure 5.
Failure to reexpress IL-7Rα in the absence of IL-2 correlates with reduced memory cell survival. 2 × 106 naive WT and CD25−/− T cells were adoptively transferred to BALB/c mice and primed in vivo with 3 × 106 OVA-pulsed DCs. Lymph nodes and spleen of recipient mice were harvested on day 3. (A) Naive (continuous line) and primed (bold line) KJ1-26+CD4+ T cells were stained for the activation markers CD44, CD62L, and CD25 and analyzed by flow cytometry. Histograms represent pooled cell samples from recipients of WT (n = 10) and CD25−/− (n = 20) T cells. Data are representative of three separate experiments. (B) Histograms compare IL-7Rα expression on naive (continuous line) with primed (bold line) WT and CD25−/− DO.11 T cells. MFI is indicated. Dot plots compare IL-7Rα expression on KJ1-26+ with KJ1-26− cells from the CD4+ T cell population. Numbers refer to percentages of IL-7Rαlow vs. IL-7Rαhigh cells within the CD4+ population. Histograms and dot plots represent pooled cell samples from recipients of WT (n = 10) and CD25−/− (n = 20) T cells. One representative experiment out of three is shown. (C) CD4+ T cells were purified from the lymph nodes and spleens 3 d after priming, and 10 × 106 primed WT and CD25−/− DO.11 T cells were adoptively transferred to secondary BALB/c recipients. DO.11 memory cell numbers in the lymph nodes (P = 0.02), spleen, and lung (P = 0.03) were analyzed as described for Fig. 4 B. Data points represent individual mice from one representative experiment out of two. Horizontal lines represent mean cell numbers.
Figure 6.
Phenotypic and functional properties of memory cells generated in the absence of IL-2. 106 WT and CD25−/− DO.11 T cells were primed in vivo with 3 × 106 OVA-pulsed DCs and analyzed 4 wk later. (A) Histograms show the phenotype of the WT (open) and CD25−/− (shaded) memory T cells recovered from the spleen. One representative mouse out of three is shown. (B) Some recipients were rechallenged with 3 × 106 OVA-pulsed DCs, and 3 d later splenocytes were stained for cytokine production after brief in vitro restimulation. Dot plots show the frequency of IL-2–, IFN-γ–, and IL-4–producing cells within the CD4+ KJ1-26+ population. The bar graph shows total numbers of cytokine-producing DO.11 T cells. Error bars display SD (n = 3).
Retrovirus-mediated expression of IL-7Rα in IL-2−/− T cells restores memory cell survival
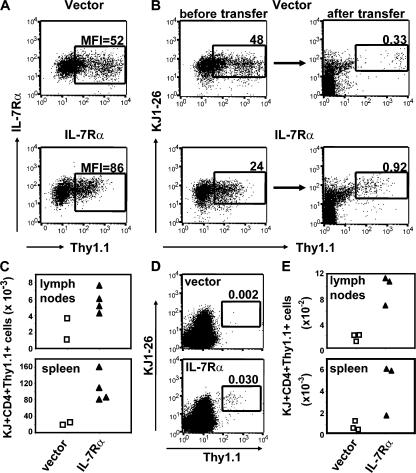
To formally demonstrate a causal relationship between diminished IL-7Rα expression and the failure of primed IL-2−/− T cells to generate a memory population, we restored IL-7Rα expression in DO.11 IL-2−/− T cells and followed the generation of memory cells. To do this, a bicistronic retroviral vector, containing an IL-7Rα cDNA and Thy1.1 reporter gene, was introduced into IL-2−/− DO.11 T cells during in vitro priming, resulting in enhanced expression of IL-7Rα in the Thy1.1+ cell population (Fig. 7 A). 3 × 106 IL-2−/− DO.11 T cells, transduced with either a control vector (48% of transferred cells being Thy1.1+) or an IL-7Rα–containing vector (24% Thy1.1+), were adoptively transferred into BALB/c recipients, and the number of DO.11 cells positive for Thy1.1 was determined 3 wk later. DO.11 IL-2−/− cells that were transduced with IL-7Rα survived markedly better than cells that received the control vector (Fig. 7 B). The difference was even more striking after normalizing cell numbers to equal input cell numbers (Fig. 7 C). In an additional experiment, Thy1.1+ IL-2−/− DO.11 T cells were purified from the transfection cultures by cell sorting, and equal numbers of cells expressing IL-7Rα or control vector were transferred into BALB/c recipients. 2 wk later, markedly higher numbers of DO.11 T cells were present in the lymph nodes and spleen of recipients that received cells expressing IL-7Rα versus control vector (Fig. 7, D and E). Transfer of sorted DO.11 T cells resulted in lower cell recoveries than transfer of unsorted cells (Fig. 7, C and E), perhaps because of the loss of antibody-coated cells. Thus, these data demonstrate that overexpression of IL-7Rα in IL-2−/− cells is sufficient to restore their capacity for long-term survival by enhancing their competitiveness to receive IL-7 survival signals.
Figure 7.
Retrovirus-mediated expression of IL-7Rα in IL-2−/− T cells restores memory cell generation. (A) IL-2−/− DO.11 T cells were retrovirally transduced with IL-7Rα or empty vector, and IL-7Rα and Thy1.1 expression were evaluated on day 4 by antibody staining and flow cytometry. MFI of IL-7Rα staining on the gated (Thy1.1+) population is indicated. (B) 3 × 106 IL-2−/− DO.11 T cells, of which 48% (empty vector) and 24% (IL-7Rα) expressed the Thy1.1 reporter gene, were adoptively transferred to BALB/c recipients, and the presence of Thy1.1+ DO.11 T cells was analyzed 3 wk after transfer. (right) Dot plots show the percentage of KJ1-26+Thy1.1+ cells within the CD4+ population in the spleen of one representative recipient. (C) To normalize for the difference in the number of Thy1.1+ cells transferred between both groups, the number of KJ1-26+Thy1.1+ memory cells recovered from the lymph nodes and spleen per 106 KJ1-26+Thy1.1+ cells transferred was calculated. Data points represent individual mice. (D) IL-2−/− DO.11 T cells were retrovirally transduced as in A, and Thy1.1+KJ1-26+CD4+ cells were purified from the cultures on day 4 by cell sorting. 2 × 106 Thy1.1+KJ1-26+CD4+ cells expressing either IL-7Rα or control vector were adoptively transferred into BALB/c recipients, and the presence of Thy1.1+ DO.11 T cells was analyzed 2 wk after transfer. Dot plots show the percentage of KJ1-26+Thy1.1+ cells within the CD4+ population in the spleen of one representative recipient. (E) Total number of KJ1-26+Thy1.1+CD4+ cells recovered from the lymph nodes and spleen per 2 × 106 purified cells transferred. Data points represent individual mice.
DISCUSSION
The cytokines IL-2 and IL-7 both use the γc for transducing survival- and growth-promoting signals in T cells. Specificity of the response to IL-2 and IL-7 is assured by integrating a cytokine-specific high affinity receptor chain in the receptor complex, the IL-2Rα or IL-7Rα, respectively. In this paper, we demonstrate that the expression of IL-2Rα and IL-7Rα is reciprocally regulated during a T cell response to antigen. This expression pattern provides a mechanism for the cell to respond to each cytokine at the appropriate time during the response, avoiding competition between the cytokines for γc usage. Naive T cells, which are dependent on IL-7 for their survival (26), express IL-7Rα on the cell surface. Because naive T cells do not produce IL-2, they cannot use IL-2 as an autocrine survival factor and, predictably, they do not express high levels of IL-2Rα. Upon TCR stimulation, IL-7Rα is down-regulated, whereas IL-2Rα expression is induced. TCR stimulation also induces production of IL-2, which T cells use in an autocrine fashion for clonal expansion and differentiation. Furthermore, the decline of IL-7Rα expression means that the T cells cannot be maintained by the constitutively produced survival cytokine, IL-7, and become dependent on the newly secreted growth factor, IL-2. Such a pattern of receptor expression would promote preferential clonal expansion of the antigen-stimulated (and, therefore, antigen-specific) T cells, the goal of the adaptive immune response. At the end of the acute phase of the response, IL-2 production ceases, IL-2Rα expression wanes, and IL-7Rα is reexpressed. Because it has recently been demonstrated that IL-7 is required for the survival of CD4+ memory T cells, it can be postulated that activated T cells reexpressing IL-7Rα will preferentially enter the memory T cell pool. Similarly, it has been demonstrated that the subpopulation of CD8+ effector T cells expressing IL-7Rα at the peak of an antiviral response was most likely to develop into long-lived memory cells (30). Our data suggest that, also for CD4+ T cells, high levels of IL-7Rα at the peak of the response predict enhanced potential for memory cell generation (Figs. 4 B and 5 C). Importantly, high IL-7Rα expression, although required, was not the sole determinant of memory cell generation, because the majority of primed, IL-7Rαhigh cells still died massively (8 × 106 on day 4 vs. 6 × 105 on day 18 in the spleen; Fig. 4 B). Thus, either a subset of committed memory cells exists within the IL-7Rαhigh primed CD4+ T cells, or the ensuing memory cell population has, in a stochastic manner, escaped deletion mechanisms operating during the contraction phase of the response.
Because the IL-7–IL-7Rα system is essential for CD4+ and CD8+ memory T cell homeostasis, it is important to identify the factors that regulate IL-7Rα expression during a T cell response. Based on our previous data showing that IL-2 is needed for memory responses, as well as the results of a microarray study cataloging IL-2–dependent gene expression in primed T cells (unpublished data), we hypothesized that IL-2 was required for IL-7Rα expression on primed T cells. We found that DO.11 T cells primed with antigen and APCs in vitro and in vivo in the absence of IL-2 signals failed to reexpress IL-7Rα with the same kinetics as WT cells after initial TCR-triggered down-regulation of the receptor (Fig. 2). IL-7Rα reexpression was more dramatically dependent on IL-2 in T cells primed in vitro than in vivo, suggesting that additional factors controlling IL-7Rα expression may be present in vivo. IL-15, sharing the IL-2/15Rβ for signaling with IL-2, is a possible candidate for this function. Our results contrast with those from an earlier report indicating that IL-2 negatively regulates IL-7Rα expression on activated T cells (25). However, whereas we used a physiologically relevant T cell priming system with antigen and APCs in vitro and, more importantly, during an in vivo T cell response, these authors relied on in vitro stimulation with anti-CD3 and anti-CD28 antibodies. It is possible that continuous TCR engagement by the immobilized antibodies may lead to persistent down-regulation of IL-7Rα.
We have previously shown that T cells primed with IL-2 acquire a competitive survival advantage over cells primed in the absence of IL-2 (17). Thus, IL-2−/− DO.11 cells primed in vitro and subsequently adoptively transferred to BALB/c mice survived poorly in comparison with WT DO.11 cells. However, primed IL-2−/− DO.11 T cells persisted efficiently after adoptive transfer into lymphopenic Rag−/− mice, where competition with other lymphocytes was absent (17). The differential long-term survival capacity of primed IL-2−/− T cells in intact versus lymphopenic mice can be explained by deficient IL-7Rα expression: CD4+ memory T cells are dependent on IL-7 to persist in intact hosts (20, 21), whereas survival and homeostatic proliferation of memory CD4+ T cells in lymphopenic animals is IL-7 independent (31). Now we also show that if DO.11 T cells are unable to receive IL-2 signals caused by deletion of the IL-2Rα gene, the cells develop poorly into long-lived memory cells after priming with antigen and APCs in vivo.
There has been ongoing discussion about the role of IL-2 in T cell responses. Although initially described as the prototypic T cell growth factor (32), analysis of mice deficient in IL-2 or IL-2Rα/β surprisingly showed a lymphoproliferative phenotype leading to autoimmune disease (33–35). This generated considerable interest in the function of IL-2 as a negative regulator of the immune system. The dependence of T regulatory cells on IL-2 for development and maintenance (36–39) and the capacity of IL-2 to sensitize repeatedly stimulated T cells for cell death by activating the Fas/FasL death pathway (40–42) have both been proposed as explanations for the negative regulatory role of IL-2. On the other hand, priming of IL-2−/− (43) or CD25−/− (44) T cells in vivo showed only a limited impact of IL-2 deficiency on initial T cell clonal expansion. These studies have raised uncertainties about the obligatory role of IL-2 as a T cell growth factor in vivo. By using CD25−/− T cells to exclude the effects of bystander IL-2, priming with antigen and APCs, and separating the acute and memory phases of the T cell response, we have further elucidated the role of IL-2 during an in vivo CD4+ T cell response. Our data demonstrate in a definitive way that IL-2 signals during priming are not required for initial cell proliferation but are more important to endow primed T cells with long-term survival potential once the antigen has been cleared. Confirming our previous studies with in vitro primed IL-2−/− T cells (17), IL-2 is not required to drive T cells through the cell cycle in vivo (Fig. 4 C). Although CD25−/− T cells expanded considerably after antigenic stimulation, we generally recovered less primed CD25−/− T cells than WT cells at days 3–4, in accordance with another study (44). Whether this diminished clonal expansion of primed T cells is a direct effect of the lack of IL-2 growth factor activity or is caused by a failure to receive IL-7 signals remains an open question. However, the most striking consequence of IL-2 deprivation during priming is the subsequent diminished capacity to generate a memory T cell population. This defect is likely caused by failed reexpression of IL-7Rα on late-activated T cells, because we (Fig. 3) and others have shown that survival of CD4+ memory T cells is dependent on IL-7Rα (19–21) and that ectopic expression of IL-7Rα in primed IL-2−/− T cells restores memory cell generation (Fig. 7). Identification of the IL-2–induced signaling pathway that leads to IL-7Rα reexpression will be an important goal of future research. Also of interest is to investigate whether GA binding protein, a transcription factor recently implicated in IL-7Rα expression in T cells, is induced by IL-2 (45).
Finally, these results demonstrate that optimal generation of memory T cell responses will require strategies for maximizing the expression of IL-7Rα on primed cells. It is also likely that analyzing IL-7Rα expression on antigen-specific T cells after vaccination may predict the efficacy of the vaccine. Such prospective studies may help improve the success of prophylactic vaccination, based on experimental results.
MATERIALS AND METHODS
Mice.
BALB/c mice were purchased from Charles River Laboratories and used as recipients for adoptive transfer experiments at 6–8 wk of age. DO.11.10 transgenic mice, expressing a TCR specific for the hen egg albumin peptide OVA323-339 presented by MHC class II molecule I-Ad, were a gift from K. Murphy (Washington University, St. Louis, MO). DO.11.10 × IL-2−/− mice were generated in our laboratory by crossing DO.11.10 mice with IL-2−/− mice (backcrossed for >10 generations onto the BALB/c background; The Jackson Laboratory). Generation of DO.11.10 × CD25−/− mice has been previously described (46). Transgenic mice were genotyped using PCR and flow cytometry. All mice were bred and maintained in accordance with the guidelines of the Laboratory Animal Resource Center of the University of California, San Francisco.
Antibodies, CFSE labeling, and flow cytometry.
DO.11.10 T cells were detected by staining with allophycocyanin- or PE-conjugated KJ1-26 TCR clonotypic antibody, generated in our lab from the hybridoma cell line (a gift from P. Marrack, National Jewish Research and Medical Center, Denver, CO), and peridinin chlorophyllprotein–conjugated anti-CD4 mAb after blocking Fc receptors with anti-CD16/CD32 (both purchased from BD Biosciences). The following antibodies coupled to the indicated fluorochromes were used for detection of activation markers and cytokine receptors: anti-CD44–PE (IM7), anti-CD62L–PE or –FITC (MEL-14/L-selectin), anti-CD25–allophycocyanin or –FITC (PC61/IL-2Rα; all obtained from BD Biosciences), and anti-CD127–biotin/streptavidin–PE (A7R34/IL-7Rα; Chemicon International). Fluorescence intensities were measured with a flow cytometer (FACSCalibur; Becton Dickinson), and data were analyzed with CellQuest software (Becton Dickinson). For in vivo blocking experiments, mAbs specific for IL-7Rα (clone A7R34) produced by the hybridoma cell line were purified with Protein G Sepharose 4 Fast Flow (GE Healthcare). Rat IgG (Jackson ImmunoResearch Laboratories) was used as a control. To follow cell division during priming, naive CD4+ T cells were labeled with 1 μM CFSE (Invitrogen) for 10 min at room temperature in serum-free medium. Excess CFSE was quenched with 50% FCS, followed by three washes with PBS. On days 3–4 after adoptive transfer and priming, spleens from the recipient mice were harvested, and CFSE content of KJ1-26+CD4+ cells was determined by flow cytometry. For intracellular cytokine staining, splenocytes were restimulated ex vivo for 4 h with 1 μg/ml OVA peptide in the presence of 10 μg/ml BrefeldinA (Epicentre Biotechnologies) for the last 3 h and stained with anti–IL-2, anti–IL-4, and anti–IFN-γ antibodies (BD Biosciences) using a Cytofix/Cytoperm Kit (BD Biosciences), according to the manufacturer's instructions.
T cell priming and adoptive transfers.
In vitro T cell priming and adoptive transfer of WT and IL-2−/− DO.11 T cells were performed as previously described (17). In brief, 2.5–5 × 105 CD4+ T cells from WT and IL-2−/− DO.11 mice were primed with 2.5 × 106 mitomycin C–treated BALB/c splenocytes and 1 μg/ml OVA323-339 in 24-well plates for 4–5 d. IL-2−/− splenocytes were used for cultures with IL-2−/− DO.11 cells to avoid the effects of bystander IL-2. In some experiments, 10 ng/ml recombinant mIL-2 (R&D Systems) was added to the cultures. Primed T cells were harvested on day 4, and dead cells were removed by centrifugation over a density gradient (Lympholyte-M; Cedarlane Laboratories). Equal numbers of viable KJ1-26+CD4+ T cells were adoptively transferred into unmanipulated BALB/c mice by tail vein injection. For in vivo priming, 1–5 × 106 naive WT or CD25−/− DO.11 T cells were adoptively transferred to sex-matched BALB/c mice and primed the next day with 1–3 × 106 mature bone marrow–derived DCs. DCs were generated by culturing 5–10 × 106 bone marrow cells in 100-mm suspension culture dishes (Corning) for 8–11 d with 20 ng/ml GM-CSF in Iscove's modified Dulbecco's medium (Sigma-Aldrich) supplemented with 1 mM l-glutamine, penicillin, streptomycin (all obtained from Life Technologies), 5 × 10−5 M 2-ME, and 10% fetal bovine serum (Sigma-Aldrich). On days 5 and 8, nonadherent and loosely adherent cells were harvested by treatment with 3 mM EDTA in PBS and transferred to new plates. 1 ng/ml IL-4 was added for the last 3 d of culture. DCs were matured with 1 μg/ml LPS (Escherichia coli O26:B6; Sigma-Aldrich) and pulsed with 1 μg/ml OVA peptide 24 h before injection. For the experiments described in Fig. 5, lymph nodes and spleen were harvested on days 3–4 after in vivo priming, and CD4+ T cells were magnetically purified with anti–mouse CD4-coated Dynabeads, according to the instructions of the manufacturer (Dynal). Equal numbers of viable KJ1-26+CD4+ T cells were transferred to a second set of BALB/c recipients by tail vein injection.
Analysis of memory cells in lymphoid and nonlymphoid tissues.
DO.11 memory T cells in spleen, pooled peripheral lymph nodes (submandibular, axillary, brachial, inguinal, and popliteal), liver, and lung were quantified 2–4 wk after priming. To recover lymphocytes from nonlymphoid tissues, mice were perfused with PBS, and lungs and liver were excised. Lung and liver tissue was mechanically dissociated and treated with 400 U/ml and 100 U/ml, respectively, collagenase VIII (C2139; Sigma-Aldrich) for 30 min at 37°C, regularly pipetting the suspension up and down. The resulting suspension was passed through a 70-μM nylon screen, and lymphocytes were isolated by density gradient centrifugation using Lympholyte-M. The percentage of KJ1-26+CD4+ memory T cells in the cell suspensions was determined by flow cytometry. Absolute numbers of DO.11 T cells in lymph nodes and spleen were calculated from this percentage and counts of total cell numbers in a hemocytometer (Improved Neubauer; Reichert) using trypan blue dye exclusion. Recall responses of WT and CD25−/− memory T cells were evaluated by rechallenging previously primed recipients of WT and CD25−/− DO.11 T cells with 3 × 106 OVA-pulsed DCs and staining splenocytes for cytokine production 3 d later using intracellular cytokine staining and flow cytometry.
Cloning and retrovirus-mediated transfection of IL-7Rα.
A full-length cDNA clone of the mouse IL-7Rα (clone 6758487; I.M.A.G.E. Consortium) (47) was obtained from Open Biosystems. The IL-7Rα coding sequence was isolated by restriction digest with the enzyme BsrG I (New England Biolabs, Inc.) and ligated into the BsrG I site of the retroviral vector MSCV 1.1 containing an IRES-Thy1.1 reporter gene (a gift from W. Sha, University of California, Berkeley, Berkeley, CA). Retroviral transfections of DO.11 T cells were performed as previously described (48). Cultures of retrovirally infected cells were harvested on day 4, and the percentage of transfected cells was determined by staining with an anti-Thy1.1 antibody (BD Biosciences) and flow cytometry before adoptive transfer to BALB/c recipients. In one experiment, Thy1.1+KJ1-26+CD4+ cells were sorted (>98% purity) from the transfection cultures with a cell sorter (MoFlo; DakoCytomation) before adoptive transfer.
Statistics.
The statistical significance of differences between groups was determined using the two-tailed Student's t test.
Acknowledgments
We thank Carlos Benitez for mouse genotyping, Cliff McArthur for expert cell sorting, and Drs. Lewis Lanier and Alejandro Villarino for reading the manuscript and giving valuable suggestions. Members of the Abbas lab are acknowledged for helpful discussions.
This work was supported by grants from the National Institutes of Health, including grant R01 AI45845.
The authors have no conflicting financial interests.
Abbreviations used: CFSE, carboxyfluorescein diacetate succinimidyl ester; γc, γ chain; MFI, mean fluorescence intensity.
References
- 1.Kaech, S.M., and R. Ahmed. 2001. Memory CD8+ T cell differentiation: initial antigen encounter triggers a developmental program in naive cells. Nat. Immunol. 2:415–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Stipdonk, M.J., E.E. Lemmens, and S.P. Schoenberger. 2001. Naive CTLs require a single brief period of antigenic stimulation for clonal expansion and differentiation. Nat. Immunol. 2:423–429. [DOI] [PubMed] [Google Scholar]
- 3.Lau, L.L., B.D. Jamieson, T. Somasundaram, and R. Ahmed. 1994. Cytotoxic T-cell memory without antigen. Nature. 369:648–652. [DOI] [PubMed] [Google Scholar]
- 4.Murali-Krishna, K., L.L. Lau, S. Sambhara, F. Lemonnier, J. Altman, and R. Ahmed. 1999. Persistence of memory CD8 T cells in MHC class I-deficient mice. Science. 286:1377–1381. [DOI] [PubMed] [Google Scholar]
- 5.Bourgeois, C., B. Rocha, and C. Tanchot. 2002. A role for CD40 expression on CD8+ T cells in the generation of CD8+ T cell memory. Science. 297:2060–2063. [DOI] [PubMed] [Google Scholar]
- 6.Janssen, E.M., E.E. Lemmens, T. Wolfe, U. Christen, M.G. von Herrath, and S.P. Schoenberger. 2003. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature. 421:852–856. [DOI] [PubMed] [Google Scholar]
- 7.Shedlock, D.J., and H. Shen. 2003. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science. 300:337–339. [DOI] [PubMed] [Google Scholar]
- 8.Sun, J.C., and M.J. Bevan. 2003. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science. 300:339–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun, J.C., M.A. Williams, and M.J. Bevan. 2004. CD4+ T cells are required for the maintenance, not programming, of memory CD8+ T cells after acute infection. Nat. Immunol. 5:927–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kennedy, M.K., M. Glaccum, S.N. Brown, E.A. Butz, J.L. Viney, M. Embers, N. Matsuki, K. Charrier, L. Sedger, C.R. Willis, et al. 2000. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15–deficient mice. J. Exp. Med. 191:771–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ku, C.C., M. Murakami, A. Sakamoto, J. Kappler, and P. Marrack. 2000. Control of homeostasis of CD8+ memory T cells by opposing cytokines. Science. 288:675–678. [DOI] [PubMed] [Google Scholar]
- 12.Lodolce, J.P., D.L. Boone, S. Chai, R.E. Swain, T. Dassopoulos, S. Trettin, and A. Ma. 1998. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity. 9:669–676. [DOI] [PubMed] [Google Scholar]
- 13.Swain, S.L., H. Hu, and G. Huston. 1999. Class II-independent generation of CD4 memory T cells from effectors. Science. 286:1381–1383. [DOI] [PubMed] [Google Scholar]
- 14.Kassiotis, G., S. Garcia, E. Simpson, and B. Stockinger. 2002. Impairment of immunological memory in the absence of MHC despite survival of memory T cells. Nat. Immunol. 3:244–250. [DOI] [PubMed] [Google Scholar]
- 15.Gett, A.V., F. Sallusto, A. Lanzavecchia, and J. Geginat. 2003. T cell fitness determined by signal strength. Nat. Immunol. 4:355–360. [DOI] [PubMed] [Google Scholar]
- 16.Hu, H., G. Huston, D. Duso, N. Lepak, E. Roman, and S.L. Swain. 2001. CD4(+) T cell effectors can become memory cells with high efficiency and without further division. Nat. Immunol. 2:705–710. [DOI] [PubMed] [Google Scholar]
- 17.Dooms, H., E. Kahn, B. Knoechel, and A.K. Abbas. 2004. IL-2 induces a competitive survival advantage in T lymphocytes. J. Immunol. 172:5973–5979. [DOI] [PubMed] [Google Scholar]
- 18.Lantz, O., I. Grandjean, P. Matzinger, and J.P. Di Santo. 2000. Gamma chain required for naive CD4+ T cell survival but not for antigen proliferation. Nat. Immunol. 1:54–58. [DOI] [PubMed] [Google Scholar]
- 19.Li, J., G. Huston, and S.L. Swain. 2003. IL-7 promotes the transition of CD4 effectors to persistent memory cells. J. Exp. Med. 198:1807–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kondrack, R.M., J. Harbertson, J.T. Tan, M.E. McBreen, C.D. Surh, and L.M. Bradley. 2003. Interleukin 7 regulates the survival and generation of memory CD4 cells. J. Exp. Med. 198:1797–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seddon, B., P. Tomlinson, and R. Zamoyska. 2003. Interleukin 7 and T cell receptor signals regulate homeostasis of CD4 memory cells. Nat. Immunol. 4:680–686. [DOI] [PubMed] [Google Scholar]
- 22.Khaled, A.R., and S.K. Durum. 2002. Lymphocide: cytokines and the control of lymphoid homeostasis. Nat. Rev. Immunol. 2:817–830. [DOI] [PubMed] [Google Scholar]
- 23.Waldmann, T.A. 1989. The multi-subunit interleukin-2 receptor. Annu. Rev. Biochem. 58:875–911. [DOI] [PubMed] [Google Scholar]
- 24.Park, J.H., Q. Yu, B. Erman, J.S. Appelbaum, D. Montoya-Durango, H.L. Grimes, and A. Singer. 2004. Suppression of IL7Ralpha transcription by IL-7 and other prosurvival cytokines: a novel mechanism for maximizing IL-7-dependent T cell survival. Immunity. 21:289–302. [DOI] [PubMed] [Google Scholar]
- 25.Xue, H.H., P.E. Kovanen, C.A. Pise-Masison, M. Berg, M.F. Radovich, J.N. Brady, and W.J. Leonard. 2002. IL-2 negatively regulates IL-7 receptor alpha chain expression in activated T lymphocytes. Proc. Natl. Acad. Sci. USA. 99:13759–13764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tan, J.T., E. Dudl, E. LeRoy, R. Murray, J. Sprent, K.I. Weinberg, and C.D. Surh. 2001. IL-7 is critical for homeostatic proliferation and survival of naive T cells. Proc. Natl. Acad. Sci. USA. 98:8732–8737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Malek, T.R., and A.L. Bayer. 2004. Tolerance, not immunity, crucially depends on IL-2. Nat. Rev. Immunol. 4:665–674. [DOI] [PubMed] [Google Scholar]
- 28.Dooms, H., and A.K. Abbas. 2006. Control of CD4+ T-cell memory by cytokines and costimulators. Immunol. Rev. 211:23–38. [DOI] [PubMed] [Google Scholar]
- 29.Williams, M.A., A.J. Tyznik, and M.J. Bevan. 2006. Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature. 441:890–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaech, S.M., J.T. Tan, E.J. Wherry, B.T. Konieczny, C.D. Surh, and R. Ahmed. 2003. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat. Immunol. 4:1191–1198. [DOI] [PubMed] [Google Scholar]
- 31.Tan, J.T., B. Ernst, W.C. Kieper, E. LeRoy, J. Sprent, and C.D. Surh. 2002. Interleukin (IL)-15 and IL-7 jointly regulate homeostatic proliferation of memory phenotype CD8+ cells but are not required for memory phenotype CD4+ cells. J. Exp. Med. 195:1523–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith, K.A. 1988. Interleukin-2: inception, impact, and implications. Science. 240:1169–1176. [DOI] [PubMed] [Google Scholar]
- 33.Sadlack, B., H. Merz, H. Schorle, A. Schimpl, A.C. Feller, and I. Horak. 1993. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell. 75:253–261. [DOI] [PubMed] [Google Scholar]
- 34.Willerford, D.M., J. Chen, J.A. Ferry, L. Davidson, A. Ma, and F.W. Alt. 1995. Interleukin-2 receptor alpha chain regulates the size and content of the peripheral lymphoid compartment. Immunity. 3:521–530. [DOI] [PubMed] [Google Scholar]
- 35.Suzuki, H., T.M. Kundig, C. Furlonger, A. Wakeham, E. Timms, T. Matsuyama, R. Schmits, J.J. Simard, P.S. Ohashi, H. Griesser, et al. 1995. Deregulated T cell activation and autoimmunity in mice lacking interleukin-2 receptor beta. Science. 268:1472–1476. [DOI] [PubMed] [Google Scholar]
- 36.Malek, T.R., A. Yu, V. Vincek, P. Scibelli, and L. Kong. 2002. CD4 regulatory T cells prevent lethal autoimmunity in IL-2Rbeta-deficient mice. Implications for the nonredundant function of IL-2. Immunity. 17:167–178. [DOI] [PubMed] [Google Scholar]
- 37.Setoguchi, R., S. Hori, T. Takahashi, and S. Sakaguchi. 2005. Homeostatic maintenance of natural Foxp3+ CD25+ CD4+ regulatory T cells by interleukin (IL)-2 and induction of autoimmune disease by IL-2 neutralization. J. Exp. Med. 201:723–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fontenot, J.D., J.P. Rasmussen, M.A. Gavin, and A.Y. Rudensky. 2005. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat. Immunol. 6:1142–1151. [DOI] [PubMed] [Google Scholar]
- 39.D'Cruz, L.M., and L. Klein. 2005. Development and function of agonist-induced CD25+Foxp3+ regulatory T cells in the absence of interleukin 2 signaling. Nat. Immunol. 6:1152–1159. [DOI] [PubMed] [Google Scholar]
- 40.Lenardo, M.J. 1991. Interleukin-2 programs mouse alpha beta T lymphocytes for apoptosis. Nature. 353:858–861. [DOI] [PubMed] [Google Scholar]
- 41.Kneitz, B., T. Herrmann, S. Yonehara, and A. Schimpl. 1995. Normal clonal expansion but impaired Fas-mediated cell death and anergy induction in interleukin-2-deficient mice. Eur. J. Immunol. 25:2572–2577. [DOI] [PubMed] [Google Scholar]
- 42.Refaeli, Y., L. Van Parijs, C.A. London, J. Tschopp, and A.K. Abbas. 1998. Biochemical mechanisms of IL-2-regulated Fas-mediated T cell apoptosis. Immunity. 8:615–623. [DOI] [PubMed] [Google Scholar]
- 43.Khoruts, A., A. Mondino, K.A. Pape, S.L. Reiner, and M.K. Jenkins. 1998. A natural immunological adjuvant enhances T cell clonal expansion through a CD28-dependent, interleukin (IL)-2–independent mechanism. J. Exp. Med. 187:225–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leung, D.T., S. Morefield, and D.M. Willerford. 2000. Regulation of lymphoid homeostasis by IL-2 receptor signals in vivo. J. Immunol. 164:3527–3534. [DOI] [PubMed] [Google Scholar]
- 45.Xue, H.H., J. Bollenbacher, V. Rovella, R. Tripuraneni, Y.B. Du, C.Y. Liu, A. Williams, J.P. McCoy, and W.J. Leonard. 2004. GA binding protein regulates interleukin 7 receptor alpha-chain gene expression in T cells. Nat. Immunol. 5:1036–1044. [DOI] [PubMed] [Google Scholar]
- 46.Van Parijs, L., A. Biuckians, A. Ibragimov, F.W. Alt, D.M. Willerford, and A.K. Abbas. 1997. Functional responses and apoptosis of CD25 (IL-2R alpha)-deficient T cells expressing a transgenic antigen receptor. J. Immunol. 158:3738–3745. [PubMed] [Google Scholar]
- 47.Lennon, G., C. Auffray, M. Polymeropoulos, and M.B. Soares. 1996. The I.M.A.G.E. Consortium: an integrated molecular analysis of genomes and their expression. Genomics. 33:151–152. [DOI] [PubMed] [Google Scholar]
- 48.Andres, P.G., K.C. Howland, A. Nirula, L.P. Kane, L. Barron, D. Dresnek, A. Sadra, J. Imboden, A. Weiss, and A.K. Abbas. 2004. Distinct regions in the CD28 cytoplasmic domain are required for T helper type 2 differentiation. Nat. Immunol. 5:435–442. [DOI] [PubMed] [Google Scholar]