Abstract
Mammalian 2-Cys peroxiredoxin II (Prx II) is a cellular peroxidase that eliminates endogenous H2O2. The involvement of Prx II in the regulation of lipopolysaccharide (LPS) signaling is poorly understood. In this report, we show that LPS induces substantially enhanced inflammatory events, which include the signaling molecules nuclear factor κB and mitogen-activated protein kinase (MAPK), in Prx II–deficient macrophages. This effect of LPS was mediated by the robust up-regulation of the reactive oxygen species (ROS)–generating nicotinamide adenine dinucleotide phosphate (NADPH) oxidases and the phosphorylation of p47phox. Furthermore, challenge with LPS induced greater sensitivity to LPS-induced lethal shock in Prx II–deficient mice than in wild-type mice. Intravenous injection of Prx II–deficient mice with the adenovirus-encoding Prx II gene significantly rescued mice from LPS-induced lethal shock as compared with the injection of a control virus. The administration of catalase mimicked the reversal effects of Prx II on LPS-induced inflammatory responses in Prx II–deficient cells, which suggests that intracellular H2O2 is attributable, at least in part, to the enhanced sensitivity to LPS. These results indicate that Prx II is an essential negative regulator of LPS-induced inflammatory signaling through modulation of ROS synthesis via NADPH oxidase activities and, therefore, is crucial for the prevention of excessive host responses to microbial products.
Peroxiredoxin (Prx), which is a scavenger of H2O2 and alkyl hydroperoxides in living organisms (1), exerts a protective antioxidant role in cells through its peroxidase activity, whereby H2O2, peroxynitrite, and a wide range of organic hydroperoxides (ROOH) are reduced and detoxified (2, 3). The mammalian Prx family members can be divided into six distinct groups (types I through VI) (4). Although different Prx family proteins exhibit distinct tissue and organellar distributions (5), they have been shown to have strong antioxidant activities in vitro (4). In addition to their antioxidant activities, Prx's participate in various biological functions, such as cell proliferation, differentiation, apoptosis, gene expression, and intracellular signaling (6–8). Of note, Prx's have received a great deal of attention owing to their role in regulating levels of H2O2, which is an intracellular signaling molecule that is common to many cytokine-induced signal transduction pathways (2, 6, 9). Prx I and Prx II are prime candidates for regulators of H2O2 signaling initiated by cell-surface receptors, because they are abundant in the cytosol and exhibit high affinity for H2O2 (K m values <20 μM) (10). Indeed, Prx II enhances the activation of platelet-derived growth factor (PDGF) receptor and phospholipase Cγ1 in PDGF signaling via the modulation of H2O2 (11). Recent studies have indicated that Prx II inhibits general immune cell responsiveness, which may be regulated by scavenging low levels of reactive oxygen species (ROS) (12). However, the specific contribution of Prx II to the regulation of inflammatory and innate immune responses has not been elucidated.
LPS is a major recognition marker for the detection of Gram-negative bacteria by the host and a powerful initiator of the inflammatory response to infection. The interactions between pathogens and their multicellular hosts are initiated by the activation of pathogen recognition receptors, among which members of the Toll-like receptor (TLR) family recognize specific pathogen-associated molecular patterns. TLR4 is involved in the activation of the immune system by LPS through the specific recognition of its endotoxic moiety (lipid A). This is a critical event in the immune response to Gram-negative bacteria as well as in the etiology of endotoxic shock (13). The endotoxin shed from an invading pathogen can prime host phagocytes for enhanced cellular responses (13). In these responses, LPS up-regulates nicotinamide adenine dinucleotide phosphate (NADPH) oxidase assembly by inducing flavocytochrome b558 translocation from the specific granules to the plasma membrane, which results in enhanced ROS release (14).
There exists a growing body of evidence that ROS can be purposely made within cells to serve as signaling molecules (15). ROS are produced in mammalian cells in response to the activation of various cell surface receptors (16) and are involved in the modulation of diverse receptor–ligand intracellular signaling pathways (17, 18). The intracellular cytosolic pathways, in turn, regulate a host of transcriptional changes through classical signaling molecules, such as the mitogen-activated protein kinases (MAPKs; e.g., MAPK, c-Jun N-terminal kinase [JNK], and the p38 kinase family), depending on cellular redox conditions. In addition, several lines of evidence indicate that the activation of NF-κB can be controlled by ROS, such as superoxide and H2O2 (19, 20). It has been demonstrated that the proximal events in TLR4 signaling are oxidant dependent and that ROS can modulate NF-κB–dependent transcription through involvement in early TLR4-mediated cellular responses (20).
Major questions persist concerning the function of Prx II in the regulation of endotoxin-induced host responses and the mechanisms by which Prx II makes a contribution to intracellular ROS signaling in TLR4/LPS-mediated cellular activation. In the present report, we demonstrate, using Prx II knockout cells and mice, that Prx II is essential for the regulation of LPS-induced inflammatory gene expression and for the activation of NF-κB and MAPKs through the modulation of NADPH oxidase activities and ROS signaling. Furthermore, we show that the loss of Prx II leads to an increased sensitivity to endotoxemia, which amplifies inflammation and contributes to mortality. Our findings indicate that Prx II is an essential component in the control of normal innate inflammatory responses through endogenous ROS-dependent activation of the NF-κB and MAPK pathways.
RESULTS
Prx II negatively regulates the synthesis of H2O2 in macrophages stimulated with LPS
Prx II has a high affinity for H2O2 and eliminates the intracellular H2O2 generated in response to cell stimulation with growth factors (5). We investigated whether intracellular H2O2 synthesis was up-regulated in Prx II−/− macrophages stimulated with LPS. Upon LPS/TLR4 stimulation, Prx II−/− bone marrow–derived macrophages (BMDMs) exhibited significantly higher (greater than twofold) levels of H2O2 than Prx II+/+ BMDMs. In addition, Prx II−/− BMDMs showed significantly higher baseline levels of H2O2 production than Prx II+/+ BMDMs, as measured by 2′,7′-dichlorofluorescein fluorescence (Fig. 1). Interestingly, the levels of H2O2 production elicited by the TLR2 agonist peptidoglycan (PGN) did not differ between BMDMs from Prx II+/+ and Prx II−/− mice (Fig. 1).
Figure 1.
LPS-induced up-regulation of ROS generation in Prx II–deficient macrophages. Cells were incubated with DCFH-DA in the presence or absence of 1 μg/ml LPS or 10 μg/ml PGN for 30 min. (left) Live cells were washed with serum-free medium and imaged on a confocal microscope. (right) Quantitative data for LPS- or PGN-induced DCF fluorescence in BMDMs from Prx II+/+ and Prx II −/− mice (n = 4). *, P < 0.05; and ***, P < 0.001 compared with WT cell cultures. Bar, 50 μm.
The expression levels of proinflammatory cytokines and cyclooxygenase (COX)-2 and the production of NO are significantly elevated in LPS-stimulated Prx II−/− macrophages
We also investigated the regulatory role of Prx II in LPS-induced inflammatory responses in BMDMs from Prx II+/+ and Prx II−/− mice. Stimulation of BMDMs with 1 μg/ml LPS resulted in significantly higher TNF-α and IL-6 levels (mRNA and protein) in BMDMs from Prx II−/− mice compared with Prx II+/+ mice at different time points (Fig. 2, A and B). We also examined the COX-2 expression levels of LPS-stimulated BMDMs, as COX-2 is an important inflammatory mediator that is induced by various stimuli, including LPS and cytokines (21). The LPS-induced expression of COX-2 was substantially enhanced in Prx II–deficient cells as compared with control cells (Fig. 2, C and D), which confirms that Prx II negatively modulates inflammatory factor production in response to LPS. LPS stimulation also induced higher NO production in Prx II−/− than in Prx II+/+ BMDMs. Of note, neither PGN nor the synthetic bacterial lipoprotein (BLP) lipopeptide (Pam3Cys-Ser-Lys4- OH) produced any differences in cytokine production between BMDMs from Prx II+/+ and Prx II−/− mice (Fig. 2 F). LPS induced significant production of proinflammatory cytokines in BMDMs and splenocytes in both dose- and time-dependent manners (Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20061849/DC1). These results imply a significant role for Prx II in the specific control of LPS-induced proinflammatory responses in macrophages and splenocytes.
Figure 2.
Proinflammatory mediators are up-regulated in Prx II–deficient macrophages. (A and B) BMDMs from Prx II+/+ and Prx II−/− mice with the 129/SvJ or C57BL/6 background were stimulated with 1 μg/ml LPS, and cell lysates and supernatants were harvested at the times indicated. Total RNA was assessed by PCR for TNF-α, IL-6, and COX-2 mRNA (mice with the C57BL/6 background; A), and protein expression was measured by ELISA (B). (C and D) BMDMs from Prx II+/+ and Prx II−/− mice were stimulated with 1 μg/ml LPS for the times indicated. COX-2 levels were determined by immunoblotting with the anti–COX-2 Ab (mice with the C57BL/6 background; C). Immunofluorescence images for COX-2 in BMDMs are shown (D). Bar, 100 μm. (E) Nitrite levels in response to LPS were measured by the Griess reagent in BMDMs from mice with the C57BL/6 background. (F) BMDMs were stimulated with 10 μg/ml PGN, 10 μg/ml BLP, or 1 μg/ml LPS, and the supernatants were harvested at 18 h. Cytokine production was measured by ELISA. Data shown are the mean ± SD of three experiments. *, P < 0.05; and ***, P < 0.001 compared with WT cell cultures stimulated with LPS. MC, media control.
Prx II negatively modulates NF-κB activation and the phosphorylation of MAPK pathway kinases in response to LPS
The above data (Fig. 2) indicate that Prx II plays a regulatory role in LPS-activated signaling cascades (22). Thus, the role of Prx II in regulating LPS-induced NF-κB activation was addressed using primary mouse macrophages and Raw264.7 cells transfected with Prx II constructs. The expression level of IκBα was dramatically attenuated in Prx II–deficient BMDMs after LPS treatment for 0–15 min, whereas it was sustained to a certain extent until 120–240 min in WT cells (Fig. 3 A). In addition, the phosphorylation of IKKα/β was more rapid and higher in Prx II−/− BMDMs from 15 min after LPS stimulation than in WT cells (Fig. 3 A). In addition, we performed electrophoretic mobility shift assays (EMSAs) with a 32P-labeled NF-κB consensus sequence using nuclear extracts from LPS-stimulated primary mouse splenocytes. The results show a time-dependent increase in NF-κB DNA binding activity in nuclear extracts from both cells, although the Prx II−/− cells exhibited greater DNA binding activity for NF-κB as compared with the WT cells (Fig. 3 B). These findings correlate with those showing differential patterns of degradation of IκBα and IKKα/β phosphorylation between cells from Prx II+/+ and Prx II−/− mice.
Figure 3.
Prx II acts as a physiological negative regulator of LPS signaling. (A) BMDMs from Prx II–deficient and WT mice were stimulated with 1 μg/ml LPS, and cell lysates were harvested at the times indicated. Western blot analysis was conducted using the anti-IκBα and anti–phospho-IKKα/β Abs. The detection of actin in each sample serves as a loading control. (B) NF-κB activation was measured by EMSA with 10 μg of nuclear extracts of isolated splenocytes from Prx II–deficient and WT mice. The specificity of NF-κB binding was assessed by incubating the nuclear extracts with a 100-fold excess of unlabeled specific probe (lanes 5 and 10; C, cold). (C) RAW264.7 cells were transfected with WT–Prx II, DN–Prx II, or empty vector. After a 24-h incubation period in normal culture medium, the transfected cells were stimulated with LPS at the times indicated. The lysates of 5 × 105 cell equivalents from each transfectant were immunoblotted with the specific Abs, as indicated. The same blots were stripped and reprobed with the anti–β-actin Ab. (D) The experimental conditions followed the pattern outlined in A. The cells were harvested and subjected to Western blot analysis for phosphorylated SAPK/JNK, ERK1/2, and p38 MAPK. The same blots were washed and blotted for total MAPKs as the loading controls. Data shown are the mean ± SD and are representative of three independent experiments that gave similar results (top). The bar graph representing mean phospho-MAPK/total MAPK protein expression in cytoplasmic extracts of BMDMs was obtained by densitometric analysis (see Materials and methods).
We also examined LPS-mediated NF-κB activation in Raw264.7 cells transfected with Myc-tagged Prx II–WT and Prx II–dominant-negative (DN) constructs or empty vector, respectively. Consistent with the findings in Fig. 3 B, the IκBα levels were considerably decreased from the earliest time point of measurement, whereas IKKα/β phosphorylation was substantially increased in Prx II–DN–transfected cells, when compared with cells transfected with the empty vector (Fig. 3 C). In contrast, IKKα/β phosphorylation was totally abrogated, and the IκBα levels were almost consistent in Prx II–WT–transfected cells during the 60-min period of LPS treatment (Fig. 3 C).
Activation of the MAPK pathways plays an essential role in the mediation of macrophage responses to proinflammatory stimuli, such as LPS and cytokines (21, 23, 24). Therefore, we investigated whether Prx II could affect MAPK signaling cascades, including p38 MAPK (p38) and extracellular signal–regulated kinase (ERK) 1/2, during LPS treatment. Notably, the levels of phosphorylation of p38 and ERK1/2 were negligible in LPS-stimulated cells transfected with the Prx II–WT construct, whereas they were considerably increased in cells transfected with the Prx II–DN construct, as compared with those that carried the empty vector (Fig. 3 C). Consistent with the findings in Fig. 3 C, primary BMDMs from Prx II–deficient mice showed lower activation of MAPKs, which included JNK, ERK 1/2, and p38, than the primary BMDMs from WT mice, although the kinetics of activation during LPS treatment were similar for cells for the two types of mice (Fig. 3 D). Other TLR agonists, such as TLR2 (PGN or BLP), TLR3 (poly I:C), or TLR9 (CpG-ODN), did not produce any differences in the phosphorylated levels of MAPKs between BMDMs from Prx II+/+ and Prx II−/− mice (Fig. S2 A, available at http://www.jem.org/cgi/content/full/jem.20061849/DC1). Similar patterns of MAPK activation were observed for LPS-stimulated splenocytes (Fig. S2 B). Therefore, these data demonstrate that Prx II plays a key role in regulating NF-κB and MAPK activation in response to LPS.
Endogenous ROS induced by NADPH oxidase is specifically involved in the LPS-induced activation of MAPK and proinflammatory responses of Prx II–deficient cells
ROS, whether endogenously produced or exogenously added, have been shown to activate critical signaling pathways to promote cell activation or growth responses in a growing number of cell systems (25, 26). Therefore, we investigated the potential contribution of ROS to the activation of MAPK and proinflammatory responses using selective ROS inhibitors in BMDMs from Prx II+/+ and Prx II−/− mice. Pretreatment with 10 and 30 mM of the antioxidant N-acetyl-l-cysteine (NAC) significantly reduced the LPS-mediated activation of MAPK and IκBα degradation in cells from both Prx II+/+ and Prx II−/− mice (Fig. 4, A and B). Intriguingly, both 10 and 20 μM of an inhibitor of NADPH oxidase (diphenylene iodonium [DPI]) and 1 and 10 μM of an inhibitor of mitochondrial electron transfer chain subunit I (rotenone) were capable of blocking LPS-induced MAPK and NF-κB activation in a dose-dependent manner, albeit only in Prx II–deficient cells (Fig. 4, A and B). On the other hand, pretreatment with 10 and 100 μM of a xanthine oxidase inhibitor (allopurinol) or 100 and 500 μM of NO synthase inhibitors N G-monomethyl-l-arginine (l-NMMA) and Nω-nitro-l-arginine methyl ester (l-NAME) did not significantly modulate MAPK activation or IκBα degradation in cells from either Prx II+/+ or Prx II−/− mice (Fig. 4 A).
Figure 4.
Endogenous ROS induced by NADPH oxidase is specifically involved in LPS signaling and proinflammatory responses in Prx II–deficient cells. (A) After preincubation for 30 min with 10 and 30 mM NAC, 10 and 20 μM DPI, 10 and 100 μM allopurinol, 1 and 10 μM rotenone, 100 and 500 μM l-NMMA, or 100 and 500 μM l-NAME, BMDMs were stimulated with 1 μg/ml LPS for 30 min. The cells were harvested and subjected to Western blot analysis for IκBα, phosphorylated ERK1/2, and p38 MAPK. The same blots were washed and blotted for β-actin as the loading control. Data shown are representative of three independent experiments that gave similar results. (B) The experimental conditions followed the pattern outlined in A. Culture supernatants were harvested after stimulation with LPS for 18 h, and the TNF-α and IL-6 expression levels were measured by ELISA. Data shown are the mean ± SD of three experiments. *, P < 0.05; **, P < 0.01; and ***, P < 0.001 compared with WT cell cultures stimulated with LPS. MC, media control.
We also examined the effects of various ROS inhibitors on the LPS-stimulated proinflammatory cytokine responses of BMDMs from Prx II+/+ and Prx II−/− mice. As shown in Fig. 4 B, pretreatment with either DPI or rotenone significantly attenuated the LPS-induced secretion of TNF-α and IL-6 in Prx II−/− BMDMs but not in WT cells. These data suggest that the augmented responses of LPS-induced MAPK activation and proinflammatory cytokine release in Prx II–deficient cells are probably mediated via ROS generated by NADPH oxidase and the mitochondria.
LPS stimulation induces greater ROS generation and activation of NADPH oxidases in Prx II–deficient BMDMs than in WT BMDMs
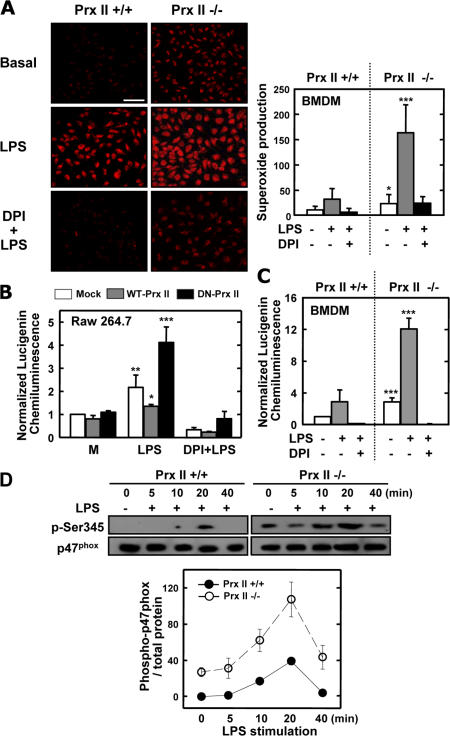
The data (Fig. 4) suggest that both NADPH oxidase and the generation of superoxide contribute to LPS-mediated inflammatory signaling in Prx II–deficient cells. Therefore, we evaluated the superoxide generation and NADPH oxidase activities of Prx II+/+ and Prx II−/− cells. BMDMs from Prx II+/+ and Prx II−/− mice were labeled with the ROS-sensitive indicator dihydroethidium (DHE), and the generation of oxidized fluorescent DHE was monitored after LPS stimulation (Fig. 5 A). As early as 10 min after LPS simulation, an increase in ROS generation was observed in both types of cells, although significantly elevated responses were detected in the Prx II–deficient cells. The ROS levels remained elevated for several hours, as assessed by determinations of DHE fluorescence 2, 6, and 18 h after LPS stimulation (unpublished data). In addition, this increase in ROS was inhibited by the presence of 10 μM of the NADPH oxidase inhibitor DPI (Fig. 5 A).
Figure 5.
ROS-generating NADPH oxidase activities are up-regulated in Prx II–deficient macrophages. (A) DHE fluorescence image after 10 min of stimulation. (right) Quantitative data shown are the mean ± SD of values from five random fields and are representative of two independent experiments. Bar, 50 μm. (B and C) NADPH oxidase activity measured by lucigenin luminescence in RAW264.7 cells transfected with WT–Prx II, DN–Prx II, or empty vector (B), and in BMDMs from Prx II–deficient and WT mice (C). MC, media control. (D) BMDMs were lysed, and proteins from 5 × 105 cells were analyzed by SDS-PAGE and immunoblotting with anti–phospho-Ser345–p47phox Ab (p-Ser345) or anti-p47phox Ab (p47phox). The Western blots from different experiments were scanned, phosphorylated, total p47phox was quantified by densitometry, and the intensity of phosphorylated p47phox was corrected for the amount of p47phox. Results shown are the mean ± SD of three experiments. *, P < 0.05; **, P < 0.01; and ***, P < 0.001 compared with WT cell cultures.
We also examined whether NADPH oxidase activities were differentially modulated in cells from Prx II+/+ and Prx II−/− mice in response to LPS stimulation. As shown in Fig. 5 B, higher levels of NADPH-induced lucigenin chemiluminescence (CL) were detected in Prx II−/− BMDMs than in WT cells after stimulation with LPS. We also studied the effect of transient overexpression of Prx II–DN in RAW264.7 cells stimulated with LPS; cells that lacked Prx II showed 4.1-fold higher levels of NADPH-induced lucigenin CL than cells that expressed an abundance of Prx II (Fig. 5 C). Significant inhibition of NADPH-induced lucigenin CL was recorded after pretreatment with DPI (Fig. 5, B and C).
Phosphorylation of p47phox is one of the key intracellular events associated with NADPH oxidase activation, and Ser345 phosphorylation plays a functional role in the potentiation of NADPH oxidase activation (27). Therefore, we examined the levels of LPS-induced p47Phox phosphorylation in the BMDMs and splenocytes of Prx II+/+ and Prx II−/− mice using the anti–phospho-Ser345–p47phox antibody (Ab). BMDMs were incubated with LPS for 5–40 min, and the levels of phosphorylation of Ser345 in p47phox were analyzed by Western blot analysis. In the absence of added LPS, weak basal phosphorylation of p47Phox was detected, and phosphorylation of p47phox peaked after 20 min of LPS stimulation. LPS-induced phosphorylation of p47phox was higher in the lysates of Prx II–deficient cells than in those of WT cells (Fig. 5 D). Western blots using an Ab directed against p47phox showed that equivalent amounts of protein had been loaded in the wells. These data suggest that Prx II–deficient macrophages generate higher levels of ROS in response to LPS because of their higher phagocyte oxidase activities.
In vivo function of Prx II during endotoxin-induced lethal shock
We performed an in vivo experiment to assess whether Prx II deficiency affects susceptibility to endotoxic shock. Prx II+/+ and Prx II−/− mice were injected i.p. with LPS (10 and 20 mg/kg body weight), and survival was monitored for 5 d. On day 5 after injection, the lower dose had killed 80% of the Prx II−/− mice but only 10% of the Prx II+/+mice (Fig. 6 A). The higher dose of LPS had killed 100% of the Prx II−/− mice and only 35% of the Prx II+/+mice by day 5 after injection (Fig. 6 A). Consistent with these findings, the LPS-induced levels of TNF-α, IL-6, and NO were significantly elevated in the sera of Prx II–deficient mice (Fig. 6 B). On the other hand, there were no remarkable differences in the survival rates and cytokine production levels of Prx II+/+ and Prx II−/− mice after i.p. injection of Staphylococcus aureus (Fig. S3, A and B, available at http://www.jem.org/cgi/content/full/jem.20061849/DC1). When LPS-induced COX-2 expression of Prx II+/+ and Prx II−/− mice was examined in spleens, the COX-2 expression of Prx II−/− mice was found to be substantially higher than that of WT mice (Fig. 6 C). On gross examination, the spleens from Prx II−/− mice were observed to be larger in size than those from Prx II+/+ mice (Fig. S3 C).
Figure 6.
Prx II–deficient mice show enhanced sensitivity to LPS-induced lethal shock. (A) LPS (10 and 20 mg/kg body weight) was injected i.p. into WT mice (+/+; n = 10) and Prx II–deficient mice (−/−; n = 10). Viability was assessed every 2 h for the first 10 h and every 10 h thereafter. There was no further increase in death after 80 h. (B) Serum concentrations of TNF-α, IL-6, and NO in WT and Prx II–deficient mice (n = 10 per group), as measured by ELISA (for TNF-α and IL-6) and by nitrate and nitrite colorimetric assay (for NO) 18 h after LPS injection. Results shown are the mean ± SD of three experiments (n = 10). *, P < 0.05; **, P < 0.01; and ***, P < 0.001 compared with WT mice. (C) COX-2 immunoreactivity in spleens from WT and Prx II–deficient mice shows an enhanced cytoplasmic signal for COX-2 in Prx II–deficient mice 18 h after LPS injection. Images are representative spleen sections from five mice per group. Bars: (top) 300 μm; (bottom) 75 μm. (D) i.v. administration of 1011 Prx II viral particles into Prx II–deficient mice (n = 8) significantly increases the survival rate from a lethal dose of LPS (20 mg/kg body weight) compared with control virus-injected Prx II–deficient mice (n = 8).
Furthermore, an “add-back” rescue experiment was performed using a recombinant adenovirus expression system in Prx II+/+ and Prx II−/− mice. After i.v. injection of adenoviral vectors that carry the Prx II gene (Ad–Prx II), Prx II was expressed in the spleens of the Prx II−/− mice, whereas control mice injected with mock virus did not express Prx II (unpublished data). The reintroduction of Prx II via adenovirus delivery significantly improved survival from 0% in the mock virus–injected control mice to 50% in the Ad–Prx II–injected mice on day 5 (P = 0.002; Fig. 6 D). These results demonstrate that Prx II plays an essential role in regulating animal sensitivity to endotoxin-induced lethal shock.
Intracellular H2O2 plays an important role in the increased responses of Prx II−/− cells to LPS-induced inflammation
Based on the aforementioned observations, we investigated whether the up-regulated responses to LPS were related to increased intracellular H2O2 production in Prx II−/− cells. To answer this question, we transduced Prx II−/− cells with adenoviral vectors that carry the catalase gene (Ad-CAT) or Ad–Prx II. H2O2 production was significantly abrogated in BMDMs that were transduced with Ad-CAT or Ad–Prx II, both at baseline and after LPS stimulation, as compared with BMDMs that were transduced with the control virus (Fig. 7 A). Catalase overexpression using adenoviral vectors significantly inhibited LPS-induced MAPK and NF-κB activation (Fig. 7 B) and decreased inflammatory cytokine release (Fig. 7 C) in Prx II–deficient cells. In addition, the administration of the H2O2 scavengers (catalase or pyruvate) reduced, whereas the catalase inhibitor (3-amino-1,2,4-triazole) treatment enhanced, the LPS-induced inflammatory responses of Prx II−/− cells in a dose-dependent manner (Fig. S4, A and B, available at http://www.jem.org/cgi/content/full/jem.20061849/DC1). Further, the administration of catalase gave significantly improved survival in Prx II−/− mice 5 d after LPS challenge (P = 0.03; Fig. S4 C). Collectively, these results suggest that increased H2O2 production plays an important role in LPS-induced inflammatory activation in Prx II–deficient cells.
Figure 7.
Intracellular H2O2 plays an important role in the increased sensitivity of Prx II−/− mice to LPS-induced inflammatory responses. (A) Prx II−/− BMDMs were infected with Ad–Prx II, Ad-CAT (catalase), or control adenovirus (mock) at 200 PFU/cell, and the cells were incubated with DCFH-DA in the presence or absence of 1 μg/ml LPS for 30 min. The results are quantitative data for DCF fluorescence in the BMDMs from Prx II−/− mice (n = 4). *, P < 0.05; and ***, P < 0.001 compared with BMDMs infected with the control virus. (B) The experimental conditions followed the protocol outlined in A. The cells were harvested and subjected to Western blot analysis for IκBα, phosphorylated ERK1/2, p38 MAPK, and SAPK/JNK. The same blots were washed and blotted for β-actin as the loading control. Data shown are representative of three independent experiments that gave similar results. (C) The experimental conditions followed the protocol outlined in A. Culture supernatants were harvested after stimulation with LPS for 18 h, and the TNF-α and IL-6 expression levels were measured by ELISA. Data shown are the mean ± SD of three experiments. ***, P < 0.001 compared with cell cultures infected with the control virus.
DISCUSSION
Accumulating evidence suggests that Prx's, a family of peroxidases initially described for their antioxidant capabilities, are important factors for the regulation of signaling pathways (11, 28). Although studies performed in other laboratories have demonstrated roles for Prx's under various treatment conditions, studies of their roles in the regulation of LPS signaling and modulation of inflammatory responses are scarce. LPS stimulation has been shown to increase ROS in monocytes/macrophages (19). ROS are key components of postreceptor intracellular signaling pathways (29), and activation of the NF-κB and MAPK pathways plays a key role in mediating macrophage responses to proinflammatory stimuli, such as LPS and cytokines (21, 23, 24). The data presented in this paper demonstrate that Prx II, which is a ubiquitous, abundant, and highly conserved thioredoxin (TRX) peroxidase, plays an essential role in regulating LPS signaling and sensitivity to endotoxin-induced lethal shock. Importantly, differential responses to LPS were conserved in Prx II+/+ and Prx II−/− cells from two genetically disparate backgrounds (Fig. 2, A, C, and E; and not depicted).
Recent studies on the roles of 2-Cys Prx's indicate that they not only abrogate the intracellular H2O2 levels that are increased by receptor stimulation but that they also suppress downstream signaling responses, which include NF-κB transcriptional activity, stress-activated protein kinase (SAPK)/JNK activity, and apoptosis (30). In the LPS signaling pathway, ROS regulation of any intermediate steps, including TLR4-dependent activation of IL-1 receptor–associated kinase and subsequent TNF-α receptor–associated factor 6–induced activation of MAPK kinase kinase family members, accounts for the redox sensitivity of the individual MAPKs, as well as that of NF-κB (31). The increased LPS-induced inflammatory responses of Prx II–deficient cells appear to be linked to the antioxidant activity of Prx II given that the overall levels of H2O2 and superoxide were significantly higher in Prx II–deficient cells, both at baseline and after LPS stimulation, than those in WT cells. In addition, the administration of the H2O2 scavenger catalase mimicked the reversal effects of Prx II on LPS-mediated inflammatory signaling and modulated endotoxin-induced lethal shock in Prx II−/− mice (Fig. 7, A–C; and Fig. S4). These data strongly suggest that intracellular H2O2 is attributable, at least in part, to the increased inflammatory responses of Prx II−/− cells to LPS.
ROS, which include H2O2, superoxide anions, and hydroxyl radicals (OH), are used as intracellular signaling molecules by pathways that are involved in proliferation, stress responses, mating behavior, and apoptosis. Potential sources of superoxide anions include the mitochondrial electron transport chain, xanthine oxidase, cytochrome P-450 enzymes, uncoupled NO synthases, the phagocytic myeloperoxidase system, and NADPH oxidases (32). The mitochondrial electron transport chain is an important source of ROS, and mitochondria are quite susceptible to oxidative damage, which can result in enhanced mitochondrial ROS production (32, 33). Our data indicate that NADPH oxidase and mitochondrion-dependent ROS generation may play important roles in Prx II regulation of LPS-induced inflammatory responses in macrophages.
NADPH oxidase activation is one of the important sources of ROS in phagocytes, including macrophages, during phagocytosis (34). In resting phagocytes, NADPH oxidase is mainly present in unassembled form on the plasma membrane or in the cytosol (35). Upon stimulation, the cytosolic components p47phox, p67phox, p40phox, and rac2 translocate to the plasma membrane or phagosome/endosome membrane and associate with the membrane-bound flavocytochrome b to constitute the intact and functioning enzyme (36). Finally, this multicomponent enzyme, which is present on the phagosomal membrane, utilizes electrons derived from cytosolic NADPH to generate superoxide anion, which subsequently dismutes to H2O2 and results in the formation of other radicals that destroy engulfed pathogens (36). In NADPH oxidase activation, the phosphorylation of several serines of p47phox plays a pivotal role in oxidase activation in intact cells (27, 37, 38). Our data show that the phosphorylation of p47phox is substantially up-regulated in Prx II–null cells. These results suggest that increased NADPH oxidase activity and excessive ROS generation are involved in the up-regulation of LPS-induced inflammation and MAPK activation in Prx II–deficient macrophages through increased p47phox-Ser345 phosphorylation. Although mouse p47phox-Ser345 is located in a different sequence than human p47phox-Ser345, this anti–human Ab recognizes phosphorylated p47phox of mouse origin (unpublished data). Recent studies have demonstrated that Ser345 is a point of convergence used by different MAPK activities to induce priming of ROS production (38). Collectively, our results indicate that Prx II attenuates the selective phosphorylation of Ser345 on p47phox, which is a novel regulatory mechanism for inflammation that may represent a target for antiinflammatory therapy.
The observed linkage of increased NADPH oxidase activity and p47phox-Ser345 phosphorylation with Prx II deficiency raises the question as to whether this activation is caused by a direct effect of Prx II as a signal regulator downstream of TLR4/LPS signaling or an indirect effect that is manifested through oxidation/inactivation of the enzymes that are involved in the dephosphorylation of several protein kinases. Previous studies have demonstrated that Prx II suppresses protein tyrosine phosphatase inactivation upon PDGF stimulation, leading to site-specific suppression of PDGFR signaling (11). In other studies, the modulation of H2O2 by Prx II indicates that H2O2 produced in response to stimulation of cells with epiderman growth factor or PDGF potentiates the accumulation of phosphatidyl inositol-3,4,5-triphosphate and subsequent activation of the phosphatidyl inositol 3 kinase/Akt signaling pathway through oxidation/inactivation of PTEN molecules (28). It is possible that Prx II influences the modulation of the cellular phosphatase oxidation/inactivation system in response to TLR4/LPS signaling.
It is noteworthy that the other TLR agonists tested in this study, with the exception of TLR4/LPS, did not induce different levels of H2O2 production and downstream signaling responses in the BMDMs of Prx II+/+ and Prx II−/− mice. In addition, there were no significant differences in the survival rates of Prx II+/+ and Prx II−/− mice after i.p. injection with S. aureus (Fig. S3, A and B). This phenomenon may be attributable to the selective role of Prx II in TLR4/LPS signaling through the modulation of NADPH-dependent ROS generation. Prx proteins are capable of serving as peroxidases and involve TRX and/or glutathione as the electron donor (39). TRX interacts with apoptosis signal–regulating kinase 1 (ASK1), which is a mitogen-activated protein 3 kinase and a sensor of oxidative stress (39). Previous studies have shown that ASK1 is selectively required for LPS-induced MAPK activation and that the induction of proinflammatory cytokines is dependent on TLR4 but not on any other TLR (40). In addition, recent studies have shown that the ROS-dependent MAPK activation is based on the ability of reduced TRX to bind to and inhibit both ASK1 and the levels of TRX–ASK1 complex upon oxidation (41). Based on these studies, it is possible that TLR4/LPS stimulation specifically alters the oxidation/reduction of TRX–ASK1 complex in association with Prx II and makes it available to activate downstream signaling targets. Future studies should reveal the precise molecular mechanisms by which Prx II regulates the fine control of TLR4/LPS signaling in the context of ROS generation.
As there is increasing evidence that the innate response can be dramatically influenced by cellular redox factors, a better understanding of oxidative regulation of innate immunity could lead to new treatments for sepsis. The increase in ROS after LPS challenge has been demonstrated in different models of septic shock using peritoneal macrophages and lymphocytes (42, 43). The injection of NAC, which decreases the levels of ROS and TNF-α and prevents the activation of nuclear translocation of NF-κB, may increase mouse survival from lethal endotoxemia (44). In addition, a recent report has shown that disruption of nuclear factor–erythroid 2–related factor 2 (Nrf2), which is a basic leucine zipper transcription factor that regulates redox balance and stress responses, dramatically increases mouse mortality from endotoxin-induced septic shock (45). Several in vitro and in vivo studies have implicated 2-Cys Prx's as either therapeutic targets or diagnostic biomarkers for major diseases. For example, a previous study showing the specific involvement of Prx II in smooth muscle cell proliferation (11) suggests the potential therapeutic use of Prx II for the inhibition of atherogenic lesion progression. In addition, Prx III knockdown by RNA interference appears to sensitize cervical cancer cells to death receptor– and stress-mediated apoptosis, possibly through the disruption of mitochondrial function (46). The therapeutic implications of our observations are highlighted by the finding that rescue of Prx II function by injection of purified Ad–Prx II increased the survival rate of Prx II−/− mice from endotoxic shock. Our data emphasize the essential role of Prx II in the regulation of endotoxic shock susceptibility, which could be useful in the development of a novel treatment modality for patients with septic peritonitis.
In summary, the present study suggests a novel key role for Prx II in regulating LPS-induced activation of signaling cascades that are involved in inflammatory responses and establishes a link between this role and the observed generation of ROS and NADPH oxidase activation. We demonstrate for the first time the importance of Prx II in the regulation of sensitivity to endotoxic shock and the role of Prx II in NADPH oxidase–derived ROS generation within the signaling cascade. Although further studies are needed to elucidate the precise mechanisms underlying TLR4-specific regulation of ROS and inflammatory factor activation, our findings may encourage the design and discovery of Prx II–selective drugs for infectious diseases and inflammation.
MATERIALS AND METHODS
Mice and cells.
All animal-related procedures were reviewed and approved by the Institutional Animal Care and Use Committee of the Korea Research Institute of Bioscience and Biotechnology. WT and Prx II–deficient mice with the 129/SvJKist background were maintained under standard laboratory conditions on a 12-h light/dark cycle, with free access to food and water. Except when specified otherwise, all of the experiments described in this study were performed using mice with the 129/SvJKist background. We also maintained WT and Prx II–deficient mice with the C57BL/6 background for some control experiments. All of the animal procedures were conducted in accordance with the guidelines of the Institutional Animal Care and Use Committee of Chungnam National University. The genotyping of animals was performed as described previously (47). Mice were used for LPS challenge at 8–10 wk of age. The experimental groups were age and sex matched. Escherichia coli O26:B6 LPS (Sigma-Aldrich) was diluted in sterile PBS and injected i.p. into the animals.
BMDMs were differentiated for 5–7 d in M-CSF–containing media, as described previously (48). The culture medium consisted of DMEM that was supplemented with 10% L929 cell-conditioned medium (as a source of M-CSF), 10% heat-inactivated FCS, 1 mM sodium pyruvate, 50 U/ml penicillin, 50 μg/ml streptomycin, and 5 × 10−5 M 2-mercaptoethanol. Splenocytes were isolated by mechanical disruption followed by differential centrifugation and resuspension in DMEM. The mouse macrophage cell line RAW264.7 was purchased from the American Type Culture Collection and grown in DMEM/glutamax supplemented with 10% FCS.
Reagents, DNA, and Abs.
For the in vitro experiments, LPS from E. coli (0111:B4), PGN (Sigma-Aldrich) from S. aureus (Fluka), and poly I:C (GE Healthcare) were used. BLP, which is a synthetic bacterial lipopeptide (Pam3Cys-Ser-Lys4-OH) derived from the immunologically active N terminus of BLP, was purchased from Boehringer. The mouse-specific oligodeoxynucleotide CpG-ODN1668 (5′-TCCATGACGTTCCTGATGCT-3′) was a kind gift from S.J. Lee (Seoul National University, Seoul, Korea). The following reagents were purchased from Sigma-Aldrich and were used at the indicated concentrations unless specified otherwise: NAC (10 and 30 mM), DPI (10 and 20 μM), allopurinol (10 and 100 μM), rotenone (1 and 10 μM), l-NMMA (100 and 500 μM), and l-NAME (100 and 500 μM).
The expression plasmids that encode Myc–Prx II–WT and Myc–Prx II–DN (mutant forms a dimer with endogenous Prx II–WT) were generous gifts from S.-W. Kang (Ewha Womans University, Seoul, Korea). Cells were transfected using Lipofectamine, as indicated by the manufacturer (Invitrogen). Specific Abs against ERK1/2, phospho-(Thr202/Tyr204)-ERK1/2, p38, phospho-(Thr180/Tyr182)-p38, SAPK/JNK, phospho-(Thr183/Tyr185)-SAPK/JNK, and phospho-IKKα/β were purchased from Cell Signaling. Abs reactive with Prx II were obtained from Lab Frontier. Abs to IκBα, COX-2, and p47phox were purchased from Santa Cruz Biotechnology, Inc. The anti–phospho-(Ser345)-p47phox Ab has been described previously (38). The Ab to β-actin was purchased from Sigma- Aldrich. All other reagents were purchased from Sigma-Aldrich unless otherwise indicated.
Enzyme-linked immunosorbent assay, RT-PCR, and Western blots.
BMDMs were treated as indicated in the figures and processed for analysis by sandwich ELISA, RT-PCR, and Western blot, as described previously (48). For the sandwich ELISA, serum and cell-culture supernatants were analyzed for cytokine content using Duoset Ab pairs (R&D Systems) for the detection of IL-6 and TNF-α. RT-PCR was performed after total RNA was extracted from the cells using TRIzol (Invitrogen). For Western blot analysis, Abs to phosphorylated and total p38, SAPK/JNK, ERK1/2, and β-actin were used at 1:1,000 dilutions. Specific bands were developed by enhanced CL (GE Healthcare).
Immunostaining.
COX-2 expression in BMDMs was assessed by fluorescence microscopy. The cells were plated on glass coverslips. When the cells were 70–80% confluent, they were fixed with cold methanol for 10 min at −20°C. Fixed cells were washed three times with PBS and blocked by incubation in PBS with 3% BSA for 1 h at room temperature. The cells were washed once with 0.3% PBS/BSA and incubated overnight at 4°C with the anti–smooth muscle α-actin primary Ab (1:100). The coverslips were washed three times with PBS and incubated for 1 h at room temperature with a secondary Ab that was conjugated to the green fluorescent Alexa Flour 488 dye. Immunofluorescence images were taken using a confocal laser-scanning microscope (FV500; Olympus).
For immunostaining of tissue sections, spleens were fixed by inflating the tissues with neutral-buffered formalin and sectioned, and the slides were assessed for immunohistochemistry of COX-2 expression, as previously described (49).
H2DCFDA and DHE assays.
After LPS stimulation, the cells were incubated with either 10 μM H2DCFDA or 2 μM DHE (Invitrogen) for 15 min at 37°C in 5% CO2. The cells were collected and washed once with RPMI 1640 that contained 10 mM Hepes (Invitrogen). The cells were examined under a laser-scanning confocal microscope (LSM 510; Carl Zeiss MicroImaging, Inc.). The images were digitized and stored at a resolution of 512 × 512 pixels. Five groups of cells were randomly selected from each sample, and the mean relative fluorescence intensity for each group of cells was measured with a vision system (LSM 510, version 2.3; Carl Zeiss MicroImaging, Inc.) and averaged for all the groups. All of the experiments were repeated at least three times.
Measurement of NADPH oxidase activity.
NADPH oxidase activity was measured using the lucigenin CL method described previously (50), with some modifications. In brief, the reaction mixture contained 50 mM phosphate buffer (pH 7), 1 mM EGTA, 150 mM sucrose, and 500 μM lucigenin as the electron acceptor, and either 100 μM NADPH or 100 μM NADH as the electron donor. The reaction was initiated by the addition of cell homogenate (150–200 μg of protein). Luminescence was monitored on a bioluminescence plate.
EMSA.
EMSA were performed as described previously (51). In brief, nuclear extracts (2 μg of protein content) were incubated with a consensus double- stranded NF-κB oligonucleotide (5′-AGT TGAGGGGACTTTCCCAGG-3′; Promega) that was end labeled with γ-[33P]ATP (PerkinElmer). The products were separated by electrophoresis, as described previously (51).
Adenovirus production.
Recombinant replication-deficient adenoviruses were used in all the experiments. The adenovirus that encodes the Prx II cDNA was a gift from S.-W. Kang, and the adenovirus that encodes the catalase cDNA was a gift from K.-J. Lee (Ewha Womans University, Seoul, Korea). Large-scale amplification of adenovirus was performed as previously described (52). In brief, HEK293 cells were transfected with adenoviral vector (multiplicity of infection = 2), and the replicated virus particles were concentrated by CsCl gradient ultracentrifugation. Viral titers were determined as previously described (52). Purified and concentrated adenoviruses that had titers in the range of 109–1011 PFU/ml were suspended in 10 mM Tris-HCl (pH 8.), 2 mM MgCl2, and 5% sucrose. For in vivo delivery of adenovirus, 8–10-wk-old Prx II−/− mice were injected i.v. with Ad–Prx II (1011 viral particles) before 24 h of LPS challenge. Another cohort of Prx II−/− mice (n = 8) was injected with mock adenovirus. For in vitro infection with adenovirus, the BMDMs were plated at 5 × 105 cells per well in 96-well tissue culture plates with RPMI 1640 plus 2% FCS medium that contained the recombinant adenovirus at a concentration of 200 PFU per cell, according to the method described previously (53).
Statistical analyses.
For parametric data, the results are expressed as the mean ± SEM and compared with the two-tailed Student's t test for paired samples. For nonparametric data, the results are expressed as the median ± quartiles and compared with the Wilcoxon signed rank test. Where indicated, an adjusted Bonferroni correction for multiple comparisons was used to reach an overall p-value of <0.05.
Online supplemental material.
Supplemental materials and methods details the preparation of S. aureus and injections and describes reagents, DNA, and Abs. Fig. S1 shows the dose-dependent production of TNF-α and IL-6 by Prx II+/+ and Prx II −/− BMDMs (A) and splenocytes (B) in response to LPS. Fig. S2 shows the MAPK phosphorylation in Prx II+/+ and Prx II−/− BMDMs treated with various TLR agonists (A), and MAPK phosphorylation and IκBα degradation in LPS-treated Prx II+/+ and Prx II−/− splenocytes (B). Fig. S3 shows viability (A) and serum concentrations of inflammatory mediators (TNF-α, IL-6, and NO; B) in S. aureus–injected Prx II+/+ and Prx II−/− mice, as well as gross examination of spleens (C) from Prx II+/+ and Prx II−/− mice after LPS injection. Fig. S4 shows the LPS-induced inflammatory responses after the administration of the H2O2 scavengers or the catalase inhibitor (A and B), and viability in catalase-injected Prx II−/− mice after LPS challenge (C). Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20061849/DC1.
Acknowledgments
We thank Dr. Sang-Won Kang for critical review of the paper and for kind provision of constructs.
These studies were supported in part by a grant from the Korea Health 21 R&D Project, Ministry of Health & Welfare, Republic of Korea (A060312), and a grant R01-2005-000-10561-0 (2005) from the Basic Research Program of the Korea Science & Engineering Foundation. These studies were supported in part by the Korea Research Institute of Bioscience and Biotechnology Research Initiative Program Grant.
There are no potential conflicts of interest.
Abbreviations used: Ab, antibody; Ad-CAT, adenoviral vectors that carry the catalase gene; Ad–Prx II, adenoviral vectors that carry the Prx II gene; ASK1, apoptosis signal–regulating kinase 1; BLP, bacterial lipoprotein; BMDM, bone marrow–derived macrophage; CL, chemiluminescence; DHE, dihydroethidium; DN, dominant negative; DPI, diphenylene iodonium; EMSA, electrophoretic mobility shift assay; ERK, extracellular signal– regulated kinase; JNK, c-Jun N-terminal kinase; l-NAME, Nω-nitro-l-arginine methyl ester; l-NMMA, N G-monomethyl-l-arginine; MAPK, mitogen-activated protein kinase; NAC, N-acetyl-l-cysteine; NADPH, nicotinamide adenine dinucleotide phosphate; PDGF, platelet-derived growth factor; PGN, peptidoglycan; Prx II, peroxiredoxin II; ROS, reactive oxygen species; SAPK, stress-activated protein kinase; TLR, Toll-like receptor; TRX, thioredoxin.
C.-S. Yang and D.-S. Lee contributed equally to this work.
References
- 1.Chae, H.Z., I.H. Kim, K. Kim, and S.G. Rhee. 1993. Cloning, sequencing, and mutation of thiol-specific antioxidant gene of Saccharomyces cerevisiae. J. Biol. Chem. 268:16815–16821. [PubMed] [Google Scholar]
- 2.Hofmann, B., H.J. Hecht, and L. Flohe. 2002. Peroxiredoxins. Biol. Chem. 383:347–364. [DOI] [PubMed] [Google Scholar]
- 3.Bryk, R., P. Griffin, and C. Nathan. 2000. Peroxynitrite reductase activity of bacterial peroxiredoxins. Nature. 407:211–215. [DOI] [PubMed] [Google Scholar]
- 4.Rhee, S.G., S.W. Kang, T.S. Chang, W. Jeong, and K. Kim. 2001. Peroxiredoxin, a novel family of peroxidases. IUBMB Life. 52:35–41. [DOI] [PubMed] [Google Scholar]
- 5.Kang, S.W., H.Z. Chae, M.S. Seo, K. Kim, I.C. Baines, and S.G. Rhee. 1998. Mammalian peroxiredoxin isoforms can reduce hydrogen peroxide generated in response to growth factors and tumor necrosis factor-alpha. J. Biol. Chem. 273:6297–6302. [DOI] [PubMed] [Google Scholar]
- 6.Fujii, J., and Y. Ikeda. 2002. Advances in our understanding of peroxiredoxin, a multifunctional, mammalian redox protein. Redox Rep. 7:123–130. [DOI] [PubMed] [Google Scholar]
- 7.Kim, H., T.H. Lee, E.S. Park, J.M. Suh, S.J. Park, H.K. Chung, O.Y. Kwon, Y.K. Kim, H.K. Ro, and M. Shong. 2000. Role of peroxiredoxins in regulating intracellular hydrogen peroxide and hydrogen peroxide- induced apoptosis in thyroid cells. J. Biol. Chem. 275:18266–18270. [DOI] [PubMed] [Google Scholar]
- 8.Wen, S.T., and R.A. Van Etten. 1997. The PAG gene product, a stress-induced protein with antioxidant properties, is an Abl SH3-binding protein and a physiological inhibitor of c-Abl tyrosine kinase activity. Genes Dev. 11:2456–2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jin, D.Y., and K.T. Jeang. 2000. Peroxiredoxins in cell signaling and HIV infection. In Antioxidant and Redox Regulation of Genes. C.K. Sen, H. Sies, and P.A. Baeuerle, editors. Academic Press, San Diego. 381–407.
- 10.Chae, H.Z., H.J. Kim, S.W. Kang, and S.G. Rhee. 1999. Characterization of three isoforms of mammalian peroxiredoxin that reduce peroxides in the presence of thioredoxin. Diabetes Res. Clin. Pract. 45:101–112. [DOI] [PubMed] [Google Scholar]
- 11.Choi, M.H., I.K. Lee, G.W. Kim, B.U. Kim, Y.H. Han, D.Y. Yu, H.S. Park, K.Y. Kim, J.S. Lee, C. Choi, et al. 2005. Regulation of PDGF signalling and vascular remodelling by peroxiredoxin II. Nature. 435:347–353. [DOI] [PubMed] [Google Scholar]
- 12.Moon, E.Y., Y.W. Noh, Y.H. Han, S.U. Kim, J.M. Kim, D.Y. Yu, and J.S. Lim. 2006. T lymphocytes and dendritic cells are activated by the deletion of peroxiredoxin II (Prx II) gene. Immunol. Lett. 102:184–190. [DOI] [PubMed] [Google Scholar]
- 13.Iliev, D.B., J.C. Roach, S. Mackenzie, J.V. Planas, and F.W. Goetz. 2005. Endotoxin recognition: in fish or not in fish? FEBS Lett. 579:6519–6528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeLeo, F.R., J. Renee, S. McCormick, M. Nakamura, M. Apicella, J.P. Weiss, and W.M. Nauseef. 1998. Neutrophils exposed to bacterial lipopolysaccharide upregulate NADPH oxidase assembly. J. Clin. Invest. 101:455–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Finkel, T. 2003. Oxidant signals and oxidative stress. Curr. Opin. Cell Biol. 15:247–254. [DOI] [PubMed] [Google Scholar]
- 16.Rhee, S.G., Y.S. Bae, S.R. Lee, and J. Kwon. 2000. Hydrogen peroxide: a key messenger that modulates protein phosphorylation through cysteine oxidation. Sci. STKE. 2000:PE1. [DOI] [PubMed]
- 17.Nathan, C. 2003. Specificity of a third kind: reactive oxygen and nitrogen intermediates in cell signaling. J. Clin. Invest. 111:769–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rhee, S.G., T.S. Chang, Y.S. Bae, S.R. Lee, and S.W. Kang. 2003. Cellular regulation by hydrogen peroxide. J. Am. Soc. Nephrol. 14:S211–S215. [DOI] [PubMed] [Google Scholar]
- 19.Sanlioglu, S., C.M. Williams, L. Samavati, N.S. Butler, G. Wang, P.B. McCray Jr., T.C. Ritchie, G.W. Hunninghake, E. Zandi, and J.F. Engelhardt. 2001. Lipopolysaccharide induces Rac1-dependent reactive oxygen species formation and coordinates tumor necrosis factor-alpha secretion through IKK regulation of NF-kappa B. J. Biol. Chem. 276:30188–30198. [DOI] [PubMed] [Google Scholar]
- 20.Asehnoune, K., D. Strassheim, S. Mitra, J.Y. Kim, and E. Abraham. 2004. Involvement of reactive oxygen species in Toll-like receptor 4-dependent activation of NF-κB. J. Immunol. 172:2522–2529. [DOI] [PubMed] [Google Scholar]
- 21.Hwang, D., B.C. Jang, G. Yu, and M. Boudreau. 1997. Expression of mitogen-inducible cyclooxygenase induced by lipopolysaccharide: mediation through both mitogen-activated protein kinase and NF-kappaB signaling pathways in macrophages. Biochem. Pharmacol. 54:87–96. [DOI] [PubMed] [Google Scholar]
- 22.Akira, S., and K. Takeda. 2004. Toll-like receptor signalling. Nat. Rev. Immunol. 4:499–511. [DOI] [PubMed] [Google Scholar]
- 23.Mestre, J.R., P.J. Mackrell, D.E. Rivadeneira, P.P. Stapleton, T. Tanabe, and J.M. Daly. 2000. Redundancy in the signaling pathways and promoter elements regulating cyclooxygenase-2 gene expression in endotoxin-treated macrophage/monocytic cells. J. Biol. Chem. 276:3977–3982. [DOI] [PubMed] [Google Scholar]
- 24.Kang, S.W., T.S. Chang, T.H. Lee, E.S. Kim, D.Y. Yu, and S.G. Rhee. 2004. Cytosolic peroxiredoxin attenuates the activation of Jnk and p38 but potentiates that of Erk in Hela cells stimulated with tumor necrosis factor-alpha. J. Biol. Chem. 279:2535–2543. [DOI] [PubMed] [Google Scholar]
- 25.Droge, W. 2002. Free radicals in the physiological control of cell function. Physiol. Rev. 82:47–95. [DOI] [PubMed] [Google Scholar]
- 26.Martindale, J.L., and N.J. Holbrook. 2002. Cellular response to oxidative stress: signaling for suicide and survival. J. Cell. Physiol. 192:1–15. [DOI] [PubMed] [Google Scholar]
- 27.El Benna, J., L.P. Faust, and B.M. Babior. 1994. The phosphorylation of the respiratory burst oxidase component p47phox during neutrophil activation. Phosphorylation of sites recognized by protein kinase C and by proline-directed kinases. J. Biol. Chem. 269:23431–23436. [PubMed] [Google Scholar]
- 28.Kwon, J., S.R. Lee, K.S. Yang, Y. Ahn, Y.J. Kim, E.R. Stadtman, and S.G. Rhee. 2004. Reversible oxidation and inactivation of the tumor suppressor PTEN in cells stimulated with peptide growth factors. Proc. Natl. Acad. Sci. USA. 101:16419–16424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DeYulia, G.J., Jr., J.M. Carcamo, O. Borquez-Ojeda, C.C. Shelton, and D.W. Golde. 2005. Hydrogen peroxide generated extracellularly by receptor-ligand interaction facilitates cell signaling. Proc. Natl. Acad. Sci. USA. 102:5044–5049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kang, S.W., S.G. Rhee, T.S. Chang, W. Jeong, and M.H. Choi. 2005. 2-Cys peroxiredoxin function in intracellular signal transduction: therapeutic implications. Trends Mol. Med. 11:571–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pawate, S., Q. Shen, F. Fan, and N.R. Bhat. 2004. Redox regulation of glial inflammatory response to lipopolysaccharide and interferongamma. J. Neurosci. Res. 77:540–551. [DOI] [PubMed] [Google Scholar]
- 32.Ray, R., and A.M. Shah. 2005. NADPH oxidase and endothelial cell function. Clin. Sci. (Lond.). 109:217–226. [DOI] [PubMed] [Google Scholar]
- 33.Duchen, M. 2004. Mitochondria in health and disease: perspectives on a new mitochondrial biology. Mol. Aspects Med. 25:365–451. [DOI] [PubMed] [Google Scholar]
- 34.Bokoch, G. 1995. Regulation of the phagocyte respiratory burst by small GTP-binding proteins. Trends Cell Biol. 5:109–113. [DOI] [PubMed] [Google Scholar]
- 35.DeLeo, F.R., L.A. Allen, M. Apicella, and W.M. Nauseef. 1999. NADPH oxidase activation and assembly during phagocytosis. J. Immunol. 163:6732–6740. [PubMed] [Google Scholar]
- 36.Dusi, S., M. Donini, and F. Rossi. 1996. Mechanisms of NADPH oxidase activation: translocation of p40phox, Rac1 and Rac2 from the cytosol to the membranes in human neutrophils lacking p47phox or p67phox. Biochem. J. 314:409–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.El Benna, J., L.P. Faust, J.L. Johnson, and B.M. Babior. 1996. Phosphorylation of the respiratory burst oxidase subunit p47phox as determined by two-dimensional phosphopeptide mapping. Phosphorylation by protein kinase C, protein kinase A, and a mitogen-activated protein kinase. J. Biol. Chem. 271:6374–6378. [DOI] [PubMed] [Google Scholar]
- 38.Dang, P.M., A. Stensballe, T. Boussetta, H. Raad, C. Dewas, Y. Kroviarski, G. Hayem, O.N. Jensen, M.A. Gougerot-Pocidalo, and J. El-Benna. 2006. A specific p47phox-serine phosphorylated by convergent MAPKs mediates neutrophil NADPH oxidase priming at inflammatory sites. J. Clin. Invest. 116:2033–2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Masutani, H., S. Ueda, and J. Yo. 2005. The thioredoxin system in retroviral infection and apoptosis. Cell Death Differ. 12(Suppl. 1): 991–998. [DOI] [PubMed] [Google Scholar]
- 40.Matsuzawa, A., K. Saegusa, T. Noguchi, C. Sadamitsu, H. Nishitoh, S. Nagai, S. Koyasu, K. Matsumoto, K. Takeda, and H. Ichijo. 2005. ROS-dependent activation of the TRAF6-ASK1-p38 pathway is selectively required for TLR4-mediated innate immunity. Nat. Immunol. 6:587–592. [DOI] [PubMed] [Google Scholar]
- 41.Hsieh, C.C., and J. Papaconstantinou. 2006. Thioredoxin-ASK1 complex levels regulate ROS-mediated p38 MAPK pathway activity in livers of aged and long-lived Snell dwarf mice. FASEB J. 20:259–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Victor, V.M., and M. De la Fuente. 2003. Immune cells redox state from mice with endotoxin-induced oxidative stress. Involvement of NF-κB. Free Radic. Res. 37:19–27. [DOI] [PubMed] [Google Scholar]
- 43.Victor, V.M., M. Rocha, and M. De la Fuente. 2004. Immune cells: free radicals and antioxidants in sepsis. Int. Immunopharmacol. 4:327–347. [DOI] [PubMed] [Google Scholar]
- 44.Victor, V.M., M. Rocha, and M. De la Fuente. 2003. N-acetylcysteine protects mice from lethal endotoxemia by regulating the redox state of immune cells. Free Radic. Res. 37:919–929. [DOI] [PubMed] [Google Scholar]
- 45.Thimmulappa, R.K., H. Lee, T. Rangasamy, S.P. Reddy, M. Yamamoto, T.W. Kensler, and S. Biswal. 2006. Nrf2 is a critical regulator of the innate immune response and survival during experimental sepsis. J. Clin. Invest. 116:984–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chang, T.S., C.S. Cho, S. Park, S. Yu, S.W. Kang, and S.G. Rhee. 2004. Peroxiredoxin III, a mitochondrion-specific peroxidase, regulates apoptotic signaling by mitochondria. J. Biol. Chem. 279:41975–41984. [DOI] [PubMed] [Google Scholar]
- 47.Lee, T.H., S.U. Kim, S.L. Yu, S.H. Kim, D.S. Park, H.B. Moon, S.H. Dho, K.S. Kwon, H.J. Kwon, Y.H. Han, et al. 2003. Peroxiredoxin II is essential for sustaining life span of erythrocytes in mice. Blood. 101:5033–5038. [DOI] [PubMed] [Google Scholar]
- 48.Yang, C.S., C.H. Song, J.S. Lee, S.B. Jung, J.H. Oh, J. Park, H.J. Kim, J.K. Park, T.H. Paik, and E.K. Jo. 2006. Intracellular network of phosphatidylinositol 3-kinase, mammalian target of the rapamycin/70 kDa ribosomal S6 kinase 1, and mitogen-activated protein kinases pathways for regulating mycobacteria-induced IL-23 expression in human macrophages. Cell. Microbiol. 8:1158–1171. [DOI] [PubMed] [Google Scholar]
- 49.Klein, R.D., C.S. Van Pelt, A.L. Sabichi, J. Dela Cerda, S.M. Fischer, G. Furstenberger, and K. Muller-Decker. 2005. Transitional cell hyperplasia and carcinomas in urinary bladders of transgenic mice with keratin 5 promoter-driven cyclooxygenase-2 overexpression. Cancer Res. 65:1808–1813. [DOI] [PubMed] [Google Scholar]
- 50.Griendling, K.K., C.A. Minieri, J.D. Ollerenshaw, and R.W. Alexander. 1994. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ. Res. 74:1141–1148. [DOI] [PubMed] [Google Scholar]
- 51.Werts, C., R.I. Tapping, J.C. Mathison, T.H. Chuang, V. Kravchenko, I. Saint Girons, D.A. Haake, P.J. Godowski, F. Hayashi, A. Ozinsky, et al. 2001. Leptospiral lipopolysaccharide activates cells through a TLR2-dependent mechanism. Nat. Immunol. 2:346–352. [DOI] [PubMed] [Google Scholar]
- 52.Gupta, M., E.E. Jansen, H. Senephansiri, C. Jakobs, O.C. Snead, M. Grompe, and K.M. Gibson. 2004. Liver-directed adenoviral gene transfer in murine succinate semialdehyde dehydrogenase deficiency. Mol. Ther. 9:527–539. [DOI] [PubMed] [Google Scholar]
- 53.Yang, C.S., J.S. Lee, C.H. Song, G.M. Hur, S.J. Lee, S. Tanaka, S. Akira, T.H. Paik, and E.K. Jo. 2007. Protein kinase C zeta plays an essential role for Mycobacterium tuberculosis-induced extracellular signal-regulated kinase 1/2 activation in monocytes/macrophages via Toll-like receptor 2. Cell. Microbiol. 9:382–396. [DOI] [PubMed] [Google Scholar]