Abstract
The contribution of proliferation to B lymphocyte homeostasis and antigen responses is largely unknown. We quantified the replication history of mouse and human B lymphocyte subsets by calculating the ratio between genomic coding joints and signal joints on kappa-deleting recombination excision circles (KREC) of the IGK-deleting rearrangement. This approach was validated with in vitro proliferation studies. We demonstrate that naive mature B lymphocytes, but not transitional B lymphocytes, undergo in vivo homeostatic proliferation in the absence of somatic mutations in the periphery. T cell–dependent B cell proliferation was substantially higher and showed higher frequencies of somatic hypermutation than T cell–independent responses, fitting with the robustness and high affinity of T cell–dependent antibody responses. More extensive proliferation and somatic hypermutation in antigen-experienced B lymphocytes from human adults compared to children indicated consecutive responses upon additional antigen exposures. Our combined observations unravel the contribution of proliferation to both B lymphocyte homeostasis and antigen-induced B cell expansion. We propose an important role for both processes in humoral immunity. These new insights will support the understanding of peripheral B cell regeneration after hematopoietic stem cell transplantation or B cell–directed antibody therapy, and the identification of defects in homeostatic or antigen-induced B cell proliferation in patients with common variable immunodeficiency or another antibody deficiency.
B lymphocytes appear in peripheral blood and secondary lymphoid organs after they have differentiated from precursor B cells in bone marrow (1) and have created a unique B cell antigen receptor by V(D)J rearrangement of their Ig heavy and Ig light chain loci (2). In the periphery, multiple B cell subsets can be identified, which are either naive or have been exposed to antigen.
The mouse spleen has been extensively studied for B cell maturation (for review see reference 3). Although several models exist (4, 5), the general consensus is that recent bone marrow emigrants are AA4.1+ and defined as transitional B cells. Subsequently, these cells can give rise to either naive follicular B cells, or marginal zone (MZ) B cells, which are named based on their anatomical location. MZ B cells show a rapid and predominantly T cell–independent response to pathogens, whereas follicular B cells show a T cell–dependent response in a germinal center reaction, where they undergo somatic hypermutation (SHM) and class-switch recombination (CSR) to generate an optimized high affinity antibody response.
In man, the recent bone marrow emigrants, i.e., the transitional B lymphocytes, are CD38+CD24+IgD+ and are relatively small subset compared with the CD38−IgM+IgD+CD27− naive mature B lymphocytes in blood and represent a peripheral lymphoid organs (6, 7). In peripheral blood, a substantial fraction of the naive B lymphocyte population expresses the CD5 antigen and is prone to produce polyreactive antibodies (8). Splenic MZ B lymphocytes in man are IgM+IgD+CD27+. They show a rapid IgM response to blood-borne T cell–independent antigens (9, 10) and their Ig loci carry somatic mutations (11). Counterparts of splenic MZ B lymphocytes are found in lymph nodes, tonsils, and Peyer's patches, and they circulate in blood (12–15). Because these cells are not anatomically located in the splenic MZ, it was suggested to refer to them as natural effector B lymphocytes (16). T cell–dependent responsive B lymphocytes proliferate in a germinal center reaction as centroblasts (CD38+CD77+IgD−) (for review see reference 17). These centroblasts undergo SHM before further differentiation into centrocytes (CD38+CD77−IgD−), which are subsequently selected based on the highest affinity for their cognate antigen, undergo CSR, and give rise to either CD27+ memory B lymphocytes or CD38hi antibody–producing plasma cells (18). B cell proliferation upon antigen encounter in the germinal center is a key step in the humoral immune response, which has been extensively studied (19, 20), but the extent of antigen-induced proliferation of B lymphocytes remains largely unknown.
Recent reports suggest that in addition to antigen-induced proliferation, naive B lymphocytes can proliferate without further differentiation (21–23). This homeostatic proliferation of naive B lymphocytes has been described in mouse models in response to a B cell deficit (22). Theoretically, the absolute number of naive B lymphocytes is regulated by four processes: (a) output from bone marrow; (b) survival; (c) loss of naive B lymphocytes via further maturation after antigen encounter (24); and (d) antigen-independent (homeostatic) proliferation. However, similar to the extent of antigen-induced B cell proliferation, it remains unclear to what extent proliferation contributes to naive B lymphocyte numbers; to answer both questions, a robust assay is needed to assess the replication history of B cells.
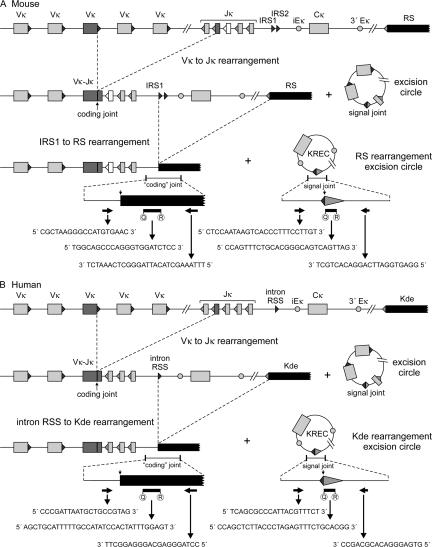
Here we introduce the use of kappa-deleting recombination excision circles (KRECs) to determine the replication history of both human and mouse mature B cell subsets. Rearrangement of the Jκ-Cκ intron recombination signal sequence (IRS1 in mouse and intronRSS in man) to the kappa-deleting element (RS in mouse and Kde in man) renders the IGK locus nonfunctional, precludes any further rearrangements in the IGK locus, and therefore the coding joint of this rearrangement remains stably present in the genome (Fig. 1, A and B) (25, 26). It is a common rearrangement because it is present in ∼30% of Igκ+ and in almost all Igλ+ mature human B lymphocytes and does not influence antigen-specificity (27, 28). The chromosomal DNA located in between the two rearranging elements is excised from the genome and circularized into stable episomal structures, which are diluted twofold by each cell division (Fig. 2 A) (29–32). Previously, we demonstrated the use of T cell receptor excision circles (TRECs) for studying rearrangement and proliferation processes (30, 31). Subsequently, TRECs of δREC-ψJα rearrangements have been used as markers for recent thymic emigrants and T cell expansion in patients treated for HIV infection (33–35).
Figure 1.
Detection of coding joints and signal joints of kappa-deleting rearrangements in mice (A) and man (B). V(D)J recombination on the IGK locus results in a Vκ-Jκ coding joint. Subsequent rearrangement between the IRS1 (mouse) or intronRSS (human) and the RS (mouse) and Kde (human) elements can make the IGK allele nonfunctional by deleting the Cκ exons and the enhancers. Consequently, the coding joint precludes any further rearrangements in the IGK locus and therefore remains present in the genome. The KREC with the corresponding signal joint is a stable double-stranded, circular DNA structure. The coding joint in the genome and the signal joint on the episomal excision circle can be quantified via RQ-PCR using the indicated primers and TaqMan probes. The murine IGK locus contains two intronRSS sequences, IRS1 and IRS2, of which IRS1 has the most conserved RSS sequence and is >10-fold more frequently found in rearrangements to RS (reference 62 and unpublished data).
Figure 2.
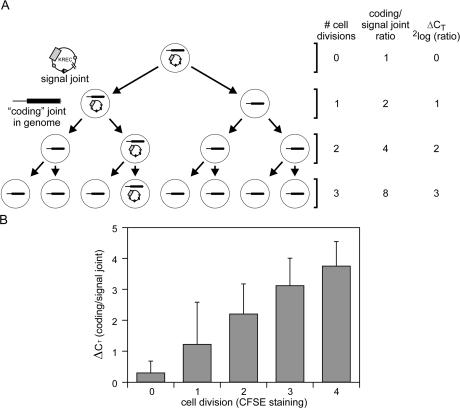
Quantification of the replication history of B cells using KRECs. (A) When a B lymphocyte with an intronRSS–Kde rearrangement divides, both daughter cells inherit the intronRSS–Kde coding joint in the genome. However, the signal joint, which is on the episomal KREC, will be inherited by only one of the two daughter cells. Crucially, the ΔCT of the PCRs detecting the coding joint and the signal joint exactly represent the number of cell divisions a B lymphocyte has undergone because both processes have an exponential increase with base number 2. (B) The ΔCT between the coding joint and the signal joint is shown for mouse splenic B cells that were cultured in vitro with polyclonal stimulation and were sorted based on the CFSE staining intensity as measure for 0, 1, 2, 3, and 4 cell divisions.
In this study, we introduce the use of KRECs to accurately assess the replication history of isolated mouse and human B cell subsets. Using this approach we demonstrate homeostatic proliferation of mature B lymphocytes, but not of transitional B lymphocytes, in healthy individuals. Furthermore, mouse MZ and human natural effector B lymphocytes, which mainly respond to T cell–independent antigens, undergo additional antigen-induced proliferation. The T cell–dependent antigen response in the germinal center was found to be much stronger with regard to both proliferation and SHM.
RESULTS
The KREC assay is a robust technique to determine the replication history of B cells
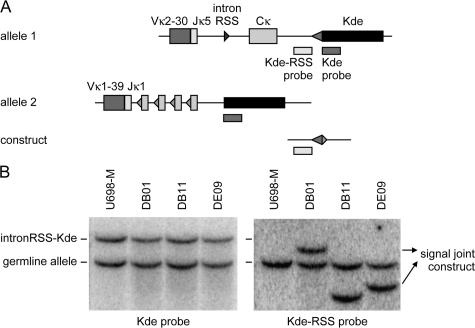
The ratio between the kappa-deleting rearrangement and the corresponding excision circle can be used as measure for the in vivo replication history of an isolated B cell subset, assuming that the excision circle is a stable DNA structure, which is diluted twofold in every cell division (Fig. 2 A). Consequently, real-time quantitative (RQ)-PCR assays were designed for detection of the coding joints and the corresponding signal joints of both the mouse IRS1–RS (Fig. 1 A) and the human intronRSS–Kde (Fig. 1 B) rearrangements. The two mouse RQ-PCR assays were tested for equal efficiency on serial dilutions of constructs containing either the coding joint or the signal joint. To minimize the chance of contamination in potential future clinical studies, a special cell line control was created for the human KREC studies. The Igκ+ B cell line U698-M was selected because it has two Vκ-Jκ–rearranged IGK alleles, one of which is out-of-frame and contains an intronRSS–Kde rearrangement (Fig. 3, A and B). To obtain an equal ratio between the intronRSS–Kde coding and signal joints, an intronRSS–Kde signal joint construct was inserted into the genome of the U698-M cell line using retroviral transduction. Individual clones were sorted, and Southern blot analysis revealed a single insertion per genome for three clones: DB01, DB11, and DE09 (Fig. 3 B). Each of these stable B cell lines contained both the coding joint and the corresponding signal joint of the intronRSS–Kde rearrangement and were therefore used as positive control for assessment of the efficiency of both human RQ-PCR assays.
Figure 3.
Generation of a human control B cell line with one intronRSS–Kde coding joint and one artificially introduced signal joint per genome. (A) Schematic representation of the Southern blot probes that recognize the IGK alleles of the U698-M cell line and the KREC signal joint construct that was introduced into the cell line. The Kde probe recognizes both IGK alleles, whereas the Kde-RSS probe recognizes allele 1, which does not harbor an intronRSS–Kde rearrangement, and the KREC construct. (B) Southern blot of the original U698-M cell line and three single clones that contain a unique insertion of the signal joint construct.
The stability and twofold dilution of excision circles were investigated by in vitro proliferation experiments. Resting B lymphocytes were isolated from wild-type mouse spleen, labeled with CFSE, and cultured with IL-4, LPS, and anti-IgM for 2–3 d. DNA was isolated from B cells before culture and from sorted B cells that had undergone 1, 2, 3, or 4 cell divisions to quantify the kappa-deleting rearrangement and the excision circle. The combined data from three individual experiments show that resting B lymphocytes contain a coding joint to signal joint ratio of ∼1:1 (ΔCT = 0). Furthermore, the ΔCT (CTcoding joint – CTsignal joint) of the RQ-PCRs corresponded well with the number of cell divisions these cells underwent in culture as observed with CFSE staining (Fig. 2 B). Therefore, the KREC assay can be used as a robust technique to determine the replication history of B cell subsets in vitro.
In vivo replication history of mouse B cell subsets
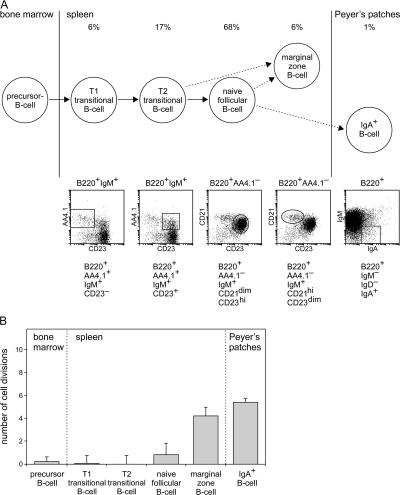
To determine the in vivo replication history using the KREC assay in a well-defined system, mouse B cell subsets (B220+) from spleen and Peyer's patches were isolated (Fig. 4 A). From spleen, T1 (AA4.1+CD23−) and T2 (AA4.1+CD23+) transitional B cells and naive follicular (AA4.1−CD21lowCD23hi) and MZ (AA4.1−CD21hiCD23low) B lymphocytes were purified, whereas IgA+ class-switched B lymphocytes were isolated from Peyer's patches. Total bone marrow samples were used to determine the replication history of precursor B cells, in which the Igκ-deleting rearrangement is initiated (Fig. 4 A).
Figure 4.
Replication history of mouse B cell subsets. (A) Scheme of the differentiation stages of mature B cell development in mice. B cell subsets were isolated based on the indicated marker expression from spleen and Peyer's patches. (B) The replication history of each B cell subset. The ΔCT between the coding joint and the signal joint PCRs represents the number of cell divisions, which are given for each subset. The data are the means of three independent sorts and are presented as mean ± SD.
Precursor B cells in bone marrow and transitional B cells in spleen did not show replication (Fig. 4 B). In contrast, naive follicular B cells showed limited proliferation (1 cell division). MZ B cells and class-switched IgA+ B cells showed a more extensive replication history of 4 and 5.5 cell divisions, respectively. As expected, the observed replication histories increased with more progressive differentiation stages. Consequently, the KREC assay is a powerful tool to study not only the in vitro replication history but also the in vivo replication history of sorted B cell subsets.
The extent of antigen-induced B cell proliferation in man
The majority of the intronRSS–Kde rearrangements in man is initiated in small pre–B-II cells (27). Therefore, B cell subsets starting with the small pre–B-II stage were isolated from bone marrow, tonsil, and peripheral blood using previously established markers (Fig. 5 A and Fig. 6 A) (6, 15, 18, 27, 36) to quantify intronRSS–Kde coding joints in the genome and the intronRSS–Kde signal joints on the KRECs. The coding joints were easily detected; 35–50% of the alleles in all peripheral B cell subsets carried this rearrangement (not depicted). In small pre–B-II and immature B cells the ΔCT was 0.4 (Fig. 5 B). This indicates that precursor B cells in human bone marrow hardly proliferate after rearrangement of intronRSS to Kde.
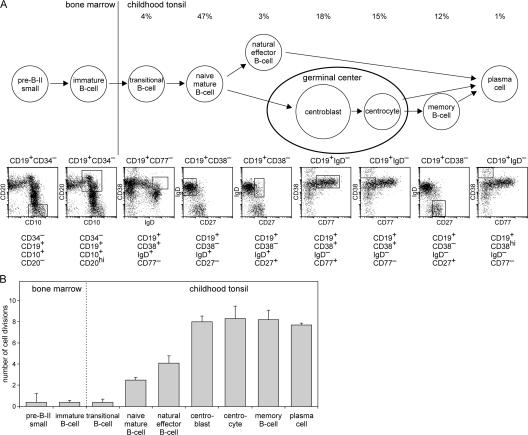
Figure 5.
Replication history of human B lymphocyte subsets from childhood tonsil. (A) Scheme of the differentiation stages of mature B cell development. The B cell subsets that contain the intronRSS–Kde rearrangement were isolated from bone marrow and tonsil samples of young children using the indicated markers. (B) The replication history of each B cell subset. The ΔCT between the coding joint and the signal joint PCRs, which represents the number of cell divisions is given for each subset. The data are the means of independently sorted subsets from three children and are presented as mean ± SD.
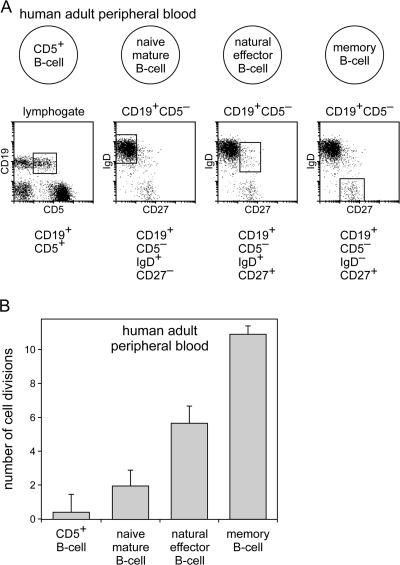
Figure 6.
Replication history of the four main B lymphocyte subsets in adult peripheral blood. (A) Schematic overview of the four main blood B lymphocyte subsets and the markers that were used to isolate them from adult peripheral blood. (B) The replication history of these subsets was determined as the ΔCT of the coding joint and the signal joint PCR of subsets isolated from four or five donor samples (represented as mean ± SD).
Transitional B lymphocytes in tonsil did not show replication, whereas naive B lymphocytes in tonsil showed a replication history of 2.5 cell divisions (Fig. 5 B), indicating a substantial proliferation in this subset. Natural effector B lymphocytes (counterparts of splenic MZ B lymphocytes) underwent more proliferation: on average 4.1 cell divisions. Centroblasts and centrocytes in the germinal center, memory B cells, and plasma cells all showed a replication history of ∼8 cell divisions. As expected by their nature, the centroblasts in human tonsil form the major proliferative subset. It can thus be concluded that naive mature B lymphocytes have already proliferated but massive proliferation is found in subsequent germinal center reactions where each cell generates 45 (2[8–2.5]) or more daughter cells.
Several mature B lymphocyte subsets circulate in blood and can be identified as counterparts of subsets found in secondary lymphoid organs (Fig. 6 A). The four main human B lymphocyte subsets were isolated from blood of healthy adults and analyzed for their replication history. Naive CD5− B lymphocytes in adult peripheral blood were found to have a replication history of 1.9 (Fig. 6 B), which is almost similar to the total bulk of naive mature B lymphocytes in tonsils from children. Furthermore, natural effector B lymphocytes and memory B lymphocytes in adult blood were found to have a replication history of 5.6 and 10.9 cell divisions, respectively. For both populations, these numbers are higher than what was found in childhood tonsil. This indicates that most memory B lymphocytes in adults have been exposed to their cognate antigen multiple times and have accordingly undergone additional proliferation in consecutive germinal center reactions.
The fourth major population isolated from blood was the CD5+ naive B lymphocyte subset. This subset had a replication history of 0.4, similar to small pre–B-II cells and immature B cells in bone marrow and transitional B lymphocytes in tonsil. Thus, contrary to CD5− naive mature B lymphocytes, CD5+ naive B lymphocytes do not proliferate in the peripheral B cell compartment.
Naive B lymphocytes undergo homeostatic proliferation in healthy individuals
Using the KREC assay, we found that the mature B lymphocyte subsets, except for the CD5+ naive B lymphocytes, had undergone proliferation in the periphery. Antigen-induced proliferation is usually accompanied by the induction of SHM. To provide a quantitative measurement for the presence of SHM, we determined the presence of mutations in a hot- spot motif in rearranged IGK (Vκ-Jκ) alleles involving the Vκ3-20 gene segment using a modified version of the Igκ restriction enzyme-based hot-spot mutation assay (IgκREHMA; Fig. 7, A–C) (37).
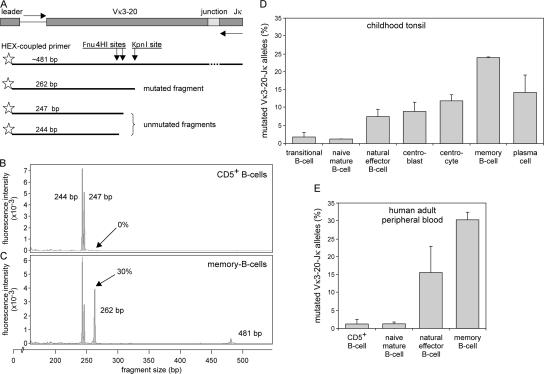
Figure 7.
Quantification of SHM in human B cell subsets. The IgκREHMA assay was modified from Andersen et al. (37) for use on genomice DNA. (A) Schematic representation of a Vκ3-20 to Jκ rearrangement on genomic DNA. The Vκ3-20 gene segment contains two Fnu4HI restriction sites in a SHM hot-spot in the CDR1 region. In addition, a KpnI site is located downstream of the Fnu4HI sites in the FR2 region. Vκ3-20–Jκ rearrangements were PCR amplified with one HEX-labeled Vκ3-20–intron forward and two Jκ reverse primers that recognize all five Jκ gene segments (61). Digestion of these PCR products with both Fnu4HI and KpnI resulted in unmutated fragments of 244 and 247 bp and mutated fragments of 262 bp. (B and C) Spectratyping of Vκ3-20–Jκ rearrangements of CD5+ B lymphocytes and memory B lymphocytes after double digest with Fnu4HI and KpnI. CD5+ B lymphocytes did not have a replication history and showed no SHM, whereas memory B lymphocytes in adult blood had undergone 11 cell cycles in the periphery and showed 30% mutated Vκ3-20–Jκ rearrangements. (D and E) The percentage of mutated Vκ3-20–Jκ rearrangements was measured in the same DNA samples that were previously used for determining the replication history (data represent means ± SD).
Neither transitional and naive mature B lymphocytes from tonsil, nor CD5+ and CD5− naive B lymphocytes from blood carried mutated Vκ3-20 to Jκ rearrangements (<1.5%; Fig. 7, D and E). In contrast, germinal center and memory B lymphocyte subsets contained substantial numbers of mutated Vκ3-20 alleles. Of the rearranged Vκ3-20 alleles, 7.5% was mutated in natural effector B lymphocytes from childhood tonsil, and 16% in natural effector B lymphocytes from adult blood. Centroblasts and centrocytes from childhood tonsil showed 9% and 12% mutated rearranged Vκ3-20 alleles, respectively. Memory B lymphocytes from childhood tonsil had 24% mutated alleles, and memory B lymphocytes from adult blood had 30% mutated alleles. Finally, plasma cells in childhood tonsil showed 14% mutated alleles.
It can thus be concluded that naive mature B lymphocytes in blood and tonsil undergo limited proliferation in the absence of SHM, indicative of antigen-independent homeostatic proliferation. In contrast, the proliferation of centroblasts in tonsil is associated with SHM. The frequency of mutated alleles was higher in centrocytes, memory B cells, and plasma cells, probably as the result of selection for antigen binding. Finally, the frequency of mutated alleles is higher in adult natural effector B lymphocytes and memory B lymphocytes than in their counterparts isolated from tonsils of children. This correlated with the higher proliferation rate, fitting with additional antigen-induced proliferation and SHM in adults.
DISCUSSION
In this study, we demonstrate the contribution of proliferation to B cell homeostasis and the extent of antigen-induced B cell expansion in mouse and man. Assessment of the replication history and the SHM status allowed us to discriminate homeostatic proliferation from antigen-induced proliferation. The homeostatic proliferation in naive B lymphocytes did not differ between human childhood and adult naive B lymphocytes, whereas the extensive proliferation and SHM of natural effector and memory B cells in adults was greater than in children, indicating additional antigen encounters in adults.
In our novel RQ-PCR–based KREC assay, we use the coding joint versus signal joint ratio of the intronRSS–Kde rearrangement to obtain highly accurate quantification of the replication history of mature B lymphocytes. This approach differs from studies in which the kinetics of the peripheral B cell pool was assessed with BrdU or 6,6-2H2-glucose (38, 39). The latter studies have provided insight into the recent turnover of B cell subsets but could not quantify the contribution of proliferation to B cell homeostasis. Because of time-lapse differences (measurement 3–4 d after deuterium glucose infusion) it was not possible to determine whether proliferation occurred in the investigated B cell differentiation stage or in an earlier (preceding) differentiation stage (39). The KREC assay allows us to assess the total number of cell divisions that B cells, in a well-defined subset isolated from lymphoid tissue, have undergone since their emigration from bone marrow. Consequently, differences in replication history between B cell subsets can be straightforwardly assessed by measuring the KREC content of the B cell subsets under study.
The applicability of KRECs depends on their stability, which has been suggested before (29) but never formally proven. In theory, the excision circles can be subject to transcriptional activity and recombinase activity in precursor B cells because of the presence of IGK enhancers. Furthermore, it is unclear whether excision circles remain stably present in the nucleus of one of the two daughter cells after cell division. We show here that there is very little or no breakdown of KRECs in precursor B cells and that the KREC content is diluted twofold with every cell division.
The use of KRECs to measure the replication history of B cell subsets is analogous to the use of δREC–ψJα rearrangements and their corresponding TRECs to study T cell dynamics. Although the use of TRECs provided insight into the effects of novel antiviral therapies on the thymic output in patients with HIV infection (33, 34), TRECs could not be used to quantify proliferation because most δREC–ψJα coding joints are deleted from the genome by subsequent Vα–Jα rearrangements (30, 31). Therefore, TREC data as measured in T lymphocytes of healthy and diseased individuals are difficult to interpret (31, 35). Our KREC assay does not have these limitations. In contrast, the intronRSS–Kde rearrangement has several advantages for studying the replication history of B lymphocytes: (a) it is a frequently occurring IGK gene rearrangement in both normal and malignant B cells (27, 28); (b) it is one of the last Ig gene rearrangements in bone marrow–derived B cells before obtaining a functional Ig molecule (27), ensuring that the corresponding KRECs are abundantly present in naive B lymphocytes; (c) it is a single-step rearrangement, which allows easy design of RQ-PCR primers and probes for accurate detection of the coding joints and signal joints (40); and (d) it is an end-stage rearrangement, precluding further rearrangements (41), which implies that the coding joint is retained in the genome while the corresponding signal joint on the KREC is diluted twofold by each cell division. These characteristics make the intronRSS–Kde rearrangement a solid RQ-PCR target for accurate quantification of mature B lymphocyte proliferation.
The intronRSS–Kde rearrangement is initiated in precursor B cells in bone marrow and is frequently found to inactivate functionally rearranged IGK loci, suggesting actively induced receptor editing in bone marrow (42). Furthermore, receptor revision has been speculated to occur in peripheral B lymphocytes, based on low levels of RAG gene transcription and the presence of double-stranded DNA breaks in Jκ gene segments (43–48). If intronRSS–Kde rearrangements would be induced in the process of receptor revision in peripheral B lymphocytes, the observed number of cell divisions measured with KRECs should be an underestimate. However, because the RAG mRNA and double-stranded DNA break levels are clearly lower in peripheral B cell subsets compared with bone marrow and RAG expression is inhibited by BCR signaling (49, 50), the influence of receptor revision on measuring proliferation using KRECs will be limited.
The contribution of proliferation to B lymphocyte homeostasis in healthy individuals has never been shown. Using the KREC assay we were able to address this issue. We found no replication history for transitional B lymphocytes in mouse spleen and childhood tonsil. In addition, no replication was seen in the CD5+ B cell subset in human peripheral blood. Recently, it has been shown that nearly all human transitional B cells express CD5, whereas only a fraction of the naive mature B lymphocytes express CD5 (7). Thus, the CD5+ B lymphocyte subset we isolated from peripheral blood mainly consists of transitional B lymphocytes. Consequently, the limited proliferation seen in CD5+ B lymphocytes is likely to be caused by the high abundance of excision circles from transitional B lymphocytes. Therefore, it remains unclear whether the absence of proliferation in the CD5+ B cell subset reflects a difference in maturation stage or is a direct effect of CD5 expression.
Naive follicular B lymphocytes in mice and naive mature B lymphocytes obtained from human blood and tonsil showed proliferation in the absence of SHM. By definition, this is homeostatic proliferation, which occurred in the periphery, because no replication history was found for precursor B cells and transitional B lymphocytes. Homeostatic proliferation has been observed before in naive T and NK cells (51–54). It has been suggested to occur in B lymphocytes also (23), but it was only observed in response to a B cell deficit (21, 22). Our results show for the first time that homeostatic proliferation of naive mature B lymphocytes occurs in healthy individuals and contributes greatly to the homeostasis of absolute B lymphocyte numbers by two- to fourfold expansion of the naive B lymphocyte pool. The factors driving this homeostatic proliferation need to be identified in future studies.
Splenic MZ B lymphocytes in mice showed more replications than naive follicular B lymphocytes. In absence of B cell influx from bone marrow, MZ B lymphocytes persist much longer than naive follicular B lymphocytes (55). Our results support earlier suggestions that this persistence partly results from an increased proliferation capacity. It remains unclear, however, whether this proliferation is antigen induced, especially because these cells carry nonmutated Ig genes (10). In contrast, human MZ B lymphocytes have a memory phenotype (CD27+) and carry mutated Ig genes generated mainly in response to T cell–independent antigens (9, 10, 15). We found that the replication history of their counterparts in childhood tonsil, natural effector B lymphocytes, was comparable to that of mouse MZ B lymphocytes. Studies in children younger than 2 yr suggest that natural effector B lymphocytes undergo SHM during ontogeny (15). This observation supports the idea that similar to mouse human MZ B lymphocytes are generated independent of antigen. However, in this study we observed increased proliferation and SHM in natural effector B lymphocytes from adult blood compared with childhood tonsil, consistent with antigen-induced maturation. Consequently, more insight into the induction of SHM is needed to solve the question whether MZ B lymphocytes and natural effector B lymphocytes in man are generated dependently or independently of antigen.
The T cell–dependent response of B lymphocytes in a germinal center reaction is thought to be more powerful than the T cell–independent response. Our analyses indeed consistently showed that the germinal center response was greater with regard to proliferation and SHM. Interestingly, the replication history of germinal center–derived B cells differed greatly among mice, children, and adults. A plausible explanation is the exposure to antigen. Mice that are 5–12 wk old and kept under specific pathogen-free conditions are exposed to a limited load and spectrum of antigens and likely have less extensive B cell maturation. Furthermore, the increased proliferation of memory B lymphocytes from adults compared with children correlated with increased frequencies of mutated IGK alleles. This could reflect additional maturation resulting from secondary and tertiary immune responses to a specific antigen, or additional activation over time induced by innate stimuli acting on innate antigen receptors, such as Toll-like receptors (56).
In this study, we introduced the robust KREC assay to quantify the replication history of mouse and human B lymphocyte subsets in vivo. We observed similar trends in both species. In combination with the IgκREHMA assay to determine SHM, this resulted in new and quantitative insights into homeostatic proliferation of naive mature B lymphocytes and the extent of the humoral immune response in healthy individuals. Importantly, the techniques and insights demonstrated in this study provide a firm basis for clinical evaluation of the regeneration of the peripheral B cell compartment after hematopoietic stem cell transplantation or B cell–directed antibody therapy. Furthermore, these new insights will contribute to the understanding of defects in homeostatic or antigen-induced B cell proliferation in patients suffering from common variable immunodeficiency or another antibody deficiency.
MATERIALS AND METHODS
Mice, B cell culture, and B cell subset isolation.
The animal studies were approved by the Institutional Animal Care and Use Committees of the Erasmus University Rotterdam and the University of California, San Diego.
Resting B cells were purified from total splenocytes of young adult C57BL/6 mice (5–12 wk) using the MACS B cell isolation kit (Miltenyi Biotec), labeled for 10 min at 37°C with 7.5 μM CFSE, and cultured for 2–3 d in RPMI medium containing 10% fetal calf serum, 200 U/ml penicillin, 200 μg/ml streptomycin, 4 mM l-glutamine, and 50 μM β-mercaptoethanol supplemented with IL-4, anti-IgM, and LPS at 1 ng/ml.
B cell subsets were purified from spleen and Peyer's patches using a FACSAria cell sorter (BD Biosciences). The following monoclonal antibodies were used: CD21-FITC (7G6), CD23-PE (B3B4), B220-PerCP (RA3-6B2), IgM-biotin (II/41), IgA-biotin (C10-1; all from BD Biosciences), AA4.1-APC, streptavidin PE-cy7, IgM-APC (II/41; all from eBiosciences), and IgD-PE (11–26; Southern Biotechnology Associates, Inc.).
Isolation of (precursor) B cell subsets from human bone marrow, peripheral blood, and tonsil.
All bone marrow, peripheral blood, and tonsil samples were obtained with informed consent and according to the guidelines of the Medical Ethics Committee of Erasmus MC.
Precursor B cells were obtained from freshly isolated bone marrow samples of three healthy children (age 3–16) as described previously (27).
Four B cell subsets were purified from blood samples of five healthy adults using a FACSDiVa cell sorter (BD Biosciences) after staining of post-Ficoll mononuclear cells that were MACS sorted using CD19 beads (Miltenyi Biotec) with CD27-FITC (LT27; Serotec), IgD-PE, CD19- PE-Cy7 (SJ25C1), and CD5-APC (L17F12; all from BD Biosciences).
Seven tonsillar B lymphocyte subsets were sorted from tonsils of three children on a FACSDiVa cells sorter after direct staining on freeze-thawed material (17). Additional monoclonal antibodies used were CD77-FITC (5B5) and CD38-APC (HB7; both from BD Biosciences).
All fractions were obtained with a purity of >95%.
Generation of control constructs and control cell line for the KREC assay.
Mouse kappa-deleting coding and signal joints were PCR amplified from total bone marrow and cloned into the pGEMT-easy vector (Promega). Efficiency was tested on dilution series of the construct. A pGEMT-easy RQ-PCR was performed to correct for input.
A human KREC signal joint was PCR amplified in two parts from bone marrow mononuclear cell DNA to introduce an EcoRI restriction site 60 bp upstream of the Kde RSS heptamer sequence, and subsequently cloned into the retroviral LZRS-IRES-eGFP vector (Fig. 3 A). The LZRS-KREC construct was transfected into the Phoenix amphotropic packaging cell lines using Fugene-6 (Roche Molecular Biochemicals). Stable high- titer producer clones were selected with puromycin (1 μg/ml). The U698-M pre–B cell line was cultured for several days in RPMI 1640 medium containing 10% FCS and antibiotics before transduction using Retronectin-coated Petri dishes (Takara) and recombinant retrovirus containing supernatant for 2 d, with daily replenishing of retroviral supernatant. GFP-positive cells were single cell sorted using a FACSDiVa cell sorter. Individual clones were selected for dim GFP expression, suggesting a single genomic integration.
Southern blot analysis of individual U698-M KREC cell line clones.
DNA isolation and Southern blot analysis was performed as previously described (57). The configuration of the IGK locus was determined using 32P-labeled probes: the IGK-Kde probe is specific for the Kde region (58), and the Kde-RSS probe is specific for the region upstream of Kde, which is deleted from the genome after an intronRSS–Kde rearrangement and are present on the KREC construct (Fig. 3 B).
RQ-PCR and calculation of the replication history of B cell populations.
Primers and probes were designed to specifically amplify the IRS1-RS and intronRSS–Kde rearrangements (coding joint) and the corresponding signal joint using TaqMan-based RQ-PCR from DNA isolated from cell lines and primary material (Fig. 1, A and B). The RQ-PCR mixture of 25 μl contained TaqMan Universal MasterMix (Applied Biosystems), 900 nM of each primer, 100 nM of each FAM-TAMRA–labeled probe, 50 ng of DNA, and 0.4 ng BSA, and was run on the ABIPRISM 7700 sequence detection system (Applied Biosystems) (59, 60). The primer-probe sets were tested for comparable efficiencies using dilution series of plasmid constructs (for mouse reagents), and DNA was isolated from the U698-M clones DB01, DB11, and DE09, which contain one intronRSS–Kde rearrangement and one KREC construct per genome (for human reagents).
In all experiments, the cycle threshold (CT) was set at 0.03, and the Ct values of the coding joint and the signal joint were compared for each sample. Because both PCR amplification and cell division are exponential multiplication processes with base 2, the ΔCT (CTintronRSS–Kde – CTKREC) from a given cell fraction represents the mean number of cell divisions (Fig. 2 A).
SHM analysis using a Vκ3-20–specific restriction enzyme hot-spot mutation assay (IgκREHMA) on genomic DNA.
To investigate the occurrence of SHM in specific B cell subsets, we modified the IgκREHMA assay developed by Andersen et al. (37) for use on genomic DNA (Fig. 7 A). In short, a PCR reaction was performed using a HEX-coupled Vκ3-20 intron forward primer and two FAM-coupled Jκ reverse primers recognizing all five Jκ gene segments (61). The PCR products (∼500 bp) were digested with KpnI and Fnu4HI and run on a Capillary Sequencer ABI 3100 (Applied Biosystems). The Fnu4HI restriction enzyme recognizes two adjacent sites in the unmutated gene product in the SHM hot-spot region of CDR1. Unmutated gene products can therefore be visualized as 244 or 247-bp HEX-coupled fragments. KpnI cuts the gene products in FR2 downstream of the Fnu4HI sites, resulting in a 262-bp HEX-coupled mutated fragment. The unmutated B cell line CLL-1 was used as a positive control for complete digestion with Fnu4HI. The digests hardly contained undigested gene products of ∼481 bp, indicating complete digestion by KpnI.
Acknowledgments
We thank Dr. C. Murre, Dr. R.W. Hendriks, and Mr. A. van Oudenaren for support with murine studies, Dr. L.J. Hoeve (Erasmus MC-Sophia, Rotterdam, Netherlands) for providing childhood tonsil material, Mr. E.F.E. de Haas for high-speed cell sorting, Ms. E.J. van Gastel-Mol for Southern blot analysis, Mrs. M. Comans-Bitter for assistance in preparing the figures, and Drs. R. Benner and J.D. Laman for critically reading the manuscript.
This work was supported by grants from the foundation “Sophia Kinderziekenhuis Fonds” (grant 349 to M.C. van Zelm, R. de Groot, and J.J.M. van Dongen) and the Dutch Organization for Scientific Research (NWO/ZonMw veni grant 916.56.107 to M. van der Burg).
The authors have no conflicting financial interests.
Abbreviations used: CSR, class switch recombination; IgκREHMA, Igκ restriction enzyme hot-spot mutation assay; KREC, kappa-deleting recombination excision circle; MZ, marginal zone; RQ-PCR real-time quantitative PCR; SHM, somatic hypermutation; TREC, T cell receptor excision circle.
References
- 1.Ghia, P., E. ten Boekel, A.G. Rolink, and F. Melchers. 1998. B-cell development: a comparison between mouse and man. Immunol. Today. 19:480–485. [DOI] [PubMed] [Google Scholar]
- 2.Tonegawa, S. 1983. Somatic generation of antibody diversity. Nature. 302:575–581. [DOI] [PubMed] [Google Scholar]
- 3.Thomas, M.D., B. Srivastava, and D. Allman. 2006. Regulation of peripheral B cell maturation. Cell. Immunol. 239:92–102. [DOI] [PubMed] [Google Scholar]
- 4.Loder, F., B. Mutschler, R.J. Ray, C.J. Paige, P. Sideras, R. Torres, M.C. Lamers, and R. Carsetti. 1999. B cell development in the spleen takes place in discrete steps and is determined by the quality of B cell receptor-derived signals. J. Exp. Med. 190:75–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allman, D., R.C. Lindsley, W. DeMuth, K. Rudd, S.A. Shinton, and R.R. Hardy. 2001. Resolution of three nonproliferative immature splenic B cell subsets reveals multiple selection points during peripheral B cell maturation. J. Immunol. 167:6834–6840. [DOI] [PubMed] [Google Scholar]
- 6.Sims, G.P., R. Ettinger, Y. Shirota, C.H. Yarboro, G.G. Illei, and P.E. Lipsky. 2005. Identification and characterization of circulating human transitional B cells. Blood. 105:4390–4398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cuss, A.K., D.T. Avery, J.L. Cannons, L.J. Yu, K.E. Nichols, P.J. Shaw, and S.G. Tangye. 2006. Expansion of functionally immature transitional B cells is associated with human-immunodeficient states characterized by impaired humoral immunity. J. Immunol. 176:1506–1516. [DOI] [PubMed] [Google Scholar]
- 8.Casali, P., S.E. Burastero, M. Nakamura, G. Inghirami, and A.L. Notkins. 1987. Human lymphocytes making rheumatoid factor and antibody to ssDNA belong to Leu-1+ B-cell subset. Science. 236:77–81. [DOI] [PubMed] [Google Scholar]
- 9.Spencer, J., M.E. Perry, and D.K. Dunn-Walters. 1998. Human marginal-zone B cells. Immunol. Today. 19:421–426. [DOI] [PubMed] [Google Scholar]
- 10.Martin, F., and J.F. Kearney. 2002. Marginal-zone B cells. Nat. Rev. Immunol. 2:323–335. [DOI] [PubMed] [Google Scholar]
- 11.Dunn-Walters, D.K., P.G. Isaacson, and J. Spencer. 1995. Analysis of mutations in immunoglobulin heavy chain variable region genes of microdissected marginal zone (MGZ) B cells suggests that the MGZ of human spleen is a reservoir of memory B cells. J. Exp. Med. 182:559–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van den Oord, J.J., C. de Wolf-Peeters, and V.J. Desmet. 1986. The marginal zone in the human reactive lymph node. Am. J. Clin. Pathol. 86:475–479. [DOI] [PubMed] [Google Scholar]
- 13.Dono, M., S. Zupo, A. Augliera, V.L. Burgio, R. Massara, A. Melagrana, M. Costa, C.E. Grossi, N. Chiorazzi, and M. Ferrarini. 1996. Subepithelial B cells in the human palatine tonsil. II. Functional characterization. Eur. J. Immunol. 26:2043–2049. [DOI] [PubMed] [Google Scholar]
- 14.Spencer, J., T. Finn, K.A. Pulford, D.Y. Mason, and P.G. Isaacson. 1985. The human gut contains a novel population of B lymphocytes which resemble marginal zone cells. Clin. Exp. Immunol. 62:607–612. [PMC free article] [PubMed] [Google Scholar]
- 15.Weller, S., M.C. Braun, B.K. Tan, A. Rosenwald, C. Cordier, M.E. Conley, A. Plebani, D.S. Kumararatne, D. Bonnet, O. Tournilhac, et al. 2004. Human blood IgM “memory” B cells are circulating splenic marginal zone B cells harboring a prediversified immunoglobulin repertoire. Blood. 104:3647–3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weill, J.C. 2006. B cell memory. Abstract book of XIIth Meeting of European Society for Immunodeficiency, Budapest, Hungary. 13.
- 17.MacLennan, I.C. 1994. Germinal centers. Annu. Rev. Immunol. 12:117–139. [DOI] [PubMed] [Google Scholar]
- 18.Pascual, V., Y.J. Liu, A. Magalski, O. de Bouteiller, J. Banchereau, and J.D. Capra. 1994. Analysis of somatic mutation in five B cell subsets of human tonsil. J. Exp. Med. 180:329–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wolniak, K.L., S.M. Shinall, and T.J. Waldschmidt. 2004. The germinal center response. Crit. Rev. Immunol. 24:39–65. [DOI] [PubMed] [Google Scholar]
- 20.MacLennan, I.C. 2005. Germinal centers still hold secrets. Immunity. 22:656–657. [DOI] [PubMed] [Google Scholar]
- 21.Agenes, F., and A.A. Freitas. 1999. Transfer of small resting B cells into immunodeficient hosts results in the selection of a self-renewing activated B cell population. J. Exp. Med. 189:319–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cabatingan, M.S., M.R. Schmidt, R. Sen, and R.T. Woodland. 2002. Naive B lymphocytes undergo homeostatic proliferation in response to B cell deficit. J. Immunol. 169:6795–6805. [DOI] [PubMed] [Google Scholar]
- 23.Woodland, R.T., and M.R. Schmidt. 2005. Homeostatic proliferation of B cells. Semin. Immunol. 17:209–217. [DOI] [PubMed] [Google Scholar]
- 24.Agenes, F., M.M. Rosado, and A.A. Freitas. 2000. Peripheral B cell survival. Cell. Mol. Life Sci. 57:1220–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siminovitch, K.A., A. Bakhshi, P. Goldman, and S.J. Korsmeyer. 1985. A uniform deleting element mediates the loss of kappa genes in human B cells. Nature. 316:260–262. [DOI] [PubMed] [Google Scholar]
- 26.Inlay, M., F.W. Alt, D. Baltimore, and Y. Xu. 2002. Essential roles of the kappa light chain intronic enhancer and 3′ enhancer in kappa rearrangement and demethylation. Nat. Immunol. 3:463–468. [DOI] [PubMed] [Google Scholar]
- 27.van Zelm, M.C., M. van der Burg, D. de Ridder, B.H. Barendregt, E.F. de Haas, M.J. Reinders, A.C. Lankester, T. Revesz, F.J. Staal, and J.J. van Dongen. 2005. Ig gene rearrangement steps are initiated in early human precursor B cell subsets and correlate with specific transcription factor expression. J. Immunol. 175:5912–5922. [DOI] [PubMed] [Google Scholar]
- 28.van der Burg, M., T. Tumkaya, M. Boerma, S. de Bruin-Versteeg, A.W. Langerak, and J.J. van Dongen. 2001. Ordered recombination of immunoglobulin light chain genes occurs at the IGK locus but seems less strict at the IGL locus. Blood. 97:1001–1008. [DOI] [PubMed] [Google Scholar]
- 29.Livak, F., and D.G. Schatz. 1996. T-cell receptor alpha locus V(D)J recombination by-products are abundant in thymocytes and mature T cells. Mol. Cell. Biol. 16:609–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Verschuren, M.C., I.L. Wolvers-Tettero, T.M. Breit, J. Noordzij, E.R. van Wering, and J.J. van Dongen. 1997. Preferential rearrangements of the T cell receptor-delta-deleting elements in human T cells. J. Immunol. 158:1208–1216. [PubMed] [Google Scholar]
- 31.Breit, T.M., M.C. Verschuren, I.L. Wolvers-Tettero, E.J. Van Gastel-Mol, K. Hahlen, and J.J. van Dongen. 1997. Human T cell leukemias with continuous V(D)J recombinase activity for TCR-delta gene deletion. J. Immunol. 159:4341–4349. [PubMed] [Google Scholar]
- 32.Kong, F.K., C.L. Chen, A. Six, R.D. Hockett, and M.D. Cooper. 1999. T cell receptor gene deletion circles identify recent thymic emigrants in the peripheral T cell pool. Proc. Natl. Acad. Sci. USA. 96:1536–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Douek, D.C., R.D. McFarland, P.H. Keiser, E.A. Gage, J.M. Massey, B.F. Haynes, M.A. Polis, A.T. Haase, M.B. Feinberg, J.L. Sullivan, et al. 1998. Changes in thymic function with age and during the treatment of HIV infection. Nature. 396:690–695. [DOI] [PubMed] [Google Scholar]
- 34.Hazenberg, M.D., S.A. Otto, J.W. Cohen Stuart, M.C. Verschuren, J.C. Borleffs, C.A. Boucher, R.A. Coutinho, J.M. Lange, T.F. Rinke de Wit, A. Tsegaye, et al. 2000. Increased cell division but not thymic dysfunction rapidly affects the T-cell receptor excision circle content of the naive T cell population in HIV-1 infection. Nat. Med. 6:1036–1042. [DOI] [PubMed] [Google Scholar]
- 35.Hazenberg, M.D., M.C. Verschuren, D. Hamann, F. Miedema, and J.J. van Dongen. 2001. T cell receptor excision circles as markers for recent thymic emigrants: basic aspects, technical approach, and guidelines for interpretation. J. Mol. Med. 79:631–640. [DOI] [PubMed] [Google Scholar]
- 36.Klein, U., Y. Tu, G.A. Stolovitzky, J.L. Keller, J. Haddad Jr., V. Miljkovic, G. Cattoretti, A. Califano, and R. Dalla-Favera. 2003. Transcriptional analysis of the B cell germinal center reaction. Proc. Natl. Acad. Sci. USA. 100:2639–2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Andersen, P., H. Permin, V. Andersen, L. Schejbel, P. Garred, A. Svejgaard, and T. Barington. 2005. Deficiency of somatic hypermutation of the antibody light chain is associated with increased frequency of severe respiratory tract infection in common variable immunodeficiency. Blood. 105:511–517. [DOI] [PubMed] [Google Scholar]
- 38.Deenen, G.J., and F.G. Kroese. 1993. Kinetics of B cell subpopulations in peripheral lymphoid tissues: evidence for the presence of phenotypically distinct short-lived and long-lived B cell subsets. Int. Immunol. 5:735–741. [DOI] [PubMed] [Google Scholar]
- 39.Macallan, D.C., D.L. Wallace, Y. Zhang, H. Ghattas, B. Asquith, C. de Lara, A. Worth, G. Panayiotakopoulos, G.E. Griffin, D.F. Tough, and P.C. Beverley. 2005. B-cell kinetics in humans: rapid turnover of peripheral blood memory cells. Blood. 105:3633–3640. [DOI] [PubMed] [Google Scholar]
- 40.van der Velden, V.H., M.J. Willemse, C.E. van der Schoot, K. Hahlen, E.R. van Wering, and J.J. van Dongen. 2002. Immunoglobulin kappa deleting element rearrangements in precursor-B acute lymphoblastic leukemia are stable targets for detection of minimal residual disease by real-time quantitative PCR. Leukemia. 16:928–936. [DOI] [PubMed] [Google Scholar]
- 41.Langerak, A.W., B. Nadel, A. De Torbal, I.L. Wolvers-Tettero, E.J. van Gastel-Mol, B. Verhaaf, U. Jager, and J.J. van Dongen. 2004. Unraveling the consecutive recombination events in the human IGK locus. J. Immunol. 173:3878–3888. [DOI] [PubMed] [Google Scholar]
- 42.Retter, M.W., and D. Nemazee. 1998. Receptor editing occurs frequently during normal B cell development. J. Exp. Med. 188:1231–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Han, S., B. Zheng, D.G. Schatz, E. Spanopoulou, and G. Kelsoe. 1996. Neoteny in lymphocytes: Rag1 and Rag2 expression in germinal center B cells. Science. 274:2094–2097. [DOI] [PubMed] [Google Scholar]
- 44.Hikida, M., M. Mori, T. Takai, K. Tomochika, K. Hamatani, and H. Ohmori. 1996. Reexpression of RAG-1 and RAG-2 genes in activated mature mouse B cells. Science. 274:2092–2094. [DOI] [PubMed] [Google Scholar]
- 45.Papavasiliou, F., R. Casellas, H. Suh, X.F. Qin, E. Besmer, R. Pelanda, D. Nemazee, K. Rajewsky, and M.C. Nussenzweig. 1997. V(D)J recombination in mature B cells: a mechanism for altering antibody responses. Science. 278:298–301. [DOI] [PubMed] [Google Scholar]
- 46.Han, S., S.R. Dillon, B. Zheng, M. Shimoda, M.S. Schlissel, and G. Kelsoe. 1997. V(D)J recombinase activity in a subset of germinal center B lymphocytes. Science. 278:301–305. [DOI] [PubMed] [Google Scholar]
- 47.Hikida, M., and H. Ohmori. 1998. Rearrangement of lambda light chain genes in mature B cells in vitro and in vivo. Function of reexpressed recombination-activating gene (RAG) products. J. Exp. Med. 187:795–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hillion, S., A. Saraux, P. Youinou, and C. Jamin. 2005. Expression of RAGs in peripheral B cells outside germinal centers is associated with the expression of CD5. J. Immunol. 174:5553–5561. [DOI] [PubMed] [Google Scholar]
- 49.Hertz, M., V. Kouskoff, T. Nakamura, and D. Nemazee. 1998. V(D)J recombinase induction in splenic B lymphocytes is inhibited by antigen-receptor signalling. Nature. 394:292–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Meffre, E., F. Papavasiliou, P. Cohen, O. de Bouteiller, D. Bell, H. Karasuyama, C. Schiff, J. Banchereau, Y.J. Liu, and M.C. Nussenzweig. 1998. Antigen receptor engagement turns off the V(D)J recombination machinery in human tonsil B cells. J. Exp. Med. 188:765–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schluns, K.S., W.C. Kieper, S.C. Jameson, and L. Lefrancois. 2000. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat. Immunol. 1:426–432. [DOI] [PubMed] [Google Scholar]
- 52.Khaled, A.R., and S.K. Durum. 2002. Lymphocide: cytokines and the control of lymphoid homeostasis. Nat. Rev. Immunol. 2:817–830. [DOI] [PubMed] [Google Scholar]
- 53.Prlic, M., B.R. Blazar, M.A. Farrar, and S.C. Jameson. 2003. In vivo survival and homeostatic proliferation of natural killer cells. J. Exp. Med. 197:967–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jamieson, A.M., P. Isnard, J.R. Dorfman, M.C. Coles, and D.H. Raulet. 2004. Turnover and proliferation of NK cells in steady state and lymphopenic conditions. J. Immunol. 172:864–870. [DOI] [PubMed] [Google Scholar]
- 55.Hao, Z., and K. Rajewsky. 2001. Homeostasis of peripheral B cells in the absence of B cell influx from the bone marrow. J. Exp. Med. 194:1151–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ruprecht, C.R., and A. Lanzavecchia. 2006. Toll-like receptor stimulation as a third signal required for activation of human naive B cells. Eur. J. Immunol. 36:810–816. [DOI] [PubMed] [Google Scholar]
- 57.van Dongen, J.J., and I.L. Wolvers-Tettero. 1991. Analysis of immunoglobulin and T cell receptor genes. Part I: Basic and technical aspects. Clin. Chim. Acta. 198:1–91. [DOI] [PubMed] [Google Scholar]
- 58.Beishuizen, A., M.A. Verhoeven, E.J. Mol, and J.J. van Dongen. 1994. Detection of immunoglobulin kappa light-chain gene rearrangement patterns by Southern blot analysis. Leukemia. 8:2228–2236 (discussion 2237-2239). [PubMed] [Google Scholar]
- 59.Moppett, J., V.H. van der Velden, A.J. Wijkhuijs, J. Hancock, J.J. van Dongen, and N. Goulden. 2003. Inhibition affecting RQ-PCR-based assessment of minimal residual disease in acute lymphoblastic leukemia: reversal by addition of bovine serum albumin. Leukemia. 17:268–270. [DOI] [PubMed] [Google Scholar]
- 60.van der Velden, V.H., A. Hochhaus, G. Cazzaniga, T. Szczepanski, J. Gabert, and J.J. van Dongen. 2003. Detection of minimal residual disease in hematologic malignancies by real-time quantitative PCR: principles, approaches, and laboratory aspects. Leukemia. 17:1013–1034. [DOI] [PubMed] [Google Scholar]
- 61.Van Dongen, J.J., A.W. Langerak, M. Bruggemann, P.A. Evans, M. Hummel, F.L. Lavender, E. Delabesse, F. Davi, E. Schuuring, R. Garcia-Sanz, et al. 2003. Design and standardization of PCR primers and protocols for detection of clonal immunoglobulin and T-cell receptor gene recombinations in suspect lymphoproliferations: Report of the BIOMED-2 Concerted Action BMH4-CT98-3936. Leukemia. 17:2257–2317. [DOI] [PubMed] [Google Scholar]
- 62.Shimizu, T., T. Iwasato, and H. Yamagishi. 1991. Deletions of immunoglobulin C kappa region characterized by the circular excision products in mouse splenocytes. J. Exp. Med. 173:1065–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]