Abstract
Hepatitis B virus (HBV) causes chronic infection in more than 350 million people worldwide. It replicates in hepatocytes but is non-cytopathic; liver damage is thought to be immune mediated. Here, we investigated the role of innate immune responses in mediating liver damage in patients with chronic HBV infection. Longitudinal analysis revealed a temporal correlation between flares of liver inflammation and fluctuations in interleukin (IL)-8, interferon (IFN)-α, and natural killer (NK) cell expression of tumor necrosis factor–related apoptosis-inducing ligand (TRAIL) directly ex vivo. A cross-sectional study confirmed these findings in patients with HBV-related liver inflammation compared with healthy carriers. Activated, TRAIL-expressing NK cells were further enriched in the liver of patients with chronic HBV infection, while their hepatocytes expressed increased levels of a TRAIL death–inducing receptor. IFN-α concentrations found in patients were capable of activating NK cells to induce TRAIL-mediated hepatocyte apoptosis in vitro. The pathogenic potential of this pathway could be further enhanced by the ability of the IFN-α/IL-8 combination to dysregulate the balance of death-inducing and regulatory TRAIL receptors expressed on hepatocytes. We conclude that NK cells may contribute to liver inflammation by TRAIL-mediated death of hepatocytes and demonstrate that this non-antigen–specific mechanism can be switched on by cytokines produced during active HBV infection.
Chronic infection with hepatitis B virus (HBV), a hepatotropic DNA virus, is a major cause of liver disease worldwide. More than 350 million people are persistently infected and at risk of developing chronic liver inflammation resulting in liver cirrhosis and hepatocellular carcinoma. The World Health Organization estimates that at least 1 million deaths each year are directly attributable to HBV-related liver disease. An increasing proportion of the chronic disease seen in many countries is due to the development of viral variants lacking the expression of “e” antigen but associated with active viral replication and liver disease, termed e antigen negative chronic hepatitis B (eAg-CHB; reference 1). Patients with eAg-CHB are prone to recurrent, spontaneous “hepatic flares,” characterized by large, unexplained fluctuations in liver inflammation and a propensity to progress rapidly to severe liver fibrosis (2). These hepatic flares provide a window of opportunity to study mechanisms involved in dynamic alterations in viral load and liver inflammation over a condensed timeframe. Because HBV is non- cytopathic, liver damage is thought to be immune mediated, but the molecular pathways leading to hepatocyte death in human HBV infection are not well understood. There is a pressing need to dissect the ways in which different components of the immune response contribute to liver disease in HBV infection. This will allow the rational development of immunotherapeutic strategies that enhance viral control while limiting or blocking liver inflammation.
Immune-mediated liver damage in patients with HBV infection has conventionally been attributed to cytolytic killing of infected hepatocytes by virus-specific CD8 T cells. However, this assumption was challenged by previous work demonstrating the presence of activated HBV-specific CD8 T cells at high frequencies in the livers of patients controlling HBV infection without any evidence of liver inflammation. Instead, the distinguishing feature between patients with or without HBV-related chronic liver disease was the presence of a large, non-antigen–specific lymphocytic infiltrate in the livers of the former group (3). The mechanisms resulting in the recruitment and activation of this nonspecific inflammatory infiltrate have been explored in the transgenic mouse model of HBV. In this model, it was possible to reduce the severity of liver damage by inhibiting the nonspecific cellular infiltrate (4, 5), reinforcing the concept that liver inflammation initiated by virus-specific CD8 is amplified by other lymphocytes (6).
One of the largest constituents of the lymphocytic infiltrate in HBV transgenic mice is NK cells (NK1.1+CD3−), with a 10–12-fold increase in their numbers in the inflammatory infiltrate compared with baseline (4, 5). NK cells (CD3−CD56+) are likewise a major component of the cellular infiltrate in the human liver, comprising 30–40% of total intrahepatic lymphocytes (7). An early rise in circulating NK cells has been documented in the incubation phase of HBV infection, suggesting that they may contribute to the initial viral containment in this setting (8). The antiviral and pathogenic potential of NK cells in patients with chronic HBV infection remain to be addressed.
The mechanism through which NK cells mediate antiviral cytotoxicity appears to be organ dependent (9). NK cytotoxicity through perforin/granzyme is now considered to be of less relevance in the liver environment, where the target hepatocytes are relatively resistant to lysis through this pathway (9, 10). Receptor-mediated cell death through ligand–receptor pairs belonging to the TNF superfamily is likely to play a more important role in liver damage (11, 12). One such pathway is mediated through TNF-related apoptosis-inducing ligand (TRAIL; reference 13) expressed on infiltrating lymphocytes interacting with TRAIL death–inducing receptors (TRAIL-R1 and TRAIL-R2; reference 14) on hepatocytes. This has been shown to be a critical mechanism of liver damage in vivo in Listeria and concanavalin A– induced hepatitis in mice (15). An essential role for NK cells in hepatic TRAIL-mediated apoptosis was highlighted in the setting of the surveillance of tumor metastases (16). Normal human hepatocytes have also been shown to be sensitive to TRAIL-mediated apoptosis (17, 18), and it has been suggested that susceptibility to this pathway may be increased during viral hepatitis (19, 20). We therefore hypothesized that NK-expressed TRAIL may play a role in non-antigen–specific mediation of liver damage in chronic HBV infection.
NK cell effector function is a result of the balance of signals through their activatory and inhibitory receptors, a balance that is influenced by the local cytokine milieu. Furthermore, NK cells can be directly activated to antiviral activity by certain cytokines, with IFN-α promoting cytotoxicity (21) and TRAIL expression (22), and IL-12 favoring IFN-γ production (21). IFN-α production characterizes the early stages of acute viral infections, but it is unclear whether its release can also be triggered by fluctuations in viral load occurring on the background of the persistent high level antigenic stimulation found in chronic HBV infection. The downstream effects of any IFN-α produced may be attenuated in antigen-activated cells (23, 24) or modified by an increase in other cytokines, such as IL-1β (25) and IL-8 (26, 27).
To understand the cytokine milieu influencing NK cell activity, we quantified IFN-α and several other key proinflammatory and immunoregulatory cytokines in patients with chronic HBV infection. We took advantage of a cohort of well-characterized patients with eAg-CHB sampled repeatedly before, during, and after multiple hepatic flares to correlate sensitive measurements of their serum cytokine levels with changes in liver inflammation. We observed large fluctuations in serum IFN-α and IL-8 concentrations in association with the hepatic flares. Increases in circulating IFN-α and IL-8 in CHB patients with liver inflammation were accompanied by an increase in NK cell activation and surface TRAIL expression measured directly ex vivo. We then explored the mechanisms underlying these ex vivo observations, which could explain the resultant liver damage. We established that the concentrations of IFN-α and IL-8 produced in vivo promoted the TRAIL pathway of NK cell killing, acting on both the ligand and the receptors. We confirmed that, in the presence of this combination of cytokines, NK cells from patients with chronic HBV infection became capable of TRAIL-mediated killing of hepatocytes.
RESULTS
Large fluctuations in circulating levels of IFN-α and IL-8 during flares of liver disease in chronic HBV infection
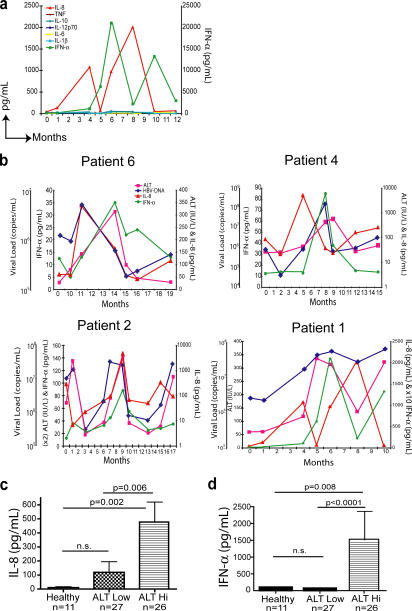
Patients with eAg-CHB are susceptible to spontaneous flares of liver inflammation associated with rapid changes in viral load. These flares provide an opportunity to investigate mechanisms of HBV-related liver damage during periods of dynamic fluctuation that are predictable enough to be captured upon longitudinal sampling. A cohort of 14 eAg-CHB patients that had previously been identified as likely to undergo recurrent hepatic flares (2) were recruited and studied longitudinally. Through frequent sampling, serum was obtained before, during, and after one or multiple flares, defined in this study as an abrupt increase in serum alanine transaminase (ALT) to more than double the baseline value and more than three times the upper limit of normal (<35 IU/L for women, <50 IU/L for men). Serum ALT was used as a surrogate marker for liver damage because studies in chimpanzees (28) and humans (29) infected with HBV have shown that it accurately predicts histological findings of hepatic inflammation. All patients included had a well-characterized disease course with clinical monitoring for at least 1 yr and between 4 and 10 serial samples available for the study (see Table II), usually taken at intervals of 1–2 mo. Using a cytometric bead array (CBA) inflammation kit and ELISA technology, it was possible to quantitate multiple cytokines simultaneously from a small volume of serum. The cytokines analyzed were IL-8, IL-1β, IL-6, IL-10, TNF, IL-12p70, and IFN-α. Of the seven cytokines examined, only IL-8 and IFN-α were consistently detected, with peak concentrations far in excess of those observed for the other five cytokines in patients and significantly higher than in healthy controls (Table I and Fig. S1, which is available at http://www.jem.org/cgi/content/full/jem.20061287/DC1). The serum levels of these two cytokines were observed to undergo large fluctuations, which were recurrent in the cases with multiple flares (Fig. 1 a), with patients consistently displaying substantial fold changes throughout the flaring events assayed (Table II). These uniform, large fluctuations were not observed with the other cytokines measured (Fig. 1 a).
Table II.
IL-8 and IFN-α serum concentrations correlate temporally with hepatic flares
| No. of samples |
Max viral load (106 copies/mL) |
Max ALT (IU/L) |
IL-8 max fold changea |
Sampling interval between ALT peak and IL-8 peakb |
IFN-α max fold change |
Sampling interval between ALT peak and IFN-α peak |
|
|---|---|---|---|---|---|---|---|
| Patient 1 | 7 | 975 | 546 | 136 | −1 | 152 | −3 |
| Patient 2 | 10 | 31.4 | 285 | 686 | 0 | 7 | 0 |
| Patient 3 | 7 | 141 | 569 | 237 | −2 | 6.8 | −1 |
| Patient 4 | 8 | 417 | 208 | 417 | −2 | 12 | na |
| Patient 5 | 8 | 16.4 | 495 | 40 | 2 | 7.2 | −5 |
| Patient 6 | 7 | 5.67 | 313 | 8.2 | −1 | 7 | 0 |
| Patient 7 | 7 | 1,510 | 214 | 29 | −1 | 20 | 0 |
| Patient 8 | 5 | 63.8 | 201 | 303 | −3 | 3.7 | 0 |
| Patient 9 | 4 | 21.4 | 565 | 11 | 0 | 2.4 | −1 |
| Patient 10 | 4 | 418 | 880 | 1.7 | −3 | 3.2 | 0 |
| Patient 11 | 5 | 600 | 403 | 5.2 | 0 | 2.3 | 2 |
| Patient 12 | 5 | 1,800 | 614 | 5.4 | −1 | 1.4 | −2 |
| Patient 13 | 5 | 19 | 158 | 32 | 0 | 8.2 | 1 |
| Patient 14 | 4 | 800 | 196 | 70 | −1 | 9.5 | 0 |
| Median | 36 | −1 | 7 | 0 |
The fold change of IL-8 or IFN-α from baseline levels to the peak of the cytokine fluctuation.
A negative value implies the cytokine peak occurs before the height of the flare, and a zero means it coincided with the flare. na, data not available.
Table I.
IL-8 and IFN-α levels are highly elevated in sera from eAg-CHB patients with flares
| Median of peak cytokine concentration (pg/mL) |
|||
|---|---|---|---|
| CHB patients | Healthy controls | p-values | |
| IL-8 | 630 | 13 | <0.0001 |
| IL-1β | 79 | blq | 0.07 |
| IL-6 | 11 | blq | 0.08 |
| IL-10 | 9 | 1.7 | 0.04 |
| TNF | 4 | blq | 0.64 |
| IL-12p70 | 17 | 2.6 | 0.1 |
| IFN-α | 253 | 20 | 0.005 |
The median value is that of the maximum cytokine concentration obtained from 12 CHB patients undergoing flares of liver inflammation and 14 healthy control donors. Significance testing was performed using the Mann-Whitney U test, with those of statistical significance highlighted in bold. blq, below level of quantification.
Figure 1.
IL-8 and IFN-α concentrations are elevated in the serum of CHB patients with liver inflammation. (a) Circulating concentrations of multiple cytokines detected in longitudinal serum samples taken from a representative patient (patient 1) assayed by CBA (IL-8, IL-1β, IL-6, IL-10, TNF, and IL-12p70) and sandwich ELISA (IFN-α). The concentrations of inflammatory cytokines were determined by CBA software or Prism. (b) Temporal relationship between serum IL-8 and IFN-α concentrations and liver inflammation (ALT) and viral load (HBV-DNA) in 4 representative patients of 14 patients assayed. Cross-sectional comparison of IL-8 (c) and IFN-α (d) levels quantitated by sandwich ELISA in healthy donors, HBV patients with low ALT (ALT < 60 IU/l for the last year), and HBV patients with raised ALT (ALT > 60 IU/L at time of sampling). Significance testing was performed using the Mann-Whitney U test.
The patients in this cohort had a marked degree of liver inflammation (indicated as maximum ALT, Table II) and high viral load (see maximum viral load, Table II) at the height of the flare. Changes in serum IFN-α and IL-8 levels showed a temporal association with fluctuations in ALT and HBV-DNA (Fig. 1 b). For the majority of patients (10/14), the peak serum level of IL-8 preceded the onset of the flare of liver inflammation (the sample just before the ALT peak) either simultaneous to or immediately after a sharp increase in viral load (Fig. 1 b and Table II). Maximal serum concentrations of IFN-α occurred concurrently with the peak of liver inflammation (Fig. 1 b and Table II; median interval between IFN-α peak and ALT peak, 0) at a time when IL-8 levels were declining but still highly elevated compared with healthy controls (Fig. 1, a and b).
To establish whether elevated levels of IL-8 and IFN-α were restricted to HBV patients with active disease or could also be found in the absence of liver inflammation, we conducted a large cross-sectional study. Serum IFN-α and IL-8 concentrations were compared in controls without HBV infection, patients with chronic HBV infection with no evidence of liver inflammation (ALT < 60 IU/L at the time of sampling and no ALT > 60 IU/L recorded in the preceding year), and patients with HBV infection with liver inflammation (ALT > 60 IU/L at the time of sampling). HBV patients with liver inflammation had significantly raised levels of both IL-8 (Fig. 1 c) and IFN-α (Fig. 1 d) compared with the control groups. In contrast, healthy donors consistently had low or undetectable levels of these two cytokines, and patients with chronic HBV without evidence of liver inflammation had no significant increases in IL-8 and IFN-α compared with healthy donors (Fig. 1, c and d). Further analysis of these data revealed a similar correlation of raised IL-8 levels and a high HBV viral load (not depicted), supporting the original observation that fluctuations in IL-8 concentrations mirrored those of HBV-DNA (Fig. 1 b).
Direct ex vivo correlation between NK cell expression of the proapoptotic ligand TRAIL and HBV-related liver inflammation
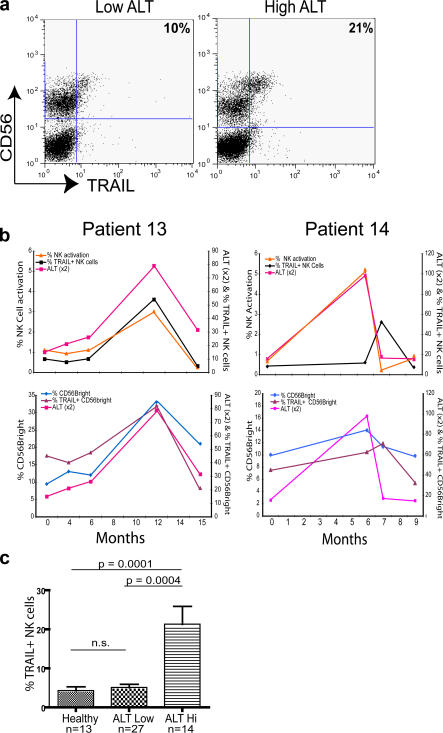
A large proportion of IFN-α–activated NK cytotoxicity is mediated through the proapoptotic ligand TRAIL (22), recently identified as a major effector in murine models of liver damage (15). Having established that the dominant cytokines during flares were IFN-α and IL-8, we investigated if there was an associated activation of the NK cell TRAIL pathway. Human NK cells have been reported to express little or no TRAIL on their surface when freshly isolated from healthy donor blood (30–32). However, some NK cell TRAIL is detectable upon permeabilization (30), and they are capable of up-regulating it upon activation in culture (31, 32). In contrast, CD3−CD56+ NK cells from an eAg-CHB patient with recurrent flares were found to have a clear population surface costaining with an anti-TRAIL mAb directly ex vivo (Fig. 2 a). The proportion of NK cells expressing surface TRAIL further increased when ALT was raised (Fig. 2 a).
Figure 2.
Direct ex vivo correlation between NK cell TRAIL expression and liver inflammation in CHB patients. (a) Representative flow cytometry dot plot from a CHB patient stained with mAb to CD3, CD56, and TRAIL, and gated on CD3− cells. The percentages denote the proportion of freshly isolated CD3−CD56+ NK cells staining with TRAIL. (b) Top: PBMCs from patients with eAg-CHB were stained ex vivo, and the percentage of NK (CD3−CD56+) -expressing TRAIL upon flow cytometry was correlated with ALT. CD69+ NK cells are presented as a percent of total lymphocytes. Bottom: The percent of CD56bright NK cells out of total CD3−CD56+ NK cells and the percent of those CD56bright NK cells that were TRAIL+ was plotted against ALT. (c) Cross-sectional analysis of ex vivo surface TRAIL expression on CD3−CD56+ NK cells from healthy donors, HBV patients with low ALT (ALT < 60 IU/l for the last year), and HBV patients with raised ALT (ALT > 60 IU/L at time of sampling). Significance testing was performed using the Mann-Whitney U test.
In a subset of five patients from our longitudinal cohort of eAg-CHB patients for whom serial PBMCs were available before, during, and after flares, we were able to make a temporal analysis of NK cell activation and TRAIL expression. As illustrated for two representative patients in Fig. 2 b, surface TRAIL expression on NK cells showed large variations ex vivo concurrent with hepatic flares (Fig. 2 b, top). The NK cell expression of CD69, a marker of activation, also correlated tightly with the hepatic flare, with peak activation coinciding with maximal ALT (Fig. 2 b, top) and with elevated levels of IL-8 and IFN-α (Table II).
The majority of TRAIL was noted to be on the CD56bright subset of NK cells (see representative sample in Fig. 2 a) rather than the larger CD56dim subset responsible for perforin-mediated cytotoxicity (33). The increase in overall NK cell TRAIL expression during flares was noted to be due to an increase in the percent of the CD56bright subset within the NK cells and an increase in the proportion of these CD56bright NK cells expressing TRAIL (Fig. 2 b, bottom).
We then compared the level of NK cell surface TRAIL expression in our larger cross-sectional cohort of healthy donors and HBV patients with or without liver inflammation. The percentage of NK cells expressing TRAIL on their surface directly ex vivo was increased more than fourfold in patients with liver inflammation compared with HBV patients with normal ALT (P < 0.001) or healthy donors (P < 0.0001; Fig. 2 c). Increased TRAIL expression in patients with liver inflammation compared with patients with no inflammation was also observed within the CD56bright subset (P < 0.0005; not depicted).
Of note, levels of TRAIL expressed on CD3+ T cells remained low upon longitudinal and cross-sectional analysis of HBV patients, regardless of the degree of liver inflammation (not depicted). In addition, levels of T cell proliferation to HBV core and surface antigens showed no increase around the time of the flare in the three patients in whom this parameter was examined longitudinally (Fig. S2, available at http://www.jem.org/cgi/content/full/jem.20061287/DC1).
These data, showing an in vivo up-regulation of activated NK cells expressing TRAIL, suggest a role for NK cells using this pathway in the pathogenesis of HBV-induced liver disease.
Intrahepatic NK cells express high levels of TRAIL and are highly activated
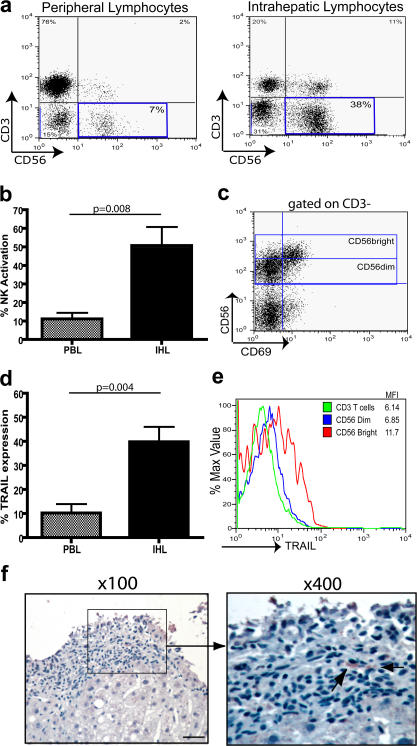
Next, we investigated whether the NK cell TRAIL pathway could operate in the liver, the site of active HBV replication. It is already well established that NK cells are enriched in healthy livers compared with the periphery (7). To identify if NK cell numbers are likewise increased in the livers of CHB patients, intrahepatic mononuclear cells were isolated from HBV-infected livers (three with cirrhosis and two with a flare), and the proportions of CD3+ T cells, CD3−CD56+ NK cells, and CD3+CD56+ NKT cells were determined by flow cytometry. As shown in Fig. 3 a, both NK and NKT cells were enriched in the liver compared with the periphery of HBV-infected patients, with CD3−CD56+ NK cells typically constituting up to 40% of total intrahepatic lymphocytes. This is in line with recent data indicating that NK cells constitute 30–40% of intrahepatic lymphocytes in HBV patients (as in healthy controls), regardless of viral load, ALT, or histology (34).
Figure 3.
Enrichment of NK cell numbers, TRAIL expression, and activation in the liver compared with periphery. (a) Mononuclear cells from the periphery and liver of a representative CHB patient were stained with antibodies to CD3 and CD56, and the proportion of CD3+ T cells, CD3+CD56+ NKT cells, and CD3−CD56+ NK cells (highlighted in box) was determined by flow cytometry. NK cells (CD3−CD56+) from liver-infiltrating (IHL) and circulating (PBL) lymphocytes from five chronically infected HBV patients were assessed ex vivo for CD69 expression (b) and TRAIL expression (d). p-values were determined by the Mann-Whitney U test. (c) Flow cytometry dot plot analysis of a representative CHB patient comparing intrahepatic NK cell activation in the CD56bright and CD56dim NK cell subsets. (e) A histogram comparing TRAIL expression on the CD56bright and CD56dim NK cell subsets and CD3+ T cells isolated from the liver. (f) Paraffin-embedded liver sections taken from seven HBV patients were stained with an anti-TRAIL mAb. The boxed area on the left indicates the field of view on the right panel. TRAIL+ cells are stained brown and are highlighted with black arrows. Bar, 40 μm.
When the activation status of these intrahepatic NK cells was assessed, a greater proportion of intrahepatic NK cells had up-regulated CD69 than peripheral NK cells from the same patient (Fig. 3 b). Of note, the most highly activated NK cell subset in the liver was the CD56bright subset (Fig. 3 c), a subset that was also preferentially enriched in the liver (not depicted and reference 32).
A recent study by Ishiyama et al. (32) showed that TRAIL was not detectable on NK cells extracted from healthy livers at the time of living donor transplantation. In contrast, we found that intrahepatic NK cells isolated from these HBV-infected livers expressed TRAIL directly ex vivo at even higher levels than seen in the periphery of the same patients (Fig. 3 d). As in the periphery, TRAIL was predominantly expressed on the preferentially activated CD56bright NK subset, and intrahepatic CD3+ T cells expressed little TRAIL (Fig. 3 e).
To further examine the relationship between intrahepatic NK TRAIL levels and liver inflammation, we compared five biopsies taken around the time of an ALT flare in patients with histologically proven eAg-CHB, with two biopsies from HBV patients with normal ALT and histology confirming inactive disease. TRAIL+ lymphocytes (presumed to be NK cells because these are the only population expressing significant TRAIL in the periphery or liver) were identified in four out of the five eAg-CHB sections by immunohistochemistry (Fig. 3 f). In contrast, sections from the two patients with inactive HBV infection resembled reports of healthy livers (32), with no TRAIL-expressing lymphocytes identifiable. The results suggest that intrahepatic NK cell TRAIL expression correlates with HBV-related liver inflammation, as noted in the periphery in Fig. 2.
NK cells from patients with chronic HBV infection can be activated and induced to express TRAIL by cytokine concentrations found during liver inflammation
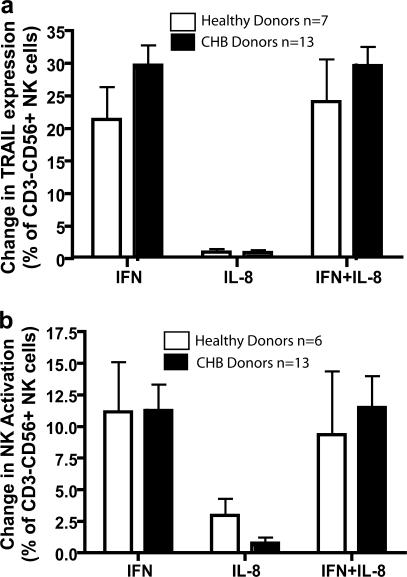
We next sought to explore possible mechanistic links between our ex vivo findings of increases in IFN-α, IL-8, and NK-expressed TRAIL in patients with raised ALT. These two cytokines found in high concentrations during HBV-related inflammation could contribute to liver damage by immunomodulatory effects on NK cells and the TRAIL pathway. IFN-α is a modulator of NK cell function, but it was unclear how this would be affected by IL-8, an inhibitor of its antiviral efficacy (26, 27). Furthermore, it was possible that NK cells could become resistant to IFN-α–mediated modulation after the recurrent stimulation likely in these patients with longstanding HBV-related inflammation. To investigate this, PBMCs or purified NK cells from healthy volunteers and patients with chronic HBV were incubated in vitro for 24 h with IFN-α or IL-8 alone or in combination at concentrations observed during hepatic flares. PBMCs or purified NK cells showed a substantial increase in the percentage of NK cells expressing TRAIL upon incubation with IFN-α (Fig. 4 a). IL-8 did not have a direct effect or inhibit the ability of IFN-α to up-regulate TRAIL expression. Rather than becoming resistant to the effects of IFN-α, NK cells from chronically infected HBV patients, including patients undergoing flares, up-regulated TRAIL by a similar amount to NK cells from healthy donors (Fig. 4 a). HBV patients with liver inflammation therefore achieved a higher total NK cell TRAIL level after in vitro IFN-α treatment as a result of their higher starting expression ex vivo.
Figure 4.
Concentrations of IFN-α observed in patient sera induce increased surface TRAIL expression and activation of NK cells isolated from CHB patients. PBMCs from healthy donors (white bars) and CHB patients (black bars) were incubated for 24 h in vitro with 1,000 U/ml IFN-α, 5 ng/ml IL-8, or IFN-α and IL-8. The effect of this incubation on TRAIL expression (a) and NK cell activation (b) was assessed by flow cytometry analysis with NK cells identified as CD3−CD56+. Graphs were plotted by subtracting baseline levels of CD69 or TRAIL observed in the untreated controls from those observed after cytokine treatment.
NK cells taken from patients with chronic HBV infection also maintained the capacity to be activated by IFN-α, with equivalent levels of CD69 up-regulation to that seen in NK cells from healthy donors and again no inhibition of this effect by IL-8 (Fig. 4 b). IFN-α induced equivalent levels of activation of highly purified NK cells, indicating a direct effect of this cytokine (not depicted). Thus, the in vivo observations of up-regulation of NK cell TRAIL and CD69 expression were mirrored in vitro using equivalent concentrations of cytokines to those circulating in CHB patients with liver inflammation.
Cytokine-modulated TRAIL receptor expression on hepatocytes in HBV infection
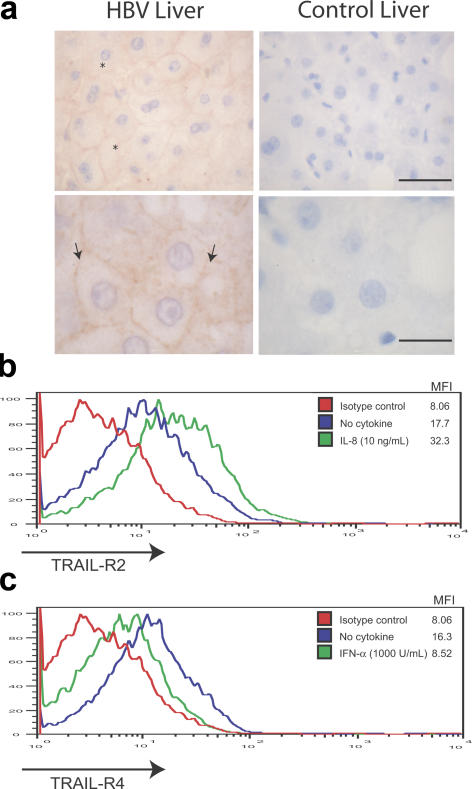
In order for TRAIL to induce receptor-mediated cell death, it needs to engage with a death domain receptor on the target cell (14). Previous studies have suggested minimal protein expression of TRAIL death–inducing receptors in healthy livers (19, 35). However, mRNA for the death-inducing receptors TRAIL-R1 and TRAIL-R2 has been isolated from human hepatocytes (14, 17), which become susceptible to TRAIL-induced apoptosis upon culture (17), suggesting a potential for up-regulation. To ascertain whether hepatocytes in HBV-infected livers express a death-inducing receptor that could engage with the NK-expressed TRAIL, paraffin-embedded liver sections from HBV-infected and control livers were stained for TRAIL-R1 and TRAIL-R2. No TRAIL-R1 was detected in the HBV-infected or control livers (not depicted). However, TRAIL-R2 was expressed by hepatocytes in 10 of the 13 HBV-infected liver sections stained (from patients with eAg-CHB, with histology showing mild to moderate inflammatory infiltrates or cirrhosis). Immunostaining for TRAIL-R2 was predominantly localized to the surface of hepatocytes (Fig. 5 a) and ranged from strong in three patients, moderate in two, and weak in six, with no clear correlation with stage of liver disease. However, TRAIL-R2 was absent in sections from two control HBV patients with normal ALT and inactive disease. TRAIL-R2 staining was detected in a control donor with hepatic steatosis but not in the other control liver sections examined, three from healthy donors and four from patients with alcoholic hepatitis (Fig. 5 a).
Figure 5.
TRAIL receptor expression on hepatocytes in HBV infection. (a) Paraffin-embedded sections from HBV-infected (left) and healthy control (right) livers were stained with an anti–TRAIL-R2 mAb. Membrane-localized (arrows) and cytoplasmic (*) TRAIL-R2 expression is indicated by the brown chromogen reactivity. Bars: top, 40 μm; bottom, 16 μm. (b) Mean fluorescence intensity of HepG2 TRAIL-R2 levels after IL-8 incubation (10 ng/ml for 24 h) compared with untreated and isotype controls. (c) Mean fluorescence intensity of HepG2 TRAIL-R4 levels after 1,000 U/ml IFN-α for 24 h compared with untreated and isotype controls. These are representative of five separate experiments.
We hypothesized that IFN-α or IL-8 might partially mediate this altered TRAIL-R expression pattern observed in HBV-infected inflamed livers to favor hepatocyte death. To investigate this, HepG2 hepatocytes were incubated with IL-8 or IFN-α at equivalent concentrations to those circulating in patients, and the levels of expression of the death-inducing (TRAIL-R1 and TRAIL-R2) and inhibitory (TRAIL-R3 and TRAIL-R4) TRAIL receptors were determined by flow cytometry. IL-8 was consistently found to induce an approximate doubling in the expression of TRAIL-R2 (Fig. 5 b), the death domain receptor observed to be up-regulated on hepatocytes of CHB patients (Fig. 5 a). Incubation with IL-8 did not alter surface expression of any of the other TRAIL receptors (not depicted). IFN-α had no effect on the expression of TRAIL-R2 (not depicted) but reproducibly and substantially decreased expression of the decoy/regulatory receptor TRAIL-R4 (Fig. 5 c). TRAIL-R4 has recently been shown to form a ligand-independent association with TRAIL-R2 to inhibit apoptosis induction (36). Collectively, these results suggest that the high concentrations of IL-8 and IFN-α can act in combination to both increase a death-inducing receptor and reduce an inhibitory receptor, thus optimally predisposing hepatocytes to TRAIL-mediated cell death.
Cytokines circulating during HBV flares can render NK cells capable of killing hepatocytes through TRAIL ligand–receptor interactions
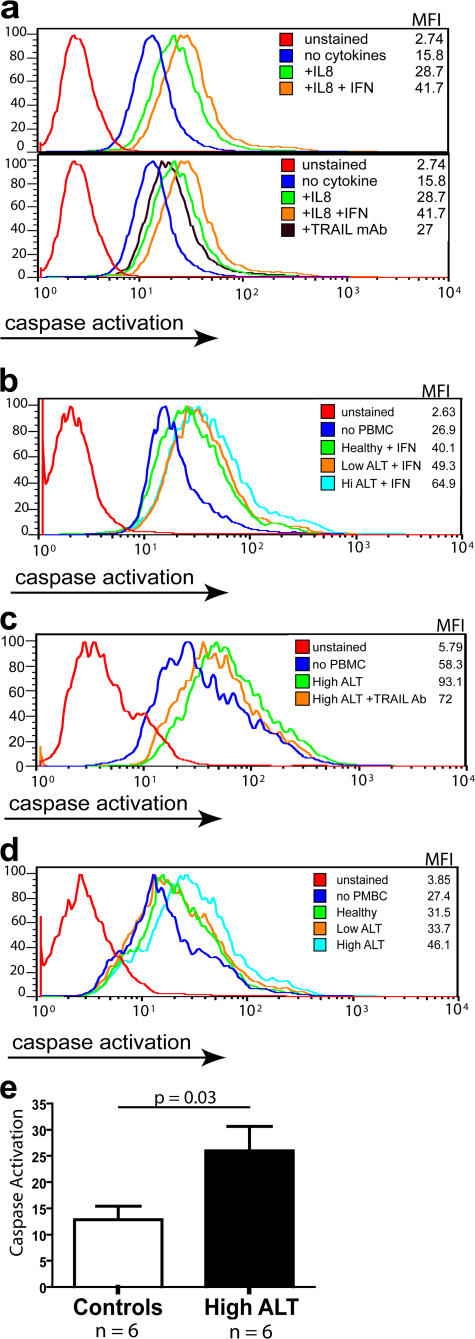
To test whether NK cells isolated from HBV patients could kill hepatocytes using TRAIL, we used an assay that can directly measure the degree of receptor-mediated cell death via the caspase cascade pathway used by TRAIL. PBMCs from patients were incubated with or without IFN-α overnight to induce maximal TRAIL expression on the NK cells. At the same time, HepG2 hepatoma cells were preincubated with or without IL-8 overnight. Activated PBMCs were then added to the HepG2 cells, and the degree of HepG2 cell caspase activation was assessed by flow cytometry. When the HepG2 cells or PBMCs were not treated with cytokines, there was little caspase activation when compared with the background HepG2 cell levels (not depicted). Pretreatment of the HepG2 cells with IL-8 increased the amount of PBMC-mediated caspase activation, which was further increased when the PBMCs were preincubated with IFN-α (Fig. 6 a, top).
Figure 6.
IFN-α–activated NK cells from CHB patients can mediate TRAIL-induced hepatocyte apoptosis. (a) HepG2 cells were incubated for 24 h with or without 10 ng/ml IL-8. Simultaneously, PBMCs were incubated for 24 h with or without 1,000 U/ml IFN-α. Top: PBMCs were then added to HepG2 at an E/T ratio of 10:1 for 4 h before visualization of caspase activation with the fluorescein-labeled Z-VAD-fmk and detection by flow cytometry, expressed as mean fluorescence intensity (MFI). Bottom: Experimental procedure as above except for the addition of 10 ng/ml of a TRAIL blocking antibody. (b) Representative results of PBMCs from healthy donors, CHB patients with low ALT, and CHB patients with high ALT incubated with 1,000 U/ml IFN-α for 24 h and then assessed for caspase activation of IL-8–treated HepG2 as above. (c) Representative HepG2 caspase induction by ex vivo PBMCs from high ALT HBV patient and reduction upon the addition of TRAIL blocking mAb. (d) Representative HepG2 caspase induction upon the addition of PBMCs taken directly ex vivo from a healthy donor, CHB patient with low ALT, and CHB patient with high ALT. (e) Summary level of HepG2 caspase induction using PBMCs directly ex vivo from HBV patients with liver injury (ALT high patients, n = 6) compared with PBMCs from controls (HBV patients without raised ALT, n = 3; healthy controls, n = 3; P = 0.03, Mann- Whitney U test).
To confirm that this IFN-α–induced caspase activation was TRAIL mediated, the HepG2 cells were preincubated with a blocking antibody to TRAIL. As can be seen in the bottom of Fig. 6 a, when TRAIL was blocked there was a reduction in IFN-α–induced caspase activation compared with the non-antibody–treated cells (varying from 50 to 100% blocking). This suggests that TRAIL plays a major role in the IFN-α–induced caspase activation but may not be the only mechanism involved. The IFN-α–induced increase in TRAIL-mediated death was maintained using purified NK cells and abrogated in NK cell–depleted fractions (not depicted).
PBMCs from HBV patients without liver inflammation or from healthy controls showed less efficient initiation of the caspase cascade after up-regulation of NK cell TRAIL with in vitro IFN-α treatment (Fig. 6 b). There was twice as much caspase induction using IFN-α–activated PBMCs from flaring patients (Fig. 6, a and b) as from healthy donors (Fig. 6 b). PBMCs taken from patients with HBV-related liver inflammation were also able to induce apoptosis when added to HepG2 directly ex vivo, partially blocked in all cases (mean of 40% blocking) upon the addition of a TRAIL-blocking mAb (Fig. 6 c). Patients with HBV liver inflammation (n = 6), showed a mean of 28% caspase induction over background, which was significantly greater than that seen using PBMCs from HBV patients with normal ALT or healthy controls (n = 6; Fig. 6, d and e).
These experiments confirm that, under the influence of cytokines induced during HBV flares, NK cells become capable of inducing death of HepG2 hepatoma cells through the TRAIL pathway.
NK cells from HBV patients with flares can initiate TRAIL-induced apoptosis of primary human hepatocytes
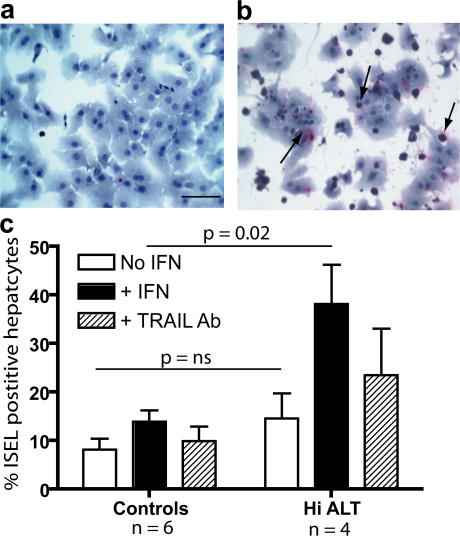
The HepG2 hepatoma cell line provided a convenient model to dissect the mechanisms of activation of this pathway, but it was important to confirm that primary human hepatocytes would also be susceptible to NK TRAIL-mediated apoptosis. Hepatocytes were isolated by perfusion of a nondiseased liver explant and cultured for 48 h, with IFN-α and IL-8 added for the last 24 h to modulate TRAIL receptor expression. Viability of hepatocytes without the addition of PBMCs was good (>80% in all wells; Fig. 7 a). In contrast, hepatocytes incubated with PBMCs from an HBV patient with a flare who had TRAIL-expressing NK cells directly ex vivo showed hepatocyte apoptosis induction (Fig. 7 b). IFN-α–treated PBMCs taken from patients expressing NK cell TRAIL during an episode of liver inflammation were more efficient at induction of apoptosis of primary human hepatocytes than PBMCs from HBV patients without a flare or healthy donors (P = 0.02, Mann-Whitney U test; Fig. 7 c). In three out of four high ALT patients, >30% of the apoptosis induced by IFN-α–treated PBMCs could be blocked through TRAIL (mean of 28% blocking for the four patients). Induction of apoptosis by PBMCs cultured without IFN-α for 24 h was less reliably elicited (showing a mean 15% increase over background levels in patients with liver disease [P = 0.04] and a nonsignificant trend to increased levels in this group compared with controls; Fig. 7 c). A larger study using PBMCs directly ex vivo will be required to confirm differences between patient groups. However, we can conclude that PBMCs from HBV patients with liver inflammation whose NK cells express TRAIL are capable of mediating death of primary human hepatocytes.
Figure 7.
NK cells from CHB patients can mediate TRAIL-induced apoptosis in primary human hepatocytes. Primary human hepatocytes were cultured for 48 h with the addition of 10 ng/ml IL-8 and 1,000 U/ml IFN-α for the last 24 h. Simultaneously, PBMCs from three healthy donors, three CHB patients with low ALT, and four CHB patients with high ALT were incubated with or without IFN-α for 24 h at 37°C. The hepatocytes and PBMCs were incubated together for 18 h at an E/T ratio of 10:1, with or without a TRAIL blocking antibody in the IFN-treated wells. The degree of apoptosis was determined by in situ DNA end labeling (ISEL) for the detection of DNA fragmentation. (a) A representative image of control hepatocytes incubated without PBMCs. Bar, 40 μm. (b) A representative image of hepatocytes after an 18-h incubation with PBMCs from a CHB patient with high ALT. The arrows represent ISEL+ hepatocytes. (c) Summary data of percentage of ISEL+ hepatocytes using PBMCs from high ALT patients (n = 4) versus controls (low ALT patients, n = 3; healthy donors, n = 3) without (white bars) or with (black bars) IFN-α treatment and with TRAIL blocking of IFN-α–treated wells (hatched bars). Results are presented after subtraction of the mean baseline level of hepatocyte apoptosis of 14% seen without the addition of PBMCs, and significance was tested with the Mann-Whitney U test.
DISCUSSION
The protracted, unpredictable natural history of the development of liver disease in chronic HBV infection makes it difficult to sample the immune correlates of liver damage longitudinally. Recurrent hepatic flares occurring on a background of chronic HBV overcome this problem by allowing capture of a compressed version of immunopathogenetic events associated with rapid changes in liver disease and viral load. Previous studies have examined the flares associated with eAg seroconversion and those found in patients undergoing therapy, demonstrating increases in serum IL-12 (37) and CD4 T cell reactivity (37–39). In this study, we focused initially on the distinct type of flare seen in patients with late reactivation of their disease, so called eAg-CHB. These patients usually have mutations in their basal core promoter region or stop codon resulting in loss of eAg expression in the face of high viral load and are at particularly high risk of progression to fibrosis and cirrhosis (1, 2). By repeatedly sampling a cohort of patients with eAg-CHB, we were able to identify raised and highly fluctuating levels of IL-8 and IFN-α during flares. The proportion of NK cells activated to express CD69 and the apoptosis-inducing TRAIL ligand directly ex vivo also fluctuated in parallel with the hepatic flares. A larger cross-sectional study extended the finding of elevated levels of serum IL-8, IFN-α, and NK cell TRAIL to patients with HBV infection with active liver inflammation as opposed to healthy HBV carriers or controls. TRAIL-expressing NK cells were further enriched and activated in the liver of HBV patients, contrasting with the lack of intrahepatic TRAIL expression ex vivo in healthy controls (32). Investigation of the possible mechanistic links between the induction of these cytokines and of the NK cell TRAIL pathway revealed that IL-8 is capable of up-regulating a death-inducing receptor for TRAIL, increased expression of which was observed in CHB livers. IFN-α, at concentrations circulating during flares, could promote cell death through the TRAIL pathway both by inducing ligand expression on NK cells and by reducing inhibition by a regulatory receptor on hepatocytes. Together, they render NK cells capable of killing hepatocytes through TRAIL.
NK cells are highly enriched in the liver of both healthy donors and HBV patients, comprising the dominant intrahepatic lymphocyte population, yet their role in HBV-related liver damage has not been well defined. Here, we present data supporting an important contribution of NK cells to HBV-related liver damage, showing activation of NK cells in parallel with flares of liver inflammation and enrichment of activated NK cells in the HBV-infected liver. The CD56dim subset expresses the majority of NK cell perforin and granzyme, but hepatocytes are relatively resistant to these classical cytolytic effector molecules (9, 10). The CD56bright subset of NK cells, noted to be selectively enriched in the periphery during flares and preferentially enriched and activated in the liver, is known for its immunoregulatory capacity, being a potent source of cytokines such as IFN-γ (33). In this study, we have concentrated on the potential of these CD56bright NK cells to mediate liver damage through an alternative cytotoxic pathway, using TRAIL to induce receptor-mediated hepatocyte death. TRAIL has been shown to be endogenously expressed by a subset of NK cells found in murine livers (16), but this is not the case in humans, where both peripheral and intrahepatic NK cells show minimal surface TRAIL expression in healthy individuals (30–32). However, human NK cells have been reported to be capable of up-regulating TRAIL expression upon stimulation in vitro with IL-2 (31, 32) or IFN-α (22); we demonstrate that NK cells retain the capacity to up-regulate TRAIL both in vitro and in vivo, despite the years of recurrent inflammation seen in these patients with chronic HBV infection. The fact that NK cell TRAIL is only elevated in those HBV patients manifesting liver inflammation (in both longitudinal and cross-sectional studies) supports a role for this ligand in hepatocyte damage.
The TRAIL pathway was originally proposed to be restricted to transformed cells, and NK-expressed TRAIL protects against tumors in the intrahepatic environment (16). However, recent human studies have highlighted a pathogenic role for this pathway outside the context of tumors, with lymphocytes mediating TRAIL-induced apoptosis of atherosclerotic plaques in acute coronary syndrome (40) and of CD4 T cells in HIV infection (41). Studies in mouse models of liver disease have reinforced the notion of NK-expressed TRAIL inducing damage of nonmalignant tissues in vivo, showing TRAIL-dependent death of hepatocytes (15) and hepatic stellate cells (42). The susceptibility of human hepatocytes to TRAIL-induced apoptosis has been an area of controversy after initial reports of lack of liver toxicity in mice and primates treated with soluble TRAIL (43, 44). However, membrane-bound TNF-related ligands have greater proapoptotic potential and liver toxicity than their soluble counterparts (35, 45). Human membrane-bound TRAIL does induce hepatocyte apoptosis in mice, resulting in widespread apoptosis, necrosis, and lymphocytic infiltration (35), compatible with the pathology of chronic HBV hepatitis. Furthermore, normal human hepatocytes have the potential to express death-inducing receptors for TRAIL and are susceptible to TRAIL-induced apoptosis in vitro (17, 18). The ratio of expression of death-inducing versus regulatory receptors has been shown to provide a means for fine-tuning the susceptibility to TRAIL-induced death (36). There is already a suggestion that this balance may be tipped in favor of death in situations of liver inflammation, such as bile acid retention (46) and viral hepatitis. Evidence for the latter comes from immunostaining of hepatitis C virus–infected livers (20) and Western blotting of total liver extracts from acute HBV-mediated liver failure (19). We show by immunostaining that expression of a death-inducing TRAIL receptor is up-regulated on hepatocytes of patients with CHB. One mechanism of modulation may be by the virus itself, based on the in vitro observations that the HBV-encoded X antigen up-regulates one of the death-inducing receptors (47) and predisposes to TRAIL-induced apoptosis through modulation of intracellular Bax (48). Here, we demonstrate an additional mechanism, whereby cytokines produced during an HBV flare may act in concert to both increase death-inducing and reduce regulatory TRAIL receptors to maximize hepatocyte apoptosis. Our data have implications for the use of soluble TRAIL in the therapy of malignancies, such as hepatocellular carcinoma. They suggest that tumor patients with coincident HBV infection and episodes of active liver inflammation might be more susceptible to hepatic toxicity from such a therapeutic approach.
The chemokine ligand–receptor pairs directing the migration of this large influx of NK cells in to the HBV- infected liver still need to be dissected and are currently under investigation. IL-8 is well known for its chemotactic function, and the high concentrations circulating during flares are likely to derive from the liver (unpublished data). In the patients studied here, IL-8 levels typically increased with the increase in HBV DNA, in keeping with the reported ability of HBV to transactivate the IL-8 gene (49). NK cells have been shown to express the high affinity IL-8 receptor CXCR1 and to migrate in response to IL-8 (50). IFNs have also been shown to regulate the trafficking of NK cells to the liver by induction of chemokines such as IFN-γ inducible protein 10 in HBV transgenic mice (4) and macrophage inflammatory protein 1α in murine CMV infection (51). We have not shown where the IFN-α surges identified in this study derive from, but likely sources are virally infected hepatocyes in addition to liver-infiltrating leukocytes, including plasmacytoid dendritic cells. We therefore speculate that IL-8 and IFN-α, in addition to activating a pathway of NK-mediated hepatocyte damage, may contribute to the chemotaxis of NK cells to the HBV liver during episodes of active inflammation.
In the transgenic mouse model of HBV infection, NK cells have potent antiviral efficacy, an effect that is attenuated in mice lacking the type I IFN receptor (52). It is likely that IFN-α–activated NK cells have a dual role in viral control and liver damage in human HBV infection too. TRAIL-induced apoptosis of HBV-infected hepatocytes by NK cells would eliminate some virally infected cells, a process that could contribute to the partial reduction in viral load often observed after a flare. However, any viral reduction by this means would always be at the expense of liver damage and would therefore be a hazardous strategy to promote therapeutically. In fact, the use of exogenous IFN-α in the treatment of HBV-associated cirrhosis is often limited by its tendency to cause a hepatic flare, which can be severe enough to precipitate hepatic decompensation. It will be important to investigate whether such flares on treatment have a similar pathogenesis to the naturally occurring flares investigated here; this would open up the potential to block IL-8 or the TRAIL pathway to limit the toxicity of IFN-α therapy. The recent finding that liver disease induced by the injection of activated lymphocytes into the HBV transgenic mouse can be abrogated with a TRAIL blocking antibody (48) lends support to this therapeutic strategy.
In summary, we demonstrate that patients with chronic HBV infection retain the capacity to activate the type I IFN system in the setting of dynamic changes in viral load and have fluctuating levels of IL-8 that may modulate the role of IFN-α in liver damage and viral control. We describe a novel mechanism of non-antigen–specific liver damage activated by these two cytokines in HBV infection and highlight the critical role of the large NK infiltrate in this process. CD3+ T cells express minimal levels of TRAIL in both the periphery and liver of these patients, despite the reported ability of IFN-α to up-regulate TRAIL on human T cells (53). However, it is likely that non-antigen–specific T cells contribute to liver damage through other pathways, such as Fas/Fas ligand (11, 12). The fact that NK-mediated killing of hepatocytes was not completely blocked by TRAIL antibodies suggests that NK cells may also use additional ligands to mediate liver damage. It remains to be seen whether the NK cell TRAIL pathway is also switched on during the large flare of liver inflammation seen during eAg seroconversion and in acute HBV infection. A further question not addressed by this study is what triggers, rather than mediates, these flares. The primary event appears to be an increase in HBV DNA, suggesting that there may be an escape from virus-specific CD8-mediated viral control. Our data support the idea that future immunotherapeutic strategies should aim to promote noncytolytic antiviral effects, such as those mediated by virus-specific CD8, while blocking the type of non-antigen– specific mechanism of liver damage described here.
MATERIALS AND METHODS
Patients and controls.
72 patients with chronic HBV infection (HBsAg+) were recruited with full ethics approval and informed consent, with 11 patients being HBeAg+ and the remainder HBeAg− and anti-HBeAb+ (measured by commercial enzyme immunoassay kits; Murex Diagnostics). HBV-DNA viral load was quantified by the Roche Amplicor Monitor Assay (Roche Laboratories). The patients were negative for antibodies to hepatitis C virus and hepatitis delta virus, and to HIV-1 and HIV-2 (Ortho Diagnostic System; Murex Diagnostics). None of the patients included in the study was taking antiviral therapy or immunosuppressive drugs. Sera were obtained and immediately frozen from 53 patients, PBMCs from 46 patients, and liver biopsies/explants or paraffin-embedded sections from 20 patients. A subset of 14 HBeAg− CHB patients was subjected to longitudinal analysis, with multiple serum and PBMC samples taken (Table II). Serum samples were analyzed in parallel, and PBMCs were analyzed directly ex vivo.
Control samples consisted of sera and PBMCs from 14 and 13 healthy donors, respectively, and paraffin-embedded liver sections from 4 healthy donors and 4 patients with alcoholic hepatitis.
Antibodies and reagents.
The antibodies CD3-Cy5.5/PerCP, CD56-FITC, TRAIL-PE, CD69-APC (BD Biosciences), TRAIL-R1-PE, TRAIL- R2-PE, TRAIL-R3-PE, TRAIL-R4-PE, and CD56-PE (R&D Systems) were used for flow cytometric analyses at the manufacturers' recommended concentrations. The anti-TRAIL antibody for neutralization of bioactivity (R&D Systems) was used at a concentration of 10 ng/ml. Recombinant human IFN-α2a (rhIFN-α; PBL Biomedical Laboratories) and recombinant human IL-8 (rhIL-8; R&D Systems) were used at concentrations stated for each experiment.
Determination of serum cytokine concentrations.
Serum cytokine concentrations were ascertained using the CBA Inflammation kit (BD Biosciences) according to the manufacturer's protocols. In brief, 50 μl of patient serum or standard recombinant protein dilutions was added to a mixture of capture beads coated with mAb to a panel of cytokines (IL-8, IL-1β, IL-6, IL-10, TNF, and IL-12p70) and a PE-conjugated detection reagent. After 3 h, the capture beads were washed and acquired on a FACSCalibur flow cytometer (BD Biosciences). Using the recombinant standards and the BD CBA Software provided, cytokine concentrations were quantified for each serum sample. Serum IFN-α was assayed using a standard sandwich ELISA kit (PBL Biomedical Laboratories), where 50 μl of patient serum was analyzed according to the manufacturer's High Sensitivity protocol.
Ex vivo staining of NK cells.
Freshly isolated PBMCs from HBV patients and healthy donors, or intrahepatic lymphocytes isolated from HBV patients as described previously (3), were incubated for 30 min at 4°C with antibodies to CD3, CD56, CD69, and TRAIL. PBMCs were washed twice with PBS plus 1% FCS and fixed with 1% paraformaldehyde before acquisition on a FACSCalibur flow cytometer. Isotype-matched control mAbs were used for defining positive population staining with the CD69 and TRAIL-specific mAbs.
Immunohistochemistry of liver samples for TRAIL and TRAIL receptors.
Archival paraffin blocks from 15 CHB, 4 alcoholic liver disease cases, and 4 healthy donors were stained for the expression of TRIAL-R1 and TRAIL-R2. Serial sections from seven eAg-CHB patients were stained for expression of TRAIL. 4-μm sections were cut onto charged slides (Surgipath) and heated for 1 h at 60°C. After deparaffinizing and rehydration, sections were treated in 0.3% H2O2 in water to block endogenous peroxidase activity. Antigen retrieval was performed using the ALTER technique as described previously (54). After a brief wash in water, sections were placed onto a Sequenza (Shandon) and washed in TBS/Tween, pH 7.6. mAbs to TRAIL-R1 (1:100 dilution; R&D Systems) or TRAIL-R2 (1:50 dilution; R&D Systems) were applied for 40 min at room temperature. Sections were washed in TBS/Tween, and antibody was detected using Dako Chemate Envision horseradish peroxidase kit (DakoCytomation). Sections were washed in water, counterstained in hematoxylin, dehydrated, placed into xylene, and mounted in DPX mountant.
Cytokine-induced NK cell activation and up-regulation of TRAIL expression.
PBMCs were resuspended in supplemented RPMI 10% FCS, plated into a round-bottom 96-well tissue culture plate at 3 × 105 cells/well, and incubated with 1,000 U/ml rhIFN-α, 5 ng/ml rhIL-8, or IFN-α and IL-8 for 24 h at 37°C. The degree of cytokine-induced NK activation and up-regulated TRAIL expression was determined by subtracting baseline CD69 or TRAIL expression from that observed after cytokine treatment.
Cytokine-induced changes in TRAIL-R expression on the HepG2 hepatoma cell line.
HepG2 hepatoma cells were trypsinized from a 75-cm2 flask and plated into a 48-well flat-bottom tissue culture plate at 2 × 105 cells/well. The cells were allowed to adhere for 5 h before the addition of 10 ng/ml rhIL-8 or 1,000 U/ml rhIFN-α and incubated for 24 h at 37°C. The wells were washed twice with PBS and incubated on ice for 45 min with 5 mM EDTA. This gentle detachment from the plate prevented the loss of surface TRAIL-R expression. The cells were then washed twice with PBS plus 1% FCS to remove the EDTA before incubation for 30 min at 4°C with mAbs to the four membrane-bound TRAIL receptors and acquisition on a FACSCalibur flow cytometer.
NK-expressed TRAIL-mediated apoptosis of HepG2 cell line.
HepG2 cells were trypsinized from a 75-cm3 flask, plated into a 48-well flat-bottom tissue culture plate at 105 cells/well, and allowed to adhere. Adhered cells were incubated with and without 10 ng/ml IL-8 or 1,000 U/ml IFN-α at 37°C for 24 h. PBMCs (or purified NK cells or NK-depleted PBMCs) from chronic HBV patients were also incubated with and without 1,000 U/ml IFN-α at 37°C for 24 h. After this incubation a TRAIL-blocking antibody was added to the relevant wells for 1 h before the addition of PBMCs to HepG2 wells at a ratio of 10:1 (PBMC/HepG2). After 4 h, the degree of caspase activation was determined using the carboxyfluorescein-FLICA apoptosis detection kit (Serotec) using the manufacturer's protocol for detection by flow cytometry.
NK-expressed TRAIL-mediated apoptosis of primary human hepatocytes.
Primary human hepatocytes were isolated from nondiseased liver explant tissue using collagenase perfusion (55), resuspended in Williams E medium containing hydrocortisone, insulin, and glutamine, plated into a 48-well flat-bottom culture plate at 105 cells per well, and allowed to adhere for 2 h. Medium was replaced, and cells rested for 24 h before stimulation for 24 h at 37°C with 10 ng/ml IL-8 and 1,000 U/ml IFN-α. PBMCs from CHB or healthy donors were incubated with or without 1,000 U/ml IFN-α at 37°C for 24 h. After this time, 10 ng/ml of a TRAIL blocking antibody was added to the relevant well for 2 h before PBMCs were added to hepatocytes at a ratio of 10:1 (PBMC/hepatocyte) and incubated for a further 18 h at 37°C before fixing with methanol. The degree of apoptosis was determined by in situ DNA end labeling (ISEL) for the detection of DNA fragmentation (55). In brief, the fixed cells were incubated with ISEL mixture (TBS, pH 7.6, plus 5 mM MgCl, 10 mM 2-mercaptoethanol, 5 mg/ml bovine serum albumin, 20 units Klenow DNA polymerase [Bioline], 0.01 M of nucleotides dATP, dCTP, and dGTP [Invitrogen], and digoxygenin-labeled dUTP [Roche Laboratories]) for 1 h at 37°C. The sections were then washed with distilled water and incubated with sheep anti-digoxygenin alkaline phosphatase–conjugated Fab fragment (1:200 dilution; Roche Laboratories) for 1 h at room temperature. After further washing in TBS, pH 7.6, sections were incubated with alkaline phophatase substrate for 15 min, counterstained with Mayers hematoxylin, and refixed in methanol at 4°C.
Induction of apoptosis was quantified by an independent observer blinded to the study design, who counted at least 200 hepatocytes in each well.
Online supplemental material.
Fig. S1 shows the individual values and means for serum concentrations of cytokines assayed by CBA (IL-8, IL-1β, IL-6, IL-10, TNF, and IL-12p70) and sandwich ELISA (IFN-α). Fig. S2 shows the results of proliferative assays performed at multiple longitudinal time points in three CHB patients with flares after 5 d of stimulation with HBcAg and HbsAg. Figs. S1 and S2 are available at http://www.jem.org/cgi/content/full/jem.20061287/DC1.
Acknowledgments
We thank the staff and patients at the Mortimer Market Centre, UCL; Spedali Riuniti di Santa Chiara, Pisa; and Division of Hepatology, University of Milan for blood samples. We are grateful to Carolina Boni, Carlo Ferrari, and Richard Tedder for their input into the study, and Andrew Copas for statistical advice.
This work was funded by The Edward Jenner Institute for Vaccine Research and the Medical Research Council (New Investigator Award to C. Dunn and Clinician Scientist Award to M.K. Maini).
The authors have no conflicting financial interests.
Abbreviations used: ALT, alanine transaminase; CBA, cytometric bead array; eAg-CHB, e antigen negative chronic hepatitis B; HBV, hepatitis B virus; TRAIL, TNF-related apoptosis-inducing ligand.
A. Bertoletti's present address is CMM, A*Star, Singapore 138668.
References
- 1.Bonino, F., and M.R. Brunetto. 2003. Chronic hepatitis B e antigen (HBeAg) negative, anti-HBe positive hepatitis B: an overview. J. Hepatol. 39:S160–S163. [DOI] [PubMed] [Google Scholar]
- 2.Brunetto, M.R., F. Oliveri, B. Coco, G. Leandro, P. Colombatto, J.M. Gorin, and F. Bonino. 2002. Outcome of anti-HBe positive chronic hepatitis B in alpha-interferon treated and untreated patients: a long term cohort study. J. Hepatol. 36:263–270. [DOI] [PubMed] [Google Scholar]
- 3.Maini, M.K., C. Boni, C.K. Lee, J.R. Larrubia, S. Reignat, G.S. Ogg, A.S. King, J. Herberg, R. Gilson, A. Alisa, et al. 2000. The role of virus-specific CD8+ cells in liver damage and viral control during persistent hepatitis B virus infection. J. Exp. Med. 191:1269–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kakimi, K., T.E. Lane, S. Wieland, V.C. Asensio, I.L. Campbell, F.V. Chisari, and L.G. Guidotti. 2001. Blocking chemokine responsive to γ-2/interferon (IFN)-γ inducible protein and monokine induced by IFN-γ activity in vivo reduces the pathogenetic but not the antiviral potential of hepatitis B virus–specific cytotoxic T lymphocytes. J. Exp. Med. 194:1755–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sitia, G., M. Isogawa, M. Iannacone, I.L. Campbell, F.V. Chisari, and L.G. Guidotti. 2004. MMPs are required for recruitment of antigen-nonspecific mononuclear cells into the liver by CTLs. J. Clin. Invest. 113:1158–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ando, K., T. Moriyama, L.G. Guidotti, S. Wirth, R.D. Schreiber, H.J. Schlicht, S.N. Huang, and F.V. Chisari. 1993. Mechanisms of class I restricted immunopathology. A transgenic mouse model of fulminant hepatitis. J. Exp. Med. 178:1541–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Norris, S., C. Collins, D.G. Doherty, F. Smith, G. McEntee, O. Traynor, N. Nolan, J. Hegarty, and C. O'Farrelly. 1998. Resident human hepatic lymphocytes are phenotypically different from circulating lymphocytes. J. Hepatol. 28:84–90. [DOI] [PubMed] [Google Scholar]
- 8.Webster, G.J., S. Reignat, M.K. Maini, S.A. Whalley, G.S. Ogg, A. King, D. Brown, P.L. Amlot, R. Williams, D. Vergani, et al. 2000. Incubation phase of acute hepatitis B in man: dynamic of cellular immune mechanisms. Hepatology. 32:1117–1124. [DOI] [PubMed] [Google Scholar]
- 9.Tay, C.H., and R.M. Welsh. 1997. Distinct organ-dependent mechanisms for the control of murine cytomegalovirus infection by natural killer cells. J. Virol. 71:267–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kafrouni, M.I., G.R. Brown, and D.L. Thiele. 2001. Virally infected hepatocytes are resistant to perforin-dependent CTL effector mechanisms. J. Immunol. 167:1566–1574. [DOI] [PubMed] [Google Scholar]
- 11.Galle, P.R., W.J. Hofmann, H. Walczak, H. Schaller, G. Otto, W. Stremmel, P.H. Krammer, and L. Runkel. 1995. Involvement of the CD95 (APO-1/Fas) receptor and ligand in liver damage. J. Exp. Med. 182:1223–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Balkow, S., A. Kersten, T.T. Tran, T. Stehle, P. Grosse, C. Museteanu, O. Utermohlen, H. Pircher, F. von Weizsacker, R. Wallich, et al. 2001. Concerted action of the FasL/Fas and perforin/granzyme A and B pathways is mandatory for the development of early viral hepatitis but not for recovery from viral infection. J. Virol. 75:8781–8791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wiley, S.R., K. Schooley, P.J. Smolak, W.S. Din, C.P. Huang, J.K. Nicholl, G.R. Sutherland, T.D. Smith, C. Rauch, C.A. Smith, et al. 1995. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 3:673–682. [DOI] [PubMed] [Google Scholar]
- 14.Sheridan, J.P., S.A. Marsters, R.M. Pitti, A. Gurney, M. Skubatch, D. Baldwin, L. Ramakrishnan, C.L. Gray, K. Baker, W.I. Wood, et al. 1997. Control of TRAIL-induced apoptosis by a family of signaling and decoy receptors. Science. 277:818–821. [DOI] [PubMed] [Google Scholar]
- 15.Zheng, S.J., P. Wang, G. Tsabary, and Y.H. Chen. 2004. Critical roles of TRAIL in hepatic cell death and hepatic inflammation. J. Clin. Invest. 113:58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takeda, K., Y. Hayakawa, M.J. Smyth, N. Kayagaki, N. Yamaguchi, S. Kakuta, Y. Iwakura, H. Yagita, and K. Okumura. 2001. Involvement of tumor necrosis factor-related apoptosis-inducing ligand in surveillance of tumor metastasis by liver natural killer cells. Nat. Med. 7:94–100. [DOI] [PubMed] [Google Scholar]
- 17.Jo, M., T.H. Kim, D.W. Seol, J.E. Esplen, K. Dorko, T.R. Billiar, and S.C. Strom. 2000. Apoptosis induced in normal human hepatocytes by tumor necrosis factor-related apoptosis-inducing ligand. Nat. Med. 6:564–567. [DOI] [PubMed] [Google Scholar]
- 18.Lawrence, D., Z. Shahrokh, S. Marsters, K. Achilles, D. Shih, B. Mounho, K. Hillan, K. Totpal, L. DeForge, P. Schow, et al. 2001. Differential hepatocyte toxicity of recombinant Apo2L/TRAIL versions. Nat. Med. 7:383–385. [DOI] [PubMed] [Google Scholar]
- 19.Mundt, B., F. Kuhnel, L. Zender, Y. Paul, H. Tillmann, C. Trautwein, M.P. Manns, and S. Kubicka. 2003. Involvement of TRAIL and its receptors in viral hepatitis. FASEB J. 17:94–96. [DOI] [PubMed] [Google Scholar]
- 20.Saitou, Y., K. Shiraki, H. Fuke, T. Inoue, K. Miyashita, Y. Yamanaka, Y. Yamaguchi, N. Yamamoto, K. Ito, K. Sugimoto, and T. Nakano. 2005. Involvement of tumor necrosis factor-related apoptosis-inducing ligand and tumor necrosis factor-related apoptosis-inducing ligand receptors in viral hepatic diseases. Hum. Pathol. 36:1066–1073. [DOI] [PubMed] [Google Scholar]
- 21.Nguyen, K.B., T.P. Salazar-Mather, M.Y. Dalod, J.B. Van Deusen, X.Q. Wei, F.Y. Liew, M.A. Caligiuri, J.E. Durbin, and C.A. Biron. 2002. Coordinated and distinct roles for IFN-alpha beta, IL-12, and IL-15 regulation of NK cell responses to viral infection. J. Immunol. 169:4279–4287. [DOI] [PubMed] [Google Scholar]
- 22.Sato, K., S. Hida, H. Takayanagi, T. Yokochi, N. Kayagaki, K. Takeda, H. Yagita, K. Okumura, N. Tanaka, T. Taniguchi, and K. Ogasawara. 2001. Antiviral response by natural killer cells through TRAIL gene induction by IFN-alpha/beta. Eur. J. Immunol. 31:3138–3146. [DOI] [PubMed] [Google Scholar]
- 23.Marrack, P., J. Kappler, and T. Mitchell. 1999. Type I interferons keep activated T cells alive. J. Exp. Med. 189:521–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dondi, E., L. Rogge, G. Lutfalla, G. Uze, and S. Pellegrini. 2003. Down-modulation of responses to type I IFN upon T cell activation. J. Immunol. 170:749–756. [DOI] [PubMed] [Google Scholar]
- 25.Tian, Z., X. Shen, H. Feng, and B. Gao. 2000. IL-1 beta attenuates IFN-alpha beta-induced antiviral activity and STAT1 activation in the liver: involvement of proteasome-dependent pathway. J. Immunol. 165:3959–3965. [DOI] [PubMed] [Google Scholar]
- 26.Khabar, K.S., F. Al-Zoghaibi, M.N. Al-Ahdal, T. Murayama, M. Dhalla, N. Mukaida, M. Taha, S.T. Al-Sedairy, Y. Siddiqui, G. Kessie, and K. Matsushima. 1997. The α chemokine, interleukin 8, inhibits the antiviral action of interferon α. J. Exp. Med. 186:1077–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Polyak, S.J., K.S. Khabar, M. Rezeiq, and D.R. Gretch. 2001. Elevated levels of interleukin-8 in serum are associated with hepatitis C virus infection and resistance to interferon therapy. J. Virol. 75:6209–6211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guidotti, L.G., R. Rochford, J. Chung, M. Shapiro, R. Purcell, and F.V. Chisari. 1999. Viral clearance without destruction of infected cells during acute HBV infection. Science. 284:825–829. [DOI] [PubMed] [Google Scholar]
- 29.Myers, R.P., M.H. Tainturier, V. Ratziu, A. Piton, V. Thibault, F. Imbert-Bismut, D. Messous, F. Charlotte, V. Di Martino, Y. Benhamou, and T. Poynard. 2003. Prediction of liver histological lesions with biochemical markers in patients with chronic hepatitis B. J. Hepatol. 39:222–230. [DOI] [PubMed] [Google Scholar]
- 30.Kashii, Y., R. Giorda, R.B. Herberman, T.L. Whiteside, and N.L. Vujanovic. 1999. Constitutive expression and role of the TNF family ligands in apoptotic killing of tumor cells by human NK cells. J. Immunol. 163:5358–5366. [PubMed] [Google Scholar]
- 31.Mirandola, P., C. Ponti, G. Gobbi, I. Sponzilli, M. Vaccarezza, L. Cocco, G. Zauli, P. Secchiero, F.A. Manzoli, and M. Vitale. 2004. Activated human NK and CD8+ T cells express both TNF-related apoptosis-inducing ligand (TRAIL) and TRAIL receptors but are resistant to TRAIL-mediated cytotoxicity. Blood. 104:2418–2424. [DOI] [PubMed] [Google Scholar]
- 32.Ishiyama, K., H. Ohdan, M. Ohira, H. Mitsuta, K. Arihiro, and T. Asahara. 2006. Difference in cytotoxicity against hepatocellular carcinoma between liver and periphery natural killer cells in humans. Hepatology. 43:362–372. [DOI] [PubMed] [Google Scholar]
- 33.Cooper, M.A., T.A. Fehniger, and M.A. Caligiuri. 2001. The biology of human natural killer-cell subsets. Trends Immunol. 22:633–640. [DOI] [PubMed] [Google Scholar]
- 34.Sprengers, D., R.G. van der Molen, J.G. Kusters, B. Hansen, H.G. Niesters, S.W. Schalm, and H.L. Janssen. 2006. Different composition of intrahepatic lymphocytes in the immune-tolerance and immune-clearance phase of chronic hepatitis B. J. Med. Virol. 78:561–568. [DOI] [PubMed] [Google Scholar]
- 35.Ichikawa, K., W. Liu, L. Zhao, Z. Wang, D. Liu, T. Ohtsuka, H. Zhang, J.D. Mountz, W.J. Koopman, R.P. Kimberly, and T. Zhou. 2001. Tumoricidal activity of a novel anti-human DR5 monoclonal antibody without hepatocyte cytotoxicity. Nat. Med. 7:954–960. [DOI] [PubMed] [Google Scholar]
- 36.Clancy, L., K. Mruk, K. Archer, M. Woelfel, J. Mongkolsapaya, G. Screaton, M.J. Lenardo, and F.K. Chan. 2005. Preligand assembly domain-mediated ligand-independent association between TRAIL receptor 4 (TR4) and TR2 regulates TRAIL-induced apoptosis. Proc. Natl. Acad. Sci. USA. 102:18099–18104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rossol, S., G. Marinos, P. Carucci, M.V. Singer, R. Williams, and N.V. Naoumov. 1997. Interleukin-12 induction of Th1 cytokines is important for viral clearance in chronic hepatitis B. J. Clin. Invest. 99:3025–3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsai, S.L., P.J. Chen, M.Y. Lai, P.M. Yang, J.L. Sung, J.H. Huang, L.H. Hwang, T.H. Chang, and D.S. Chen. 1992. Acute exacerbations of chronic type B hepatitis are accompanied by increased T cell responses to hepatitis B core and e antigens. Implications for hepatitis B e antigen seroconversion. J. Clin. Invest. 89:87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lohr, H.F., W. Weber, J. Schlaak, B. Goergen, K.H. Meyer zum Buschenfelde, and G. Gerken. 1995. Proliferative response of CD4+ T cells and hepatitis B virus clearance in chronic hepatitis with or without hepatitis B e-minus hepatitis B virus mutants. Hepatology. 22:61–68. [DOI] [PubMed] [Google Scholar]
- 40.Sato, K., A. Niessner, S.L. Kopecky, R.L. Frye, J.J. Goronzy, and C.M. Weyand. 2006. TRAIL-expressing T cells induce apoptosis of vascular smooth muscle cells in the atherosclerotic plaque. J. Exp. Med. 203:239–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Herbeuval, J.P., J.C. Grivel, A. Boasso, A.W. Hardy, C. Chougnet, M.J. Dolan, H. Yagita, J.D. Lifson, and G.M. Shearer. 2005. CD4+ T-cell death induced by infectious and noninfectious HIV-1: role of type 1 interferon-dependent, TRAIL/DR5-mediated apoptosis. Blood. 106:3524–3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Radaeva, S., R. Sun, B. Jaruga, V.T. Nguyen, Z. Tian, and B. Gao. 2006. Natural killer cells ameliorate liver fibrosis by killing activated stellate cells in NKG2D-dependent and tumor necrosis factor-related apoptosis-inducing ligand-dependent manners. Gastroenterology. 130:435–452. [DOI] [PubMed] [Google Scholar]
- 43.Ashkenazi, A., R.C. Pai, S. Fong, S. Leung, D.A. Lawrence, S.A. Marsters, C. Blackie, L. Chang, A.E. McMurtrey, A. Hebert, et al. 1999. Safety and antitumor activity of recombinant soluble Apo2 ligand. J. Clin. Invest. 104:155–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Walczak, H., R.E. Miller, K. Ariail, B. Gliniak, T.S. Griffith, M. Kubin, W. Chin, J. Jones, A. Woodward, T. Le, et al. 1999. Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nat. Med. 5:157–163. [DOI] [PubMed] [Google Scholar]
- 45.Schneider, P., N. Holler, J.L. Bodmer, M. Hahne, K. Frei, A. Fontana, and J. Tschopp. 1998. Conversion of membrane-bound Fas(CD95) ligand to its soluble form is associated with downregulation of its proapoptotic activity and loss of liver toxicity. J. Exp. Med. 187:1205–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Higuchi, H., S.F. Bronk, Y. Takikawa, N. Werneburg, R. Takimoto, W. El-Deiry, and G.J. Gores. 2001. The bile acid glycochenodeoxycholate induces trail-receptor 2/DR5 expression and apoptosis. J. Biol. Chem. 276:38610–38618. [DOI] [PubMed] [Google Scholar]
- 47.Janssen, H.L., H. Higuchi, A. Abdulkarim, and G.J. Gores. 2003. Hepatitis B virus enhances tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) cytotoxicity by increasing TRAIL-R1/death receptor 4 expression. J. Hepatol. 39:414–420. [DOI] [PubMed] [Google Scholar]
- 48.Liang, X., Y. Liu, Q. Zhang, L. Gao, L. Han, C. Ma, L. Zhang, Y.H. Chen, and W. Sun. 2007. Hepatitis B virus sensitizes hepatocytes to TRAIL-induced apoptosis through Bax. J. Immunol. 178:503–510. [DOI] [PubMed] [Google Scholar]
- 49.Mahe, Y., N. Mukaida, K. Kuno, M. Akiyama, N. Ikeda, K. Matsushima, and S. Murakami. 1991. Hepatitis B virus X protein transactivates human interleukin-8 gene through acting on nuclear factor kB and CCAAT/enhancer-binding protein-like cis-elements. J. Biol. Chem. 266:13759–13763. [PubMed] [Google Scholar]
- 50.Campbell, J.J., S. Qin, D. Unutmaz, D. Soler, K.E. Murphy, M.R. Hodge, L. Wu, and E.C. Butcher. 2001. Unique subpopulations of CD56+ NK and NK-T peripheral blood lymphocytes identified by chemokine receptor expression repertoire. J. Immunol. 166:6477–6482. [DOI] [PubMed] [Google Scholar]
- 51.Salazar-Mather, T.P., C.A. Lewis, and C.A. Biron. 2002. Type I interferons regulate inflammatory cell trafficking and macrophage inflammatory protein 1alpha delivery to the liver. J. Clin. Invest. 110:321–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kakimi, K., L.G. Guidotti, Y. Koezuka, and F.V. Chisari. 2000. Natural killer T cell activation inhibits hepatitis B virus replication in vivo. J. Exp. Med. 192:921–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kayagaki, N., N. Yamaguchi, M. Nakayama, H. Eto, K. Okumura, and H. Yagita. 1999. Type I interferons (IFNs) regulate tumor necrosis factor–related apoptosis-inducing ligand (TRAIL) expression on human T cells: a novel mechanism for the antitumor effects of type I IFNs. J. Exp. Med. 189:1451–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reynolds, G.M., L.J. Billingham, L.J. Gray, J.R. Flavell, S. Najafipour, J. Crocker, P. Nelson, L.S. Young, and P.G. Murray. 2002. Interleukin 6 expression by Hodgkin/Reed-Sternberg cells is associated with the presence of ‘B’ symptoms and failure to achieve complete remission in patients with advanced Hodgkin's disease. Br. J. Haematol. 118:195–201. [DOI] [PubMed] [Google Scholar]
- 55.Afford, S.C., S. Randhawa, A.G. Eliopoulos, S.G. Hubscher, L.S. Young, and D.H. Adams. 1999. CD40 activation induces apoptosis in cultured human hepatocytes via induction of cell surface fas ligand expression and amplifies fas-mediated hepatocyte death during allograft rejection. J. Exp. Med. 189:441–446. [DOI] [PMC free article] [PubMed] [Google Scholar]