Abstract
Ultraviolet (UV) light is absorbed by cellular proteins and DNA, promoting skin damage, aging and cancer. In this paper, we explore the UV response by cells of the Drosophila retina. We demonstrate that the retina enters a period of heightened UV sensitivity in the young developing pupa, a stage closely associated with its period of normal developmental programmed cell death. Injury to irradiated cells included morphology changes and apoptotic cell death; these defects could be completely accounted for by DNA damage. Cell death, but not morphological changes, was blocked by the caspase inhibitor P35. Utilizing genetic and microarray data, we provide evidence for the central role of Hid expression and for Diap1 protein stability in controlling the UV response. In contrast, we found that Reaper had no effect on UV sensitivity. Surprisingly, Dmp53 is required to protect cells from UV-mediated cell death, an effect attributed to its role in DNA repair. These in vivo results demonstrate that the cellular effects of DNA damage depend on the developmental status of the tissue.
Keywords: apoptosis/Drosophila/Dmp53/retina/UV
Introduction
Apoptosis is a critical developmental and homeostatic process. Misregulation of apoptosis is thought to be involved in numerous human diseases, including a number of cancers and neurodegenerative disorders. The most common cancer type in the United States, skin cancer, has been linked to exposure to ultraviolet (UV) light.
UV irradiation can lead to a number of cellular changes, including cell cycle arrest, cell death and oncogenic growth; UV light can provoke premature aging and skin cancer (Trautinger, 2001; Cleaver and Crowley, 2002). Evidence over the past decade has indicated that transformed cell lines and tissue respond through complex signaling when UV is absorbed by either DNA or cellular proteins (Norbury and Hickson, 2001; Kulms and Schwarz, 2002b). UV irradiation can be absorbed by a multitude of potential targets within a cell. One primary target is DNA; absorption results in damage that is mediated primarily by the creation of pyrimidine dimers (e.g. thymine dimers). The inability to repair these dimers efficiently and accurately can lead to cell death or abnormal proliferation.
UV-damaged DNA can be repaired by a number of mechanisms, including nucleotide excision repair (Friedberg, 2001) and photoreactivation (Carell et al., 2001). In the process of nucleotide excision repair, pyrimidine dimers are excised and replaced with undamaged nucleotides. The disorder xeroderma pigmentosum is linked to at least seven genetic loci that encode factors that participate in nucleotide excision repair (e.g. the nucleases XPF and XPG); patients exhibit hypersensitivity to UV light and a strong predisposition toward skin cancer. An alternate repair mechanism is photoreactivation. Many vertebrates and invertebrates use this system to repair pyrimidine dimers. It includes a light-dependent photolyase repair enzyme that binds to pyrimidine dimers; the dimer is then enzymatically restored to a monomeric form using 350–450 nm light as an energy source.
Several lines of evidence suggest, however, that the damage provoked by UV irradiation is mediated by more than its ability to alter DNA. Activation of a number of signaling pathways, including JNK, EGFR and TNF, can occur in a manner independent of either prior nuclear signaling or effects on DNA (e.g. Kulms et al., 1999; Kulms and Schwarz, 2002a). This broad spectrum has led to the suggestion that most receptors that are activated by oligomerization can be affected by UV (Rosette and Karin, 1996). In some cell lines, effects on cellular proteins are thought to represent the principal UV-mediated insult.
Once DNA is damaged, the tumor suppressor P53 mediates a cell’s response by regulating expression of a number of targets including signal transduction factors, cell cycle regulators, cell repair genes and cell death regulators (Vousden and Lu, 2002). P53 also binds to specific DNA sites and damaged, single-strand DNA (Liu and Kulesz-Martin, 2001). UV irradiation leads to stabilization of the P53 protein, in part due to its phosphorylation by ERK and P38 kinases (She et al., 2000; Chouinard et al., 2002). The kinases ATR and ATM have also been implicated in signaling, and perhaps even sensing DNA damage, leading to their subsequent targeting of P53 (Lakin et al., 1999; Tibbetts et al., 1999). The result is a dual role for P53: it can direct cell cycle arrest to permit DNA repair or promote cell death when this repair fails.
The Drosophila P53 ortholog Dmp53 also acts in the cellular response to DNA damage. Following ionizing radiation, Dmp53 targets expression of the pro-apoptotic effector Reaper (Brodsky et al., 2000; Jin et al., 2000; Ollmann et al., 2000; Sogame et al., 2003). Consistent with this connection, removing Reaper in the larval wing disk results in a reduction of DNA damage-induced programmed cell death (PCD; Peterson et al., 2002). Overexpression of Dmp53 in the retina can lead to extensive cell death (Jin et al., 2000; Ollmann et al., 2000). These observations have led to the suggestion that Dmp53 is promoting inappropriate Reaper expression, although genetic tests did not confirm this association (Peterson et al., 2002). Reaper belongs to the family of RHG proteins that includes Hid, Grim, and Sickle; these proteins are critical during embryonic PCD (Grether et al., 1995). The role of Grim and Hid during radiation-mediated apoptosis has not been examined. Each of these family members is active in specific tissues and responds to specific death stimuli. For example, Reaper is active during embryonic segmentation and larval CNS development (Lohmann et al., 2002; Peterson et al., 2002), whereas Hid appears necessary for PCD within the pupal retina (Yu et al., 2002).
RHG proteins direct apoptosis at least in part by targeting Diap1 (Drosophila inhibitor of apoptosis protein-1) for degradation. Diap1 normally inhibits caspase activity by direct binding, and removal of Diap1 leads to caspase activation and subsequent apoptosis. In Drosophila, regulation of Diap1 stability appears to be the primary step in the regulation of apoptosis (Martin, 2002). Its role in radiation-induced cell death, however, has yet to be explored.
In this report, we exploit the developing Drosophila retina as a model system to explore the factors that provoke UV and DNA damage response within an emerging epithelium. We utilize several advantages offered by the pupal retina as an in vivo model for UV irradiation: it is a simply constructed neuroepithelium, constituent cells are post-mitotic, the tissue is superficial and is therefore accessible (and highly sensitive) to UV irradiation, and the molecular aspects of its development have been studied extensively. We present a number of interesting features and factors associated with the retina’s response to UV, and find parallels between this response and the factors that direct normal PCD during its development.
Results
UV irradiation leads to retinal defects
The Drosophila retina is composed of ∼750 precisely patterned unit eyes, or ‘ommatidia’. By 24 h after puparium formation (APF) at 25°C, essentially all cells in the retina are post-mitotic; ommatidia are fully assembled as a composite of eight photoreceptor neurons, four glial-like ‘cone cells’ and two ‘primary pigment cells’. Between 23 and 32 h APF, the remaining ‘interommatidial cells’ are patterned by cell rearrangements and cell death; the result is an hexagonal lattice that organizes ommatidia into rows (Figure 1A and K). During this process, more than one-third of the interommatidial cells will be removed by PCD (Cagan and Ready, 1989a; Wolff and Ready, 1991). This process represents the major stage of cell death in the retina, and the factors that regulate this process typically act within this 23–32 h developmental window (e.g. Cagan and Ready, 1989b; Freeman, 1996).
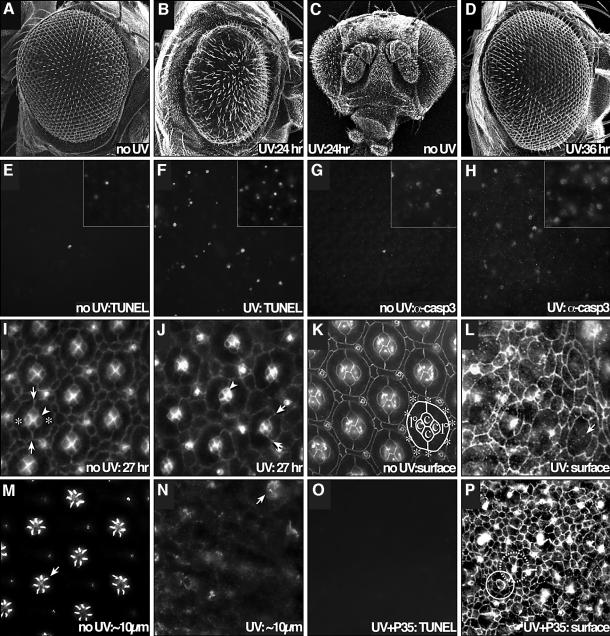
Fig. 1. UV-irradiation-directed apoptosis and cell morphology changes. (A–D) UV irradiation (40 000 µJ/cm2) led to gross alteration of retinal morphology when applied during early pupal development. (A) A non-irradiated adult retina. (B) UV irradiation at 24 h APF resulted in disturbance of the ommatidial array and overall reduction in size of the adult retina. (C) A frontal view of (B); the pupa’s right retina was irradiated. Note that only the irradiated retina was affected. (D) Irradiation later in development (36 h APF) caused significantly less damage, indicating that sensitivity of the retina is stage-dependent. (E–N) UV irradiation at 24 h APF led to an induction of caspase activation, cell death and morphological changes. (E) TUNEL staining of the central portion of a 42 h APF, non-irradiated retina detected only an occasional dying cell. Very little PCD is observed at 34 h APF (inset). (F) A 42 h APF irradiated retina showed a strong increase in TUNEL staining. The inset demonstrates an increase in TUNEL staining at 10 h after irradiation (34 h APF). (G) Similarly, active caspase was not detected in the body of the non-irradiated retina at 42 h APF, but was present in the irradiated retina (H) at the same stage (insets at 34 h APF). (I) At 27 h APF, a group of four cone cells occupy a clear niche at the center of each ommatidium (arrowhead). Arrows indicate the contacts between two enwrapping primary pigment cells (asterisks). (J) Just 3 h after irradiation (27 h APF), the apical profile of the cone cells has shrunk considerably (arrowhead) and the symmetric enwrapment of the two primary pigment cells has broken down (arrows indicate cell boundaries). (K) An apical surface view (anti-armadillo) of a 42 h APF retina showing the final, precise ommatidial array. Cone cells (c) and 1°s (1°) from one ommatidium are labeled; surrounding interommatidial 2°/3° cells are labeled with asterisks. Photoreceptor neurons are several microns below the surface and are not visible in this apical view. (L) Apical view (anti-armadillo) of a 42 h APF retina that was irradiated at 24 h APF. The overall surface morphology of the cells is relaxed and rounded. The cone cells within each ommatidium are particularly sensitive: many are not apparent at the surface, as they have released and dropped below the surface. Arrow indicates a region missing cone cells. (M) Approximately 10 µm below the surface of (K); construction of the membrane-rich rhabdomere structures has begun; an arrow indicates an example. (N) We found little evidence of rhabdomere formation at lower views of the irradiated retina. Arrow indicates a remaining, but severely disrupted, rhabdomere. (O–P) The baculovirus caspase inhibitor P35 blocked UV-induced cell death, but did not inhibit the cell morphology changes. (O) GMR-p35 blocked most UV-induced induced cell death, as measured by TUNEL at 42 h APF (compare with F). (P) Reducing caspase activity with P35 had little effect on the cell shape changes, which occurred unabated; cell morphologies are still simplified and cone cells have disappeared from the surface. The remaining damaged cells likely contribute to the overall disorder of the patterned array. An ommatidium with a single surface cone cell is indicated with a solid circle. Many of the brightly stained cells are bristle organules; one is highlighted with a dashed circle.
We have previously reported (Brachmann et al., 2000) that removal of the pupal case permits UV access to the developing retina. UV treatment of the retina 24 h APF resulted in dosage-dependent damage to the retina (Figure 1B and C; see Supplementary data for dosage response, available at The EMBO Journal Online). A dose of 40 000 µJ/cm2 was chosen for the assay as it resulted in a moderate roughening and ablation of the retina; ∼10 000 µJ/cm2 resulted in minimal defects and ∼100 000 µJ/cm2 resulted in near complete retinal ablation and eventual pupal death. The effect of radiation waned after 25 h APF (Figure 1D; see Supplementary data). By 42 h APF—the stage by which most developmental cell death is complete—the retina no longer responded to moderate UV treatment. We were unable to assess the sensitivity of the retina prior to 18 h APF as at that point the retina has yet to emerge from deeper within the developing pupa. The period of significant UV sensitivity (<25 h APF) corresponds to the early stages of cell death in the pupal retina (Cagan and Ready, 1989a; Wolff and Ready, 1991), suggesting that the signals modulating the induction of developmental cell death may regulate UV-induced cell death as well.
Some of the phenotypes observed with UV were due to induction of apoptotic cell death: we observed condensed nuclei and fragmentation of DNA as assessed by TUNEL (Figure 1F). In addition, irradiation led to activation of caspases as assessed by antibodies that target the cleaved downstream caspases caspase-3 (Figure 1H) and Drice (data not shown).
Interestingly, not all of the observed retinal defects were due to cell death. The first observed effects of UV were morphological changes in the retinal cells. For example, cone cells typically released from the epithelial surface and primary pigment cells expanded to capture their niche (Figure 1J). These morphological changes continued until, 24 h after irradiation, the ommatidial array was completely disrupted with all cell types affected (Figure 1L). In the normal retina, subcellular cytoarchitecture (e.g. rhabdomeres; Figure 1M) is readily observed in the photoreceptor neurons at later stages; these structures were disrupted or missing in irradiated neurons (Figure 1N). These morphological defects were not simply an early manifestation of apoptotic death. Most cell death was successfully blocked by expressing the caspase inhibitor P35 (Figure 1O); nevertheless, we observed no abatement of the cell morphology defects (Figure 1P). This suggests that caspase activity is not central for promoting cell shape changes upon UV irradiation although, significantly, P35 does not inhibit the apical caspase Dronc (Yoo et al., 2002).
UV phenotypes depend on DNA damage
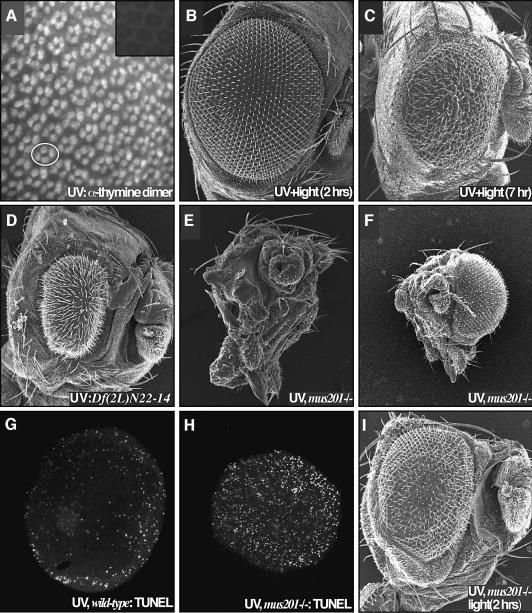
The predominant DNA lesions induced by UV irradiation are pyrimidine dimers, which are closely associated with skin cancer (Mukhtar and Elmets, 1996). In the pupal retina, UV irradiation did in fact lead to rapid induction of pyrimidine dimers (Figure 2A). Previous studies have indicated that UV irradiation can also alter cell signaling pathways through protein cross-linking (e.g. Rosette and Karin, 1996). To determine the extent by which DNA damage alone can account for UV-mediated defects, we utilized light to activate the photoreactivation repair system. Activation of photolyase in the retina resulted in complete rescue of UV-mediated damage (Figure 2B): the size and morphology of the retina was fully restored, and apoptosis was blocked as assessed by TUNEL staining (data not shown). This restoration was time-dependent: retinae were fully rescued if exposed to light within 2 h following UV irradiation. Light exposure after this time was progressively less effective (Figure 2C), indicating that cells passed a ‘commitment point’ 2 h after irradiation. Finally, membrane-bound proteins have been proposed to be affected by UV based on experiments in which their membrane movements were slowed by low temperature (Kulms et al., 1999). Consistent with the view that direct activation of proteins and protein signaling were not a primary cause of retinal damage, chilling irradiated pupae to 4°C for 90 min (beginning 30 min prior to irradiation) had no observed effect on UV-mediated damage (data not shown). We conclude from these experiments that DNA damage is the major and perhaps the sole inducer of UV-mediated cell death and morphology aberrations.
Fig. 2. Retinal defects following UV irradiation are due to DNA damage. (A) Irradiation of 24 h APF retinae resulted in a rapid induction of pyrimidine dimers. Each four-cell group is the cone cell nuclei of one ommatidium; an example is circled. The inset presents a non-irradiated control. (B) Exposure of the retina to light 2 h after irradiation resulted in a complete suppression of the UV retinal phenotype due to photoreactivation- mediated repair of the pyrimidine dimers. (C) Photoreactivating light failed to fully rescue the retina when applied 7 h after irradiation. (D) A deficiency removing the 29–30C region of the second chromosome strongly enhanced the UV phenotype (compare with Figure 1B). This region is predicted to contain xpg/mus201, along with a number of other loci. (E) Removing both functional copies of the nucleotide excision repair gene xpg/mus201, using the mus201D1 allele, resulted in a complete ablation of the retina following irradiation. The dying pupa was removed from its pupal case; few structures are identifiable as the entire retina and much of the head is affected. (F) A frontal view of this animal reveals the extent of the damage. The non-irradiated retina showed no defects. A comparison of TUNEL staining in wild type (G) and the xpg/mus201D1 mutant (H) revealed that the xpg/mus201 phenotype was due to a dramatic increase in apoptotic cell death (42 h APF). (I) Exposure of the xpg/mus201D1 retina to light following irradiation resulted in suppression of the lethality as well as retinal and head defects.
We also obtained genetic evidence for the role of DNA damage in the retina’s response to UV. During the course of this study, we irradiated mutant flies containing single copies of deficiencies or P-element insertions in order to identify dominant genetic modifiers of the UV phenotype (see Supplementary data for a complete list of modifying loci). One particularly strong, dominant enhancing locus (Figure 2D) is predicted to contain the nucleotide excision repair gene xpg/mus201, encoding a Drosophila member of the XPG family of repair proteins (Houle and Friedberg, 1999; Sekelsky et al., 2000). Irradiation of flies homozygous for the xpg/mus201 allele mus201D1 resulted in complete ablation of the retina upon irradiation (Figure 2E and F) due to a significant increase in apoptotic cell death (Figure 2H). Similar effects were observed by removing both genetic copies of the xpf ortholog mei-9 (data not shown). Photolyase treatment of both xpg/mus201 and xpf/mei-9 mutants rescued the lethality associated with the UV and partially rescued the retinal phenotype (Figure 2I, data not shown). These results provide further evidence that DNA damage is central to the UV phenotypes observed in the retina, and indicate that this damage is normally repaired through nucleotide excision repair. Failure to repair this damage leads to widespread apoptosis and ablation of the eye.
Regulators of caspases during early steps of UV damage
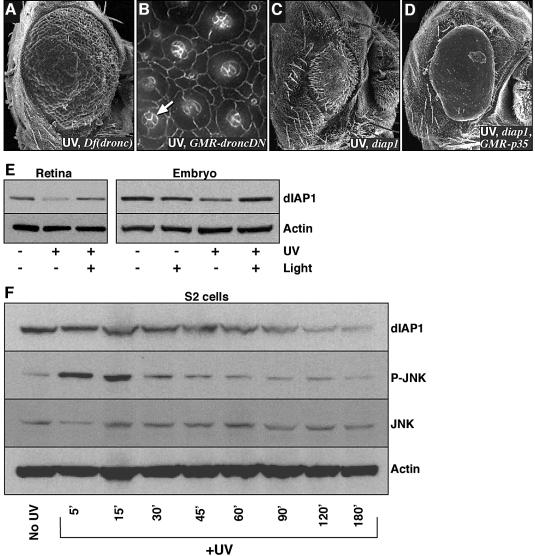
Our genetic screens also identified members of the basal cell death machinery as well as potential cell death regulators. Removing a single functional copy of the dronc locus, which encodes an upstream caspase, resulted in significant suppression of UV-induced cell death and morphological aberrations (Figure 3A). Irradiation of flies overexpressing a dominant-negative form of Dronc (Hawkins et al., 2000) led to a mild but consistent suppression of cone cell loss and morphology changes (compare Figure 3B with Figure 1L and P). These results further indicate that UV-irradiated cells undergo a caspase-dependent apoptotic death, and supports the view that Dronc may also affect cell morphology (Yu et al., 2002).
Fig. 3. The effects of UV irradiation depended on Diap1 degradation. (A–D) The UV retinal phenotype was modified by genetic alterations of the basal death machinery. (A) Removal of a single functional copy of the Drosophila upstream caspase Dronc, by utilizing the overlapping deficiency Df(3L)AC1, resulted in a significant suppression of the UV phenotype (compare with Figure 1B). (B) Overexpression of a dominant-negative form of Dronc (GMR-DroncDN) in the retina led to a suppression of cone cell changes. Note the four cone cells (arrow) that are rarely seen in irradiated wild-type retinae. (C) Removal of a functional copy of diap1, using the th7 mutation, resulted in a near complete ablation of the retina following irradiation. This phenotype was partially suppressed by the introduction of GMR-p35 (D). (E and F) UV irradiation-induced DNA damage led to a reduction of dIAP1 protein levels. (E) UV irradiation of 24 h APF pupal retinae or stage 11 embryos (40 000 µJ/cm2) resulted in a reduction of endogenous Diap1 levels that was rescued by the use of photoreactivating light. (F) Endogenous Diap1 levels in S2 cells responded in a similar fashion. In addition, JNK phosphorylation rapidly increased following UV irradiation; it then rapidly decreased, presumably as a result of negative feedback inhibition. Total levels of JNK protein were unaffected.
The major inhibitor of caspase activity in the Drosophila retina is Diap1/Thread (Hay et al., 1995). Removing a single copy of diap1 led to a dramatic enhancement of UV-mediated damage (Figure 3C). This enhancement was suppressed, in turn, by the caspase inhibitor P35 (Figure 3D). This data is reminiscent of a number of studies indicating that Diap1 protein levels must be reduced in order for cells to undergo normal PCD during development (Hay et al., 1995; Wang et al., 1999; Goyal et al., 2000; Lisi et al., 2000; Hays et al., 2002). In fact, UV irradiation of pupal retinae led to a reduction of Diap1 protein levels (Figure 3E); we observed a similar reduction when irradiating embryos or tissue culture cells (Figure 3E and F; see also Yoo et al., 2002). Diap1 levels dropped significantly within 3–5 h, coinciding with the time course of cell morphology changes and the ‘commitment point’ identified earlier through the photolyase experiments. Importantly, this loss of Diap1 protein was rescued by light in both the retina and the embryo (Figure 3E). Overexpression of Diap1 fused to a strong heterologous promoter (GMR-Diap1) had little effect on cell death or the retinal phenotype in UV-irradiated retinae; we found that despite high levels of Diap1 transcription from this promoter, Diap1 protein levels were still dramatically reduced upon irradiation (data not shown).
Together, these results indicate that DNA damage triggers a reduction of Diap1 levels, which in turn activates apoptosis. Reversal of this damage by the photoreactivation process either prevents this degradation or permits Diap1 levels to be restored. What factors connect DNA damage to the regulation of Diap1 protein levels?
Microarray analysis detects changes in hid but not reaper
The cell death effector reaper has been reported to be a major radiation-response gene in the embryo and in the larval retina (Nordstrom et al., 1996), and appears to be a target of Dmp53 (Brodsky et al., 2000; Ollmann et al., 2000). Reaper, in turn, can bind directly to Diap1 and target it for degradation as part of the cell death response (Goyal et al., 2000; Hays et al., 2002; Holley et al., 2002; Ryoo et al., 2002; Wilson et al., 2002; Wing et al., 2002; Yoo et al., 2002). Using two overlapping deficiencies (Peterson et al., 2002), however, we found that removing both functional copies of reaper resulted in no observable reduction in UV-mediated damage within the retina (data not shown). Taken together with our biochemical data, this suggests that an effector other than Reaper is regulating Diap1 during the retina’s response to UV.
To broaden our survey, we used microarray analysis to detect changes in gene expression upon UV irradiation. Pupae were irradiated at 24 h APF, aged 90 min, and retinae were removed and processed for microarray analysis by standard protocol (see Materials and methods). Non-irradiated pupae served as controls. This protocol permitted us to focus on early mediators of the UV response; a complete list of regulated transcripts is included in the Supplementary data.
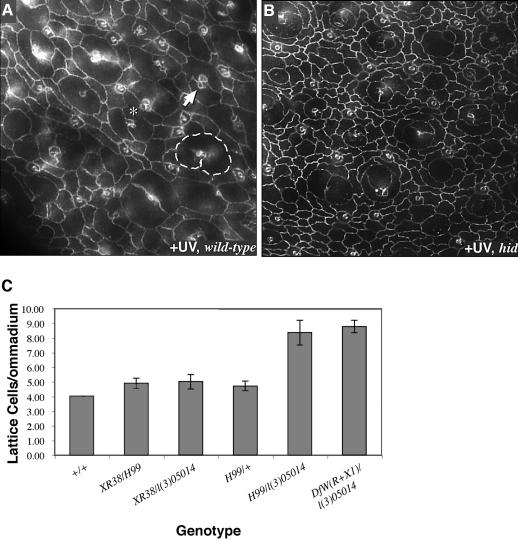
During normal development, the retina utilizes Hid, but not Reaper, as a central regulator of PCD (Figure 4C) (Kurada and White, 1998; Peterson et al., 2002). Our microarray analysis suggested that this is also true of UV-mediated cell death: within 1.5 h of UV irradiation hid expression was upregulated an average of 2.6-fold, whereas no change in reaper, grim or sickle expression was detected. Consistent with this result, irradiation of hid-deficient pupae resulted in no detectable cell death of interommatidial lattice cells (Figure 4B); these are the only cell types that normally undergo PCD in an unirradiated pupa. Remarkably, histology and TUNEL staining indicated that irradiated cells did die within the ‘ommatidial cores’: primary pigment cells, cone cells and photoreceptors all degraded, even in the absence of hid activity. These results establish an interesting parallel between development and UV response in the retina: in both instances, Hid is active specifically and exclusively within interommatidial lattice cells. The identity of the upstream factors that specifically mediate cell death cells in the ommatidial cores remains unknown. Given our inability to demonstrate a role for Reaper during the retina’s response to UV, we next asked whether Dmp53 itself has a role.
Fig. 4. Hid is responsible for lattice cell death following UV irradiation. (A and B) Lattice cell loss depended on the cell death effector, Hid. (A) Irradiation of wild-type retinae resulted in loss of cone cells (arrow points to example with only three cone cells), primary pigment cells (single primary outlined where two cells should have been), lattice cells (asterisk indicates two ommatidia in direct contact with no intervening lattice cell) and photoreceptors (not visualized in this plane) (compare with the non-irradiated retina in Figure 1K). (B) Irradiation of hid mutant retinae did not affect lattice cells. Cone cells, primary pigment cells and photoreceptors were still affected; for example, few cone cells were still present at the surface. (C) Lattice cell counts of 42 h APF pupae indicated that Hid, but not Reaper, regulates normal developmental cell death. Df(XR38) removes reaper but not hid, Df(H99) removes reaper and hid, l(3)05014 is a P-element disrupting hid, and Df(WR+X1) removes hid.
Dmp53 protects the retina from UV
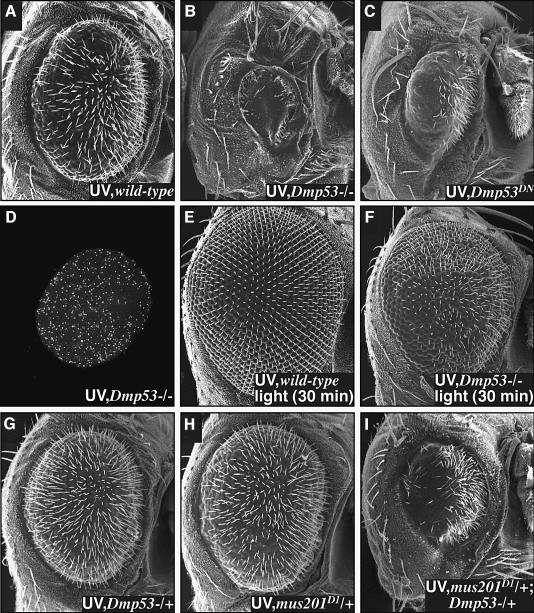
Previous work demonstrated that dominant-negative versions of Dmp53 suppressed ionizing radiation-induced cell death in larvae, and Dmp53 is able to bind to an upstream P53 consensus binding site within the reaper locus (Brodsky et al., 2000; Ollmann et al., 2000). To our surprise, flies lacking both functional copies of dmp53, due to an introduced stop codon (Rong et al., 2002), consistently displayed an enhanced retinal sensitivity to UV light and an increase in apoptotic cell death (Figure 5B and D). We were unable to assess the effect of overexpressing wild-type P53 on UV-mediated damage, as overexpression alone leads to extensive cell death in the retina (Jin et al., 2000; Ollmann et al., 2000). The protective effect of Dmp53 is likely mediated by its transcriptional activity: retina-targeted overexpression of two different dominant-negative forms of Dmp53 that lack DNA binding activity (Dmp53259 and Dmp53CT; Brodsky et al., 2000) led to a similar increase in retinal sensitivity (Figure 5C, data not shown).
Fig. 5. Dmp53 protects the retina from UV irradiation. (A–C) Dmp53 is required to protect the developing retina from UV-mediated DNA damage. (A) Wild-type retina irradiated at 24 h. (B) Removal of both functional copies of Dmp53, using the Dmp5311–1B–1 mutation, dramatically increased the retinal sensitivity to UV irradiation. (C) Overexpression of a dominant-negative form of Dmp53, Dmp53259, in the retina resulted in a similar increase in retinal sensitivity. TUNEL staining indicated that the Dmp5311–1B–1 phenotype was due to an increase in apoptotic death (D) compared with wild type (Figure 2G). (E–I) Dmp53 protects the retina by promoting DNA repair. (E) Thrity minutes of light-induced photorepair was sufficient to fully rescue the wild-type retina but not the Dmp5311–1B–1 retina (F). (G–I) xpg/mus201D1 and Dmp5311–1B–1 display a synthetic genetic interaction. Irradiation of flies containing either one copy of xpg/mus201D1 (G) or Dmp5311–1B–1 (H) mutations resulted in an eye phenotype similar to wild type. However, flies heterozygous for both mutations proved significantly more sensitive (I).
Most previous studies have found that P53 acts to promote cell death, in contrast to our observation. Potential explanations for the protective effect of Dmp53 following irradiation include: (i) Dmp53 is required for cell cycle arrest to provide the time required by cells to repair; or (ii) Dmp53 helps direct DNA damage repair. The first possibility is unlikely in our experimental paradigm, as nearly all of the cells in a 24 h APF pupal retina have been post-mitotic for >14 h. Consistent with this view, BrdU staining of untreated or irradiated retina at 24 h APF indicated no cell divisions (other than the normal, few cell divisions that complete the interommatidial bristle organules; data not shown). We therefore utilized the photoreactivation repair system to determine whether loss of Dmp53 compromised DNA repair.
Light-mediated photorepair of genotypically dmp53-null mutants for 2 h resulted in a complete reversal of the retinal phenotype (similar to Figure 5E). Reducing the dose of photorepair to 30 min still fully restored wild-type retinae, but was less efficient at restoring dmp53 mutants (Figure 5E and F). This suggests that dmp53 mutants are more sensitive to UV damage because DNA repair is impaired. Because full rescue of the retina requires intact photorepair and nucleotide excision repair (Figure 2I), this result suggests that one of these pathways is deficient in a dmp53 mutant; alternatively, Dmp53 could function at a downstream step, for example preventing Diap1 degradation. To explore further the potential connection between Dmp53 and DNA repair, we placed a single mutant copy of dmp53 and of the nucleotide excision repair mutant xpg/mus201D1 in trans to determine whether they demonstrate a dominant genetic interaction. Irradiation of flies containing a single mutant copy of either of these genes by themselves yielded a phenotype similar to irradiated wild-type retinae (Figure 5G and H). Combining a single copy of dmp53 and xpg/mus201D1 in trans resulted in a strong enhancement of the UV phenotype (Figure 5I). A similar genetic interaction was also observed between dmp53 and mei-9 (data not shown). Taken together with our repair data, this suggests that Dmp53 promotes DNA repair and cell viability, primarily by acting to enhance nucleotide excision repair.
Discussion
UV irradiation directed both morphology changes and cell death in the developing retina. Both classes of defects were fully rescued by reversal of DNA damage and exacerbated by removing DNA repair genes, indicating that DNA damage is the primary or sole source of the cells’ response. We presume the widespread apoptosis upon irradiation is a direct response to DNA damage, although we cannot rule out death as a secondary response to cells’ release from the apical surface (‘anoikis’). In either case, cell death—but not morphology defects—was fully blocked by P35, indicating that apoptotic death was caspase dependent. Based on our genetic evidence, Dronc, which is not fully inhibited by P35, may play a role in these morphology changes. If so, this activity must diverge before it reaches downstream P35-sensitive caspases.
The major inhibitor of caspase activity in Drosophila is Diap1 (Martin, 2002). Stability of Diap1 is the central point of cell death regulation in the developing retina (Hays et al., 2002) and we demonstrate that this is also true during UV irradiation in the retina. Our genetic and microarray results further suggest that the retina requires Hid as a primary regulator of Diap1 stability during UV irradiation. Hid may represent the primary regulator of Diap1 during UV (versus ionizing) irradiation response by the fly. Alternatively, the retina utilizes Hid as its major RHG factor during its development (Yu et al., 2002), and this preference may simply extend to its response to UV; other tissues may exploit different Diap1 regulators that reflect their use during development (Zhou and Steller, 2003).
This close similarity between periods of developmental cell death (Chan et al., 2002) and sensitivity to DNA damage is also seen in the developing mammalian CNS. The extreme sensitivity of the developing CNS to irradiation has limited the usefulness of radiation therapy as a treatment for pediatric CNS tumors (Packer, 1999, 2002; Kieran, 2000). Although clear links have been made between the status of a cell in the cell cycle and its response to DNA damage (Melo and Toczyski, 2002), our study, performed on a developing post-mitotic nervous system, suggests a mechanism behind this radiosensitivity. We demonstrate that Hid is both necessary and sufficient to confer radiation sensitivity to at least the interommatidial cells, mirroring its requirement during normal retinal development. Recent work in the mammalian nervous system indicate that the functionally related RGH family member Smac is capable of conferring cell death sensitivity to neurons (Deshmukh et al., 2002). We have yet to identify the factor that confers a similar sensitivity to, for example, the photoreceptor neurons in irradiated Drosophila retinae.
Reaper, also an inhibitor of Diap1 function, is thought to be of central importance during the larval wing disk’s response to ionizing radiation (Peterson et al., 2002); however, we have failed to find evidence for its use in the retina. This suggests that Dmp53—which is active in our experimental paradigm—can act in a manner independent of any regulation of Reaper. Dmp53 has been shown to be capable of targeting sequences upstream of reaper, and we do not know if Dmp53 is required for the observed upregulation in hid expression in irradiated retinae; our results suggest its primary targets may be DNA repair enzymes.
Remarkably, although all the cells in the pupal retina are sensitive to UV during the 18–25 h APF window, only the interommatidial cells are rescued when Hid activity is removed. We have ruled out Reaper as a regulator of the retina’s UV response, leaving open the question regarding what factor(s) acts to destabilize Diap1 within the ommatidial core. An equally intriguing question is why the ommatidial cores demonstrate a window of UV competence identical to the interommatidial cells; no cell death occurs within this cell population at any stage of normal development.
One of our most surprising results came with the Dmp53. Mammalian P53 can arrest proliferation to permit repair or it can promote cell death, depending on the cellular context (Vousden, 2000). In the fly retina we observed a different result: Dmp53 was required to prevent cell death following UV irradiation, but its role is unrelated to cell cycle regulation as these retinal cells are post-mitotic. Nor is it likely to be linked directly to caspase stability, as in the case of Diap1. Instead, we present genetic and photorepair evidence that Dmp53 functions to promote DNA repair and viability. There is growing data that supports the idea that P53 can direct repair of DNA damage (Hanawalt, 2002); our work provides in situ support for this proposal. Recent work has reported that dmp53 mutant larvae are more sensitive to ionizing radiation; this effect was ascribed to a block in the death of severely damaged cells (Sogame et al., 2003). We propose an alternative interpretation: the cells of irradiated larvae can not repair DNA damage proficiently, leading to an increased likelihood of cell death as well as the observed increase in mutation rates. The connection between DNA repair, Dmp53 and transcription of repair enzymes remains to be elucidated; future experiments comparing upregulated transcripts in wild-type and dmp53-null tissues should help address this issue.
Together, our results identify two points of regulation during a retinal cell’s response to UV irradiation. The early step involves pyrimidine dimers, and requires proper repair from factors that include XPG and Dmp53. The second step involves activation of caspases and requires regulation of Diap1 stability; interommatidial cells utilize Hid at this step, and the remaining cells employ a different (unknown) regulator. One challenge will be to connect these two points of regulation. Multiple signaling pathways are suggested by our microarray data (see Supplemental data). These include dEGFR/dRas1 signaling (a central regulator of Hid) (Bergmann et al., 1998; Kurada and White, 1998), JNK pathway signaling (see also Figure 3F) and TGFβ pathway signaling. The role of these factors is not known, but understanding them may help to connect early and late events.
Materials and methods
Fly strains
The following fly stocks were obtained from the Bloomington Stock Center: second and third chromosome deficiency set, third chromosome P-element set, Canton S, mus201D1, mei-9A1, mei-41RT1 and GMR-Gal4. We were kindly provided with GMR-p35, GMR-diap1 and th7 by Bruce Hay, GUS-Dmp53259 and GUS-Dmp53CT by John Abrams, Dmp5311–1B–1 by Kent Golic, Df(XR38) byfrom Kristin White, and Df(3L)WR+X1 and l(3)05014 by Nick Baker. The reaper mutant genotype was Df(XR38)/Df(H99) (Peterson et al., 2002) and the hid mutant genotype was Df(3L)WR+X1/l(3)05014 (Grether et al., 1995; Yu et al., 2002).
Pupal UV irradiation assay
White pre-pupae were collected and incubated at 25°C for the indicated time (23–24 h APF unless noted otherwise). Just prior to irradiation the pupal case was removed from around the retina. All pupal irradiations were performed using a 6 W 254 nm UVC bulb (Ultra-Violet Products, Upland, CA) at a distance of 70 cm with the exposed retina turned up (40 000 µJ/cm2). After irradiation, pupae were aged in the dark. UV-damaged DNA was repaired with a 2 h light exposure from a 65 W 10 000 K daylight lamp at a distance of 30 cm (24 mW/cm2; Custom Sealife, San Marcos, CA).
Scanning electron microscopy
Flies were prepared by ethanol fixation followed by critical point drying. Images were captured using a Hitachi S-2600H scanning electron microscope.
Immunohistochemistry
Immunohistochemistry was performed as described previously (Brachmann et al., 2000). Antibodies used were: anti-armadillo N27A1 and anti-actin (Developmental Studies Hybridoma Bank, University of Iowa), anti-caspase-3 (Cell Signaling), anti-Drice (Yoo et al., 2002), anti-thymine dimer (Kamiya Biomedical, Thousand Oaks, CA), anti-phospho-JNK and anti-total-JNK (Cell Signaling Technologies) and anti-Diap1 (gift of Kristin White).
TUNEL staining
TUNEL was performed using the Fluorescein In Situ Cell Detection Kit (Roche Diagnostics, Indianapolis, IN).
Tissue culture and western blots
S2 cells were irradiated with 40 000 µJ/cm2 UVC using a Stratalinker (Stratagene). Retinae were irradiated as described above, then either collected after 5 h of dark, or 2 h of photoreactivating dark followed by 3 h of dark. Six-hour-old embryos (approximately stage 11) were similarly irradiated with 40 000 µJ/cm2 UVC and collected after 3 h of dark or after 1 h of photoreactivation followed by 2 h of dark.
DNA microarray analysis
Twenty-three-hour APF pupae were irradiated with 40 000 µJ/cm2 UVC light and then aged for 1.5 h. Total RNA was isolated from 10 retinae using the PicoPure RNA isolation kit (Arcturus, Mountain View, CA). RNA was linearly amplified through two rounds of in vitro transcription using the RiboAmp RNA Amplification Kit (Arcturus). cRNA (30 µg) was hybridized to a Drosophila GeneChip (Affymetrix, Santa Clara, CA). Fluorescence intensity measurements and data analysis were carried out using proprietary GeneChip software. Baseline values were obtained from the similarly staged, but non-irradiated retinae. Each condition was performed in duplicate. Those transcripts that were regulated ≥2-fold in all four pair-wise comparisons were considered significant.
Lattice cell counts
Interommatidial 2°/3° lattice cells were counted from anti-armadillo stains of 42 h APF pupae. A minimum of 30 ommatidia were counted for each genotype.
Supplementary data
Supplementary data are available at The EMBO Journal Online.
Acknowledgments
Acknowledgements
We thank Julia Cordero for assistance in characterizing the Dmp53 phenotype, Dennis Poehling for technical assistance, and Carrie Baker Brachmann for helpful experimental advice and comments on the manuscript. We also greatly appreciate the Drosophila stocks and other reagents generously provided by John Abrams, Nick Baker, Kent Golic, Bruce Hay, Marek Mlodzik, Kristin White and the Bloomington Drosophila Stock Center. This work was supported by NIH grant EY11495; O.J. was further supported by NIH EY3360.
References
- Bergmann A., Agapite,J., McCall,K. and Steller,H. (1998) The Drosophila gene hid is a direct molecular target of Ras-dependent survival signaling. Cell, 95, 331–341. [DOI] [PubMed] [Google Scholar]
- Brachmann C.B., Jassim,O.W., Wachsmuth,B.D. and Cagan,R.L. (2000) The Drosophila Bcl-2 family member dBorg-1 functions in the apoptotic response to UV-irradiation. Curr. Biol., 10, 547–550. [DOI] [PubMed] [Google Scholar]
- Brodsky M.H., Nordstrom,W., Tsang,G., Kwan,E., Rubin,G.M. and Abrams,J.M. (2000) Drosophila p53 binds a damage response element at the reaper locus. Cell, 101, 103–113. [DOI] [PubMed] [Google Scholar]
- Cagan R.L. and Ready,D.F. (1989a) The emergence of order in the Drosophila pupal retina. Dev. Biol., 136, 346–362. [DOI] [PubMed] [Google Scholar]
- Cagan R.L. and Ready,D.F. (1989b) Notch is required for successive cell decisions in the developing Drosophila retina. Genes Dev., 3, 1099–1112. [DOI] [PubMed] [Google Scholar]
- Carell T., Burgdorf,L.T., Kundu,L.M. and Cichon,M. (2001) The mechanism of action of DNA photolyases. Curr. Opin. Chem. Biol., 5, 491–498. [DOI] [PubMed] [Google Scholar]
- Chan W.Y., Lorke,D.E., Tiu,S.C. and Yew,D.T. (2002) Proliferation and apoptosis in the developing human neocortex. Anat. Rec., 267, 261–276. [DOI] [PubMed] [Google Scholar]
- Chouinard N., Valerie,K., Rouabhia,M. and Huot,J. (2002) UVB-mediated activation of p38 mitogen-activated protein kinase enhances resistance of normal human keratinocytes to apoptosis by stabilizing cytoplasmic p53. Biochem. J., 365, 133–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleaver J.E. and Crowley,E. (2002) UV damage, DNA repair and skin carcinogenesis. Front. Biosci., 7, 1024–1043. [DOI] [PubMed] [Google Scholar]
- Deshmukh M., Du,C., Wang,X. and Johnson,E.M.,Jr (2002) Exogenous smac induces competence and permits caspase activation in sympathetic neurons. J. Neurosci., 22, 8018–8027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman M. (1996) Reiterative use of the EGF receptor triggers differentiation of all cell types in the Drosophila eye. Cell, 87, 651–660. [DOI] [PubMed] [Google Scholar]
- Friedberg E.C. (2001) How nucleotide excision repair protects against cancer. Nat. Rev. Cancer, 1, 22–33. [DOI] [PubMed] [Google Scholar]
- Goyal L., McCall,K., Agapite,J., Hartwieg,E. and Steller,H. (2000) Induction of apoptosis by Drosophila reaper, hid and grim through inhibition of IAP function. EMBO J., 19, 589–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grether M.E., Abrams,J.M., Agapite,J., White,K. and Steller,H. (1995) The head involution defective gene of Drosophila melanogaster functions in programmed cell death. Genes Dev., 9, 1694–1708. [DOI] [PubMed] [Google Scholar]
- Hanawalt P.C. (2002) Subpathways of nucleotide excision repair and their regulation. Oncogene, 21, 8949–8956. [DOI] [PubMed] [Google Scholar]
- Hawkins C.J., Yoo,S.J., Peterson,E.P., Wang,S.L., Vernooy,S.Y. and Hay,B.A. (2000) The Drosophila caspase DRONC is a glutamate/aspartate protease whose activity is regulated by DIAP1, HID and GRIM. J. Biol. Chem., 275, 27084–27093. [DOI] [PubMed] [Google Scholar]
- Hay B.A., Wassarman,D.A. and Rubin,G.M. (1995) Drosophila homologs of baculovirus inhibitor of apoptosis proteins function to block cell death. Cell, 83, 1253–1262. [DOI] [PubMed] [Google Scholar]
- Hays R., Wickline,L. and Cagan,R. (2002) Morgue mediates apoptosis in the Drosophila melanogaster retina by promoting degradation of DIAP1. Nat. Cell Biol., 4, 425–431. [DOI] [PubMed] [Google Scholar]
- Holley C.L., Olson,M.R., Colon-Ramos,D.A. and Kornbluth,S. (2002) Reaper eliminates IAP proteins through stimulated IAP degradation and generalized translational inhibition. Nat. Cell Biol., 4, 439–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houle J.F. and Friedberg,E.C. (1999) The Drosophila ortholog of the human XPG gene. Gene, 234, 353–360. [DOI] [PubMed] [Google Scholar]
- Jin S. et al. (2000) Identification and characterization of a p53 homologue in Drosophila melanogaster. Proc. Natl Acad. Sci. USA, 97, 7301–7306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieran M.W. (2000) Advances in pediatric neuro-oncology. Curr. Opin. Neurol., 13, 627–634. [DOI] [PubMed] [Google Scholar]
- Kulms D. and Schwarz,T. (2002a) Independent contribution of three different pathways to ultraviolet-B-induced apoptosis. Biochem. Pharmacol., 64, 837–841. [DOI] [PubMed] [Google Scholar]
- Kulms D. and Schwarz,T. (2002b) Molecular mechanisms involved in UV-induced apoptotic cell death. Skin Pharmacol. Appl. Skin Physiol., 15, 342–347. [DOI] [PubMed] [Google Scholar]
- Kulms D., Poppelmann,B., Yarosh,D., Luger,T.A., Krutmann,J. and Schwarz,T. (1999) Nuclear and cell membrane effects contribute independently to the induction of apoptosis in human cells exposed to UVB radiation. Proc. Natl Acad. Sci. USA, 96, 7974–7979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurada P. and White,K. (1998) Ras promotes cell survival in Drosophila by downregulating hid expression. Cell, 95, 319–329. [DOI] [PubMed] [Google Scholar]
- Lakin N.D., Hann,B.C. and Jackson,S.P. (1999) The ataxia-telangiectasia related protein ATR mediates DNA-dependent phosphorylation of p53. Oncogene, 18, 3989–3995. [DOI] [PubMed] [Google Scholar]
- Lisi S., Mazzon,I. and White,K. (2000) Diverse domains of THREAD/DIAP1 are required to inhibit apoptosis induced by REAPER and HID in Drosophila. Genetics, 154, 669–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y. and Kulesz-Martin,M. (2001) p53 protein at the hub of cellular DNA damage response pathways through sequence-specific and non-sequence-specific DNA binding. Carcinogenesis, 22, 851–860. [DOI] [PubMed] [Google Scholar]
- Lohmann I., McGinnis,N., Bodmer,M. and McGinnis,W. (2002) The Drosophila Hox gene deformed sculpts head morphology via direct regulation of the apoptosis activator reaper. Cell, 110, 457–466. [DOI] [PubMed] [Google Scholar]
- Martin S.J. (2002) Destabilizing influences in apoptosis: sowing the seeds of IAP destruction. Cell, 109, 793–796. [DOI] [PubMed] [Google Scholar]
- Melo J. and Toczyski,D. (2002) A unified view of the DNA-damage checkpoint. Curr. Opin. Cell Biol., 14, 237–245. [DOI] [PubMed] [Google Scholar]
- Mukhtar H. and Elmets,C.A. (1996) Photocarcinogenesis: mechanisms, models and human health implications. Photochem. Photobiol., 63, 356–357. [DOI] [PubMed] [Google Scholar]
- Norbury C.J. and Hickson,I.D. (2001) Cellular responses to DNA damage. Annu. Rev. Pharmacol. Toxicol., 41, 367–401. [DOI] [PubMed] [Google Scholar]
- Nordstrom W., Chen,P., Steller,H. and Abrams,J.M. (1996) Activation of the reaper gene during ectopic cell killing in Drosophila. Dev. Biol., 180, 213–226. [DOI] [PubMed] [Google Scholar]
- Ollmann M. et al. (2000) Drosophila p53 is a structural and functional homolog of the tumor suppressor p53. Cell, 101, 91–101. [DOI] [PubMed] [Google Scholar]
- Packer R.J. (1999) Brain tumors in children. Arch. Neurol., 56, 421–425. [DOI] [PubMed] [Google Scholar]
- Packer R.J. (2002) Radiation-induced neurocognitive decline: the risks and benefits of reducing the amount of whole-brain irradiation. Curr. Neurol. Neurosci. Rep., 2, 131–133. [DOI] [PubMed] [Google Scholar]
- Peterson C., Carney,G.E., Taylor,B.J. and White,K. (2002) reaper is required for neuroblast apoptosis during Drosophila development. Development, 129, 1467–1476. [DOI] [PubMed] [Google Scholar]
- Rong Y.S. et al. (2002) Targeted mutagenesis by homologous recombination in D. melanogaster. Genes Dev., 16, 1568–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosette C. and Karin,M. (1996) Ultraviolet light and osmotic stress: activation of the JNK cascade through multiple growth factor and cytokine receptors. Science, 274, 1194–1197. [DOI] [PubMed] [Google Scholar]
- Ryoo H.D., Bergmann,A., Gonen,H., Ciechanover,A. and Steller,H. (2002) Regulation of Drosophila IAP1 degradation and apoptosis by reaper and ubcD1. Nat. Cell Biol., 4, 432–438. [DOI] [PubMed] [Google Scholar]
- Sekelsky J.J., Hollis,K.J., Eimerl,A.I., Burtis,K.C. and Hawley,R.S. (2000) Nucleotide excision repair endonuclease genes in Drosophila melanogaster. Mutat. Res., 459, 219–228. [DOI] [PubMed] [Google Scholar]
- She Q.B., Chen,N. and Dong,Z. (2000) ERKs and p38 kinase phosphorylate p53 protein at serine 15 in response to UV radiation. J. Biol. Chem., 275, 20444–20449. [DOI] [PubMed] [Google Scholar]
- Sogame N., Kim,M. and Abrams,J.M. (2003) Drosophila p53 preserves genomic stability by regulating cell death. Proc. Natl Acad. Sci. USA, 100, 4696–4701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibbetts R.S., Brumbaugh,K.M., Williams,J.M., Sarkaria,J.N., Cliby,W.A., Shieh,S.Y., Taya,Y., Prives,C. and Abraham,R.T. (1999) A role for ATR in the DNA damage-induced phosphorylation of p53. Genes Dev., 13, 152–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trautinger F. (2001) Mechanisms of photodamage of the skin and its functional consequences for skin ageing. Clin. Exp. Dermatol., 26, 573–577. [DOI] [PubMed] [Google Scholar]
- Vousden K.H. (2000) p53: death star. Cell, 103, 691–694. [DOI] [PubMed] [Google Scholar]
- Vousden K.H. and Lu,X. (2002) Live or let die: the cell’s response to p53. Nat. Rev. Cancer, 2, 594–604. [DOI] [PubMed] [Google Scholar]
- Wang S.L., Hawkins,C.J., Yoo,S.J., Muller,H.A. and Hay,B.A. (1999) The Drosophila caspase inhibitor DIAP1 is essential for cell survival and is negatively regulated by HID. Cell, 98, 453–463. [DOI] [PubMed] [Google Scholar]
- Wilson R., Goyal,L., Ditzel,M., Zachariou,A., Baker,D.A., Agapite,J., Steller,H. and Meier,P. (2002) The DIAP1 RING finger mediates ubiquitination of Dronc and is indispensable for regulating apoptosis. Nat. Cell Biol., 4, 445–450. [DOI] [PubMed] [Google Scholar]
- Wing J.P. et al. (2002) Drosophila Morgue is an F box/ubiquitin conjugase domain protein important for grim-reaper mediated apoptosis. Nat. Cell Biol., 4, 451–456. [DOI] [PubMed] [Google Scholar]
- Wolff T. and Ready,D.F. (1991) Cell death in normal and rough eye mutants of Drosophila. Development, 113, 825–839. [DOI] [PubMed] [Google Scholar]
- Yoo S.J. et al. (2002) Hid, Rpr and Grim negatively regulate DIAP1 levels through distinct mechanisms. Nat. Cell Biol., 4, 416–424. [DOI] [PubMed] [Google Scholar]
- Yu S.Y., Yoo,S.J., Yang,L., Zapata,C., Srinivasan,A., Hay,B.A. and Baker,N.E. (2002) A pathway of signals regulating effector and initiator caspases in the developing Drosophila eye. Development, 129, 3269–3278. [DOI] [PubMed] [Google Scholar]
- Zhou L. and Steller,H. (2003) Distinct pathways mediate UV-induced apoptosis in Drosophila embryos. Dev. Cell, 4, 599–605. [DOI] [PubMed] [Google Scholar]