Abstract
Occludin is the only known integral membrane protein localized at the points of membrane– membrane interaction of the tight junction. We have used the Xenopus embryo as an assay system to examine: (a) whether the expression of mutant occludin in embryos will disrupt the barrier function of tight junctions, and (b) whether there are signals within the occludin structure that are required for targeting to the sites of junctional interaction. mRNAs transcribed from a series of COOH-terminally truncated occludin mutants were microinjected into the antero–dorsal blastomere of eight-cell embryos. 8 h after injection, the full-length and the five COOH-terminally truncated proteins were all detected at tight junctions as defined by colocalization with both endogenous occludin and zonula occludens-1 demonstrating that exogenous occludin correctly targeted to the tight junction. Importantly, our data show that tight junctions containing four of the COOH-terminally truncated occludin proteins were leaky; the intercellular spaces between the apical cells were penetrated by sulfosuccinimidyl-6-(biotinamido) Hexanoate (NHS-LC-biotin). In contrast, embryos injected with mRNAs coding for the full-length, the least truncated, or the soluble COOH terminus remained impermeable to the NHS-LC-biotin tracer. The leakage induced by the mutant occludins could be rescued by coinjection with full-length occludin mRNA. Immunoprecipitation analysis of detergent-solubilized embryo membranes revealed that the exogenous occludin was bound to endogenous Xenopus occludin in vivo, indicating that occludin oligomerized during tight junction assembly. Our data demonstrate that the COOH terminus of occludin is required for the correct assembly of tight junction barrier function. We also provide evidence for the first time that occludin forms oligomers during the normal process of tight junction assembly. Our data suggest that mutant occludins target to the tight junction by virtue of their ability to oligomerize with full-length endogenous molecules.
Tight junctions, the most apical component of the junctional complex (Farquhar and Palade, 1963), form selective permeability barriers along the paracellular pathways of epithelial and endothelial cells (Diamond, 1977; Gumbiner, 1990; Reuss, 1989). Tight junctions also act as a “fence” between the apical and lateral plasma membrane domains to prevent the mixing of membrane lipids and proteins between these two compartments (van Meer et al., 1986; Cereijido et al., 1989; Schneeberger and Lynch, 1992). In response to different stimuli, tight junctions rapidly change their permeability and functional properties, permitting dynamic fluxes of ions and solutes as well as transepithelial passage of whole cells (Claude and Goodenough, 1973; Duffey et al., 1981; Kachar and Pinto da Silva, 1981; Mazariegos et al., 1984; Milks et al., 1986; Madara and Pappenheimer, 1987; Pappenheimer, 1987, 1990; Pappenheimer and Reiss, 1987).
At least seven proteins (zonula occludens-1 and -2 [ZO-1, ZO-2], cingulin, 7H6, rab 13, occludin, and symplekin) are found to be localized at tight junctions (Citi et al., 1988; Gumbiner et al., 1991; Furuse et al., 1993; Zhong et al., 1993; Jesaitis and Goodenough, 1994; Zahraoui et al., 1994; Ando-Akatsuka et al., 1996; Keon et al., 1996). Among these proteins, occludin is the only integral membrane protein localized at the points of membrane–membrane interaction of tight junctions as revealed by immunogold labeling of thin-sections and freeze-fracture replicas (Furuse et al., 1993; Fujimoto, 1995). Hydropathy analysis predicts that occludin has four transmembrane domains, two extracellular loops, and a long COOH-terminal cytoplasmic tail consisting of 255 amino acids (Furuse et al., 1993; Ando-Akatsuka et al., 1996). By transfection of various deletion mutants of chicken occludin into Madin-Darby bovine kidney (MDBK) cells, Furuse et al. (1994) showed that the COOH-terminal ∼150 amino acids (domain E358/504) were necessary for the localization of occludin at tight junction. Their in vitro binding assay also indicated that domain E358/504 directly associated with ZO-1. However, recent data reported by Balda et al. (1996) showed that COOH-terminally truncated chicken occludin localized efficiently to the tight junction in transfected MDCK cells. The discrepancy between these experiments raises the question of whether COOH terminus of occludin is required for targeting.
Three experiments with cell culture systems indicate that occludin is directly involved in the sealing function of the tight junction. First, in the experiments outlined above (Balda et al., 1996), expression of a COOH-terminally truncated occludin resulted in an electrically tighter paracellular pathway that paradoxically had an increased paracellular flux of solutes. Second, McCarthy et al. (1996) transfected MDCK cells with chicken occludin cDNA in a Lac-inducible expression vector. Isopropyl-β-d-thiogalactoside (IPTG)1–induced expression of chicken occludin increased transepithelial resistance (TER) by 30–40%, which declined back to uninduced states after removal of IPTG from the culture medium. Freeze fracture showed an increase in the mean number and complexity of branching of tight junctional strands, together with a concomitant increase in the apical–basal width of the tight junction network. McCarthy et al. (1996) also observed the paradoxical transepithelial mannitol flux which progressively increases as TER increases. Third, Wong and Gumbiner (1997) found that a synthetic peptide (OCC2), corresponding to the second extracellular domain of occludin, reversibly disrupted the transepithelial permeability barrier when added to Xenopus kidney epithelial A6 cell monolayers. In these experiments, OCC2 decreased TER and increased the paracellular flux of tracers.
We have investigated the ability of mutant occludin molecules to assemble in tight junctions during the biogenesis of an epithelium in an intact organism. mRNAs coding for a series of COOH-terminally truncated chicken occludin molecules have been expressed in the Xenopus embryo. All exogenous proteins were FLAG tagged at the COOH terminus to permit discrimination from endogenous molecules and were seen to target to the tight junction by immunofluorescence. Four of the COOH-terminally truncated mutants caused the disruption of the tight junction solute seal as assayed by a novel surface biotinylation method. Exogenous and endogenous occludins could be coimmunoprecipitated under nondenaturing conditions as oligomeric complexes. These data indicate that the truncated, mutant occludins are targeted to the tight junction in association with intact, endogenous molecules.
Materials and Methods
Construction of Full-Length and Truncated Occludins
A full-length chicken occludin cDNA (a generous gift from S. Tsukita, Kyoto University, Japan) was subcloned into the expression vector SP64T by PCR. The sequence of PCR sense primer was as follows: 5′-TGG GCC ACC ATG TTC AGC AAG AAG-3′, which corresponded to the NH2-terminal 15 codons of chicken occludin plus 9 bp preceding the start codon. The antisense primer corresponded to the last 15 codons of the COOH terminus of occludin plus the BglII site and three restriction sites, which formed a linker between the occludin and the FLAG epitope: 5′-GCC TAC GAC AAG GTG CGG GAG ATC TTC GCG ATA TCA AGG CCT GAC TAC AAG GAC GAC GAT GAC AAG TAA-3′. These restriction sites permitted placing the FLAG-coding sequence in frame with each truncation. Using these primers, a 1.6-kb occludin cDNA with the FLAG epitope tag was amplified by Vent polymerase (New England Biolabs Inc., Beverly, MA) with full-length occludin cDNA in pBluescript SK(−) as a template. The PCR products were phosphorylated, separated by agarose gel electrophoresis, and purified by Gel Extraction Kit (QIAGEN Inc., Chatsworth, CA). The purified occludin DNA was cloned into the BglII site of the expression vector SP64T (Krieg and Melton, 1984).
A nested set of five occludin constructs truncated in the COOH-terminal domain predicted to face the cytoplasm was prepared by endonuclease digestion of the full-length occludin construct described above. Each truncated occludin was then blunt ligated after the reading frame alignment by endonuclease digestion of the restriction sites located upstream of FLAG-coding region. In addition, a construct was prepared that coded for the initial 20 amino acids of the NH2 terminus fused to the complete COOH terminus beginning at amino acid 266 and including the FLAG epitope tag. The full-length construct was sequenced using AmpliCycle Sequencing Kit (Perkin Elmer Corp., Norwalk, CT) to ensure that the occludin sequence and the FLAG epitope tag were intact and in the correct reading frame.
Production of Anti-occludin and Anti-ZO-1 Sera
The fusion protein containing glutathione-S-transferase (GST) and the COOH terminus of chicken occludin (250 amino acids) was constructed using pGEX-3 vector (Smith and Johnson, 1988). An overnight bacterial culture was diluted in Luria-Bertani medium with 0.01% ampicillin, and grown for 2–3 h at 37°C. The synthesis of the fusion protein was induced by adding 0.2 mM IPTG. After 4–5 h of induction, cells were collected by centrifugation at 2,100 g for 10 min, and resuspended in PBS. The cell suspension was sonicated 1 min on ice with 1% Triton X-100, and centrifuged at 5,100 g for 15 min. The supernatant was incubated with glutathione– agarose beads (Sigma Chemical Co., St. Louis, MO) for 30 min at 4°C, washed three times with PBS, and then the bound fusion proteins were released by adding 5 mM glutathione. The full-length fusion protein was separated on 12% SDS-polyacrylamide gel and purified by electroelution.
Two rabbits were injected with purified fusion protein to raise polyclonal antibodies (Pocono Rabbit Farm and Laboratory, Canadensis, PA). The resulting 11350 and 11351 antisera were affinity purified on a Sepharose 4B column containing 1 mg purified fusion protein. This anti– chicken occludin antibody cross-reacted with Xenopus occludin both in immunoblots and in immunohistochemistry.
The anti–ZO-1 antiserum was prepared by immunizing with a GST– ZO-1 fusion protein. A construct was made consisting of the pGEX-1 expression vector containing 1.8 kb of human ZO-1 sequence spanning the alternative splice site (the generous gift of J. Anderson, Yale University, New Haven, CT). The process of preparation and affinity purification of the antiserum against GST–ZO-1 was the same as described above for the GST–occludin construct.
In Vitro Transcription and Microinjection of mRNA into Xenopus Oocytes and Embryos
The SP64T vectors containing either wild type or mutant chicken occludins were linearized with BamHI and transcribed in vitro with SP6 RNA polymerase (Ambion Inc., Austin, TX). Adult female Xenopus laevis were obtained from a departmental frog facility (Department of Cell Biology, Harvard Medical School, Boston, MA). Oocytes were collected and defolliculated according to published protocols (Swenson et al., 1989). Oocytes were microinjected in their vegetal hemispheres with 40 nl (∼100 pg) RNA or water, and incubated at 17°C overnight for immunoblot or immunoprecipitation experiments.
Fertilized embryos were obtained as described previously (Paul et al., 1995). The anterior, dorsal blastomere of eight-cell embryos were injected with 4 nl (∼10 pg) of FLAG-tagged, full-length or truncated chicken occludin RNA. 6–8 h after injection, embryos were either directly embedded in TISSUE-TEK embedding medium (Miles Inc., Elkhart, IN) without fixation and quickly frozen in liquid propane or used for surface biotinylation experiments (see below).
Cell Culture and Metabolic Labeling
The A6 Xenopus kidney epithelial cells (kindly provided by B. Gumbiner, Sloan-Kettering Institute, New York) were grown in 61% Leibovitz L15 medium (GIBCO BRL, Gaithersburg, MD) supplemented with 5% FCS (Hyclone Laboratories, Logan, UT), 100 U/ml penicillin and 0.1 mg/ml streptomycin, and 45 mM NaHCO3, pH 7.3, at 27°C in a humidified incubator with 5% CO2. Cells were metabolically labeled for 20 h at 27°C with 150 μCi/ml Tran35S-label ([35S]met/[35S]cys; ICN Radiochemicals, Cleveland, OH) in 70% methionine-free medium (GIBCO BRL) supplemented with 5% dialyzed FCS (Sigma Chemical Co.). At the end of the labeling period cells were rinsed three times in ice-cold 0.65× PBS and extracted with the same immunoprecipitation buffer used for Xenopus embryos (vide infra).
Immunoprecipitation
RNA-injected Xenopus oocytes or embryos were rinsed three times with PBS and homogenized on ice by passing 20 times through a 22-gauge needle in lysis buffer (5 mM EDTA, 5 mM EGTA, 5 mM Tris, pH 8.0) with 10 μg/ml each of chymostatin, leupeptin, and pepstatin A, 0.01% diisopropylfluorophosphate, and 20 μM PMSF. The homogenates were centrifuged at 3,000 g for 10 min at 4°C to pellet yolk granules. The supernatants were then centrifuged at 100,000 g at 4°C for 30 min. The membrane pellet was resuspended in lysis buffer plus SDS-PAGE sample buffer to a final concentration of two oocytes per 10 μl for immunoblot. For immunoprecipitation experiments, the membrane pellet was resuspended in IP buffer (1% Triton X-100, 0.5% deoxycholic acid, 0.2% SDS, 150 mM NaCl, 10 mM Hepes, 2 mM EDTA, 10 KIU/ml Trasylol, 10 μg/ml each of chymostatin, leupeptin, and pepstatin A, and 0.01% diisopropylfluorophosphate), incubated on ice for 30 min, and then centrifuged at 100,000 g for 1 h at 4°C. The supernatants were either incubated with primary antibody directly or mixed with 35S-labeled A6 cell lysates first, and then incubated with primary antibody overnight at 4°C. The samples were incubated with either protein A–Sepharose CL-4B or protein G–Sepharose 4B (Sigma Chemical Co.) for an additional 2 h at 4°C. After three washes in IP buffer, one in high salt (0.5 M NaCl, 10 mM Tris), and one in 10 mM Tris buffer, the samples were solubilized in SDS-PAGE sample buffer.
SDS Gel Electrophoresis and Immunoblotting
Samples were boiled in SDS-PAGE sample buffer for 3 min and separated on 10 or 12% SDS-polyacrylamide minigels. The proteins were then transferred onto an Immobilon membrane (Millipore Corp., Bedford, MA). The membrane was blocked 1 h at room temperature with 5% lowfat milk in TBS plus 0.1% Tween 20, and then incubated at room temperature for 2 h with anti-occludin antibody diluted at 1:1,000 or anti-FLAG monoclonal antibody used at 10 μg/ml. The primary antibodies were detected by alkaline phosphatase–conjugated goat anti–rabbit IgG (Promega Corp., Madison, WI), or goat anti–mouse IgG (Boehringer Mannheim Biochemicals, Indianapolis, IN) used at 1:5,000 or 1:1,000. To reduce the background the secondary antibodies were preabsorbed with a crude Xenopus oocyte homogenate before reaction with the Immobilon membranes. After 1-h incubation with secondary antibody at room temperature, the blots were washed three times with TBS before the color reaction was developed according to the manufacturer's instructions.
Surface Biotinylation–Tight Junction Permeability Assay
6 h after RNA injection, Xenopus embryos were cooled to 10°C for 5 min before transferring to freshly made 1 mg/ml NHS-LC-Biotin (Pierce Chemical Co., Rockford, IL) in 0.1 × MMR solution (0.1 M NaCl, 2 mM KCl, 1 mM MgSO4, 2 mM CaCl2·2H2O, 0.1 mM EDTA, and 5 mM Hepes, pH 7.8). After 12 min of labeling with biotin at 10°C, the embryos were washed twice with 0.1 × MMR, and fixed at 4°C overnight in 3% formaldehyde made fresh from paraformaldehyde in 80 mM sodium cacodylate, pH 7.4. Embryos were rinsed with PBS and embedded in TISSUE-TEK and cryosectioned. 14-μm frozen sections were incubated in blocking buffer (1% fish skin gelatin and 1% BSA in PBS) for at least 5 h. Anti– FLAG M2 antibody diluted 1:200 in blocking buffer was then placed on the sections and incubated at 4°C overnight. After washing three times with blocking buffer, sections were then incubated with preadsorbed FITC-conjugated goat anti–mouse IgG (Boehringer Mannheim Biochemicals) and RITC-avidin (Pierce Chemical Co.), both diluted 1:500 in blocking buffer. Anti–FLAG M2 antibody was used to identify the region of RNA injection. Control embryos were warmed to 18°C after biotinylation and allowed to develop to tadpole stages. The 12-min labeling time was chosen since this was the maximum time at 10°C, which was 100% consonant with normal development of embryos to tadpole stages.
Immunofluorescence
Frozen embryos were sectioned on a cryostat HM 500 OM (MICROM; Carl Zeiss, Inc., Thornwood, NY) at 10 μm. The tissues were fixed in 100% methanol for 3 min at −20°C, and blocked with 1% fish skin gelatin plus 1% BSA in PBS for at least 5 h. The sections were double-stained with the affinity-purified anti-occludin antibody 11350 at 1:500 dilution and anti-FLAG monoclonal antibody M2 diluted at 1:200 (Eastman Kodak Co., Rochester, NY) 1 h at room temperature or 4°C overnight. Sections were washed three times, 10 min each in blocking medium, and then incubated for 1 h at room temperature with FITC-conjugated goat anti–rabbit IgG (Boehringer Mannheim Biochemicals) diluted 1:500 and rhodamine-conjugated goat anti–mouse IgG (Cappel Laboratories, Malvern, PA) diluted 1:500, which both were preabsorbed with a crude Xenopus oocyte homogenate. Sections were examined by epifluorescence using a Zeiss Axioskop (Carl Zeiss, Inc.) and photographed with TMAX-400 film (Eastman Kodak Co.).
Results
Full-Length and Truncated Chicken Occludins Can Be Expressed in Xenopus Oocytes
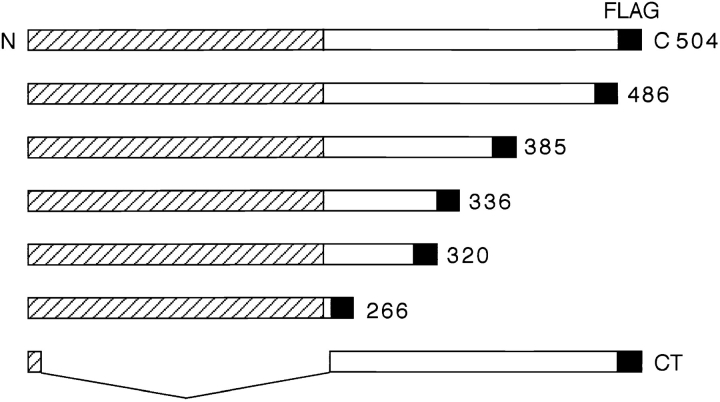
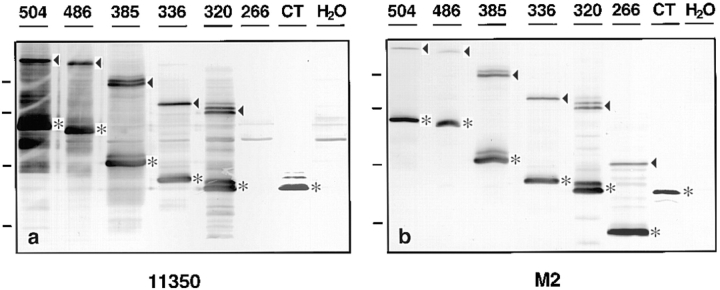
Full-length chicken occludin, a nested set of five COOH-terminally truncated occludins, and a soluble construct consisting of 20 NH2-terminal amino acids fused to the occludin COOH terminus were subcloned into the SP64T vector. These constructs are diagrammed in Fig. 1 (504, 486, 385, 336 320, 266, and CT). The nomenclature indicates the deletion site in each protein and CT represents COOH terminus. The constructs have FLAG epitope tags attached to the COOH terminus of the molecule to distinguish exogenous chicken occludin from endogenous Xenopus occludin, since our polyclonal anti–chicken occludin cross-reacted with Xenopus occludin. The mRNAs transcribed from these seven constructs were microinjected into Xenopus oocytes and the results analyzed by immunoblots shown in Fig. 2. The data in Fig. 2 a were obtained by immunoblotting with anti-occludin antibody 11350, and in Fig. 2 b with anti-FLAG antibody M2. Fig. 2 demonstrates that all proteins were equivalently expressed in Xenopus oocytes. The asterisks at the right side of each lane in both a and b indicate the predicted molecular weight of each protein. In addition, all occludin molecules that contained the four membrane-spanning domains formed presumed dimers in SDS gel sample buffer under reducing conditions as marked by arrowheads in both a and b. The aggregation of occludin in SDS is not an indication of the state of association between occludin proteins in vivo. This SDS-induced aggregation is a phenomenon shared with some of the members of the gap junction connexin family of proteins, particularly connexin 32 (Hertzberg and Gilula, 1979; Goodenough et al., 1988). Proteins 385 and 320 migrated as doublets whose identity was not investigated. It is possible that doublets were caused by posttranslational modifications in certain deletion constructs or were caused by degradation. All preparations were prepared from oocyte membrane fractions except CT, which was obtained from soluble supernatant fractions. A small amount of CT protein could be detected in membrane fractions, although full-length protein could not be detected in supernatants (data not shown). Fig. 2 also shows that all expressed proteins were recognized by both the 11350 antiserum and the anti–FLAG M2 monoclonal antibody, except 266, which lacked almost the entire COOH terminus and was not recognized by 11350.
Figure 1.
Schematic drawing of full-length (504) and six truncated chick occludin proteins that were subcloned into expression vector SP64T. Each molecule has a FLAG epitope tag (black box) at the end of COOH terminus to distinguish it from Xenopus occludin. The shaded half of the molecule is predicted to traverse the membrane four times, and contains an NH2 terminus facing the cytoplasm, two extracellular loop domains, and one cytoplasmic loop domain. The unshaded half of the protein is predicted to face the cytoplasm. The numbers on the right indicate the total number of amino acids in each protein. CT, soluble NH2-terminal 20 amino acids fused to the predicted cytoplasmic tail; N, NH2 terminus; C, COOH terminus.
Figure 2.
Western blot analysis of Xenopus oocytes injected with mRNAs corresponding to the occludin proteins diagrammed in Fig. 1. mRNAs were microinjected in the vegetal hemisphere of stage VI oocytes, and the injected oocytes were incubated at 17°C overnight. Oocyte homogenates were separated on 12% SDS-PAGE, transferred to Immobilon membranes, and then immunoblotted with either anti-occludin antibody 11350 (a) or with anti-FLAG monoclonal antibody M2 (b). The number on the top of each lane identifies each protein as named in Fig. 1. The asterisks in a and b indicate the predicted position of each expressed protein. With the exception of the soluble CT, the expressed proteins formed dimers as indicated by the arrowheads. Note that protein 266, with almost the entire COOH terminus deleted, was not recognized by 11350 antibody. H2O, control oocytes injected with water. Molecular weight markers (from top to bottom): 97.4, 66, 45, and 31 kD.
Disruption of Tight Junction Permeability Barrier by Four COOH-terminally Truncated Occludins
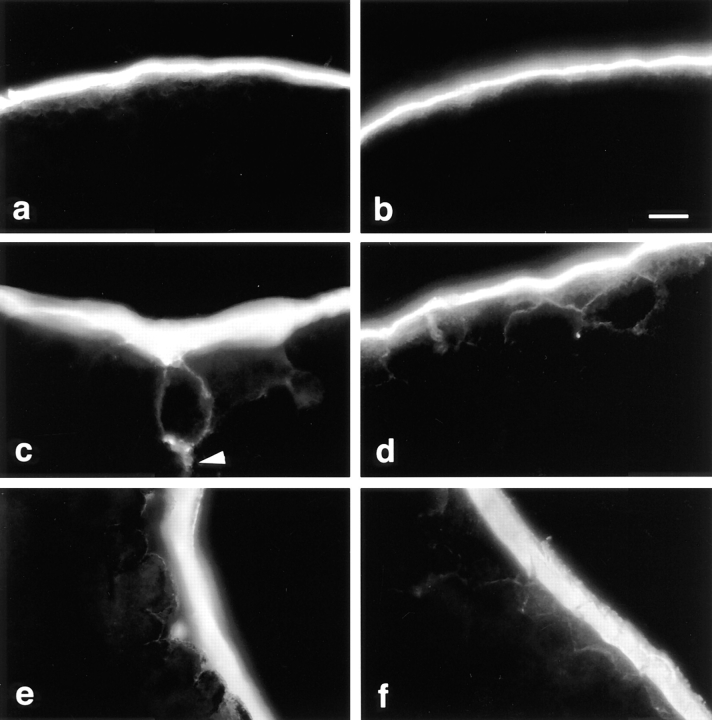
We used a novel surface biotinylation method to examine the permeability of tight junctions in the embryo. During the normal development of Xenopus embryos, biotin molecules are blocked from entry to restricted intercellular spaces beginning at the two-cell stage, suggesting that the formation of functional tight junctions and formation of the blastocoele begin at the first cleavage (Slack and Warner, 1973; Merzdorf, C., Y.-H. Chen, and D.A. Goodenough, manuscript in preparation). When mRNAs from constructs 504 (full-length), 486 (the least COOH-terminally truncated), and CT (COOH terminus only) were injected into eight-cell stage embryos, the results were identical to embryos injected with only water: tight junctions were impermeable to the biotin tracer (Fig. 3, a and b; data not shown for CT). NHS-LC-biotin labeled the vitelline envelope and apical blastomere membranes, which appear together as a single thick line at the surface of the embryo (Fig. 3, a and b). No signal was detectable between the blastomeres in the intercellular spaces. However, if the embryos were injected with mRNAs from the four COOH-terminally truncated constructs 385, 336, 320, and 266, the intercellular spaces between epithelial cells were penetrated by biotin (Fig. 3, c–f), indicating that there is a critical region between residues 486 and 385 that is required for correct assembly critical for the tight junctional seal. Fig. 3 c (arrowhead) reveals that the membranes of internal cells beneath those at the embryonic surface have also been biotinylated, indicating that the tracer penetrated beyond the first tier of cells. This observation indicates that the truncated occludin molecules must have altered the permeability function of the tight junctions. Double labeling of sections with both the avidin and anti– FLAG M2 antibody revealed that the leaks occurred only between blastomeres expressing the truncated occludin proteins; the tight junctions between the blastomeres unlabeled by M2 within the same embryo were well sealed (data not shown).
Figure 3.
Functional assay of tight junctions by surface biotinylation in Xenopus embryos injected with chicken full-length or truncated occludins. 6 h after mRNA injection (2,000 cell blastula), the embryos were labeled by incubation in 1 mg/ml NHS-LC-biotin for 12 min at 10°C, washed, and then fixed in 3% formaldehyde in 80 mM sodium cacodylate. Frozen sections were stained with RITC- avidin and observed by fluorescence microscopy. In all embryos, NHS-LC-biotin reacts with molecules in the vitelline envelope, the subvitelline space, and the apical plasma membranes of the blastomeres, which together appear as a thick, continuous line at the surface of the embryo. The tight junctions in the embryos injected with 504 (a, the full-length) or 486 (b, the least COOH-terminally truncated) occludin mRNAs deny the biotin access to the intercellular spaces, and the basolateral membranes of the blastomeres are not stained. In the embryos injected with 385 (c), 336 (d) 320 (e), and 266 (f) (four COOH-terminally truncated occludin mRNAs), the biotin molecules penetrated into intercellular spaces demonstrating the disruption of the tight junction seal. Bar, 10 μm.
These dominant-negative effects of the truncated occludin mutants were sensitive to dilution and to competition from wild-type protein. Leaks between blastomeres were observed when truncated occludin mRNAs were diluted 1: 10 but not 1:50 (data not shown). The induced tight-junctional leakage could be rescued by coinjection of an equal amount of mRNA from construct 504 (full-length) together with mRNA from construct 266. Fig. 4 shows the biotin staining of Xenopus embryos 6 h after injection of mRNAs from construct 504 (a), 266 (b), and 266 with 504 together (c). Coinjection of 504 and 266 mRNAs together resulted in no detectable leak between the blastomeres (Fig. 4 c).
Figure 4.
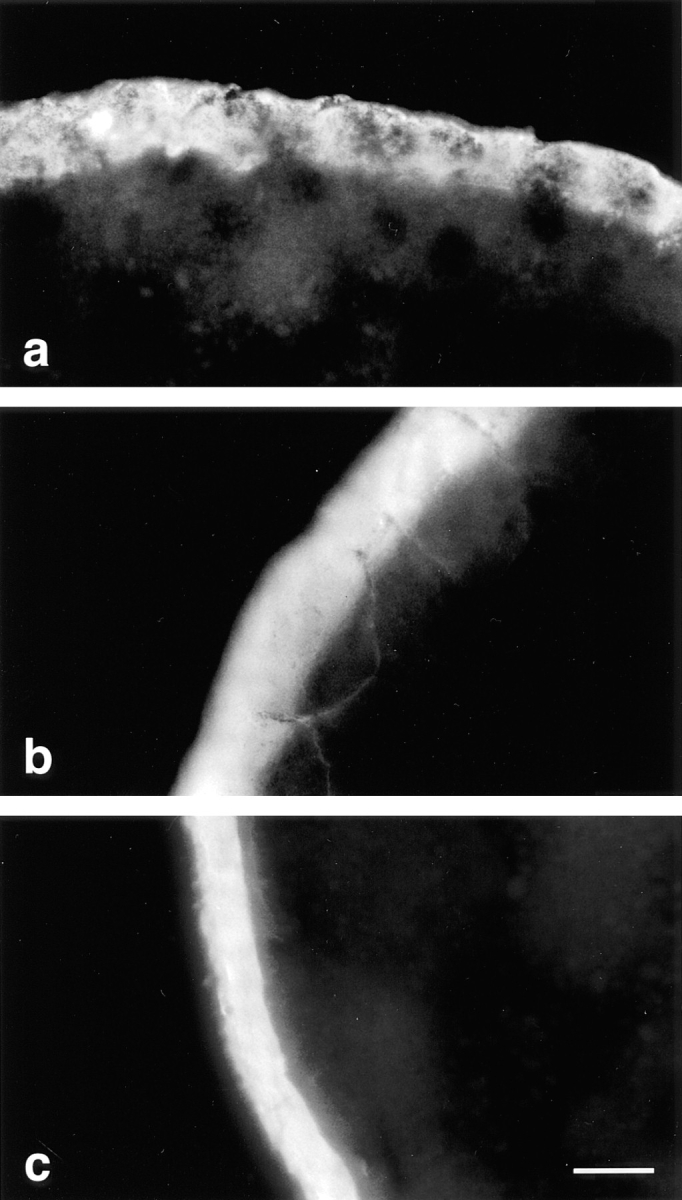
Coinjection of the full-length (504) occludin mRNA with the most COOH-terminally truncated occludin mRNA (266) rescued the tight junction leakage caused by injection of construct 266 alone. The experimental procedure was same as in Fig. 3. The frozen sections from the embryos injected with ∼2 pg of 504 (a), 266 (b), or 504 and 266 (c) construct mRNA were stained with RITC-avidin. The extraembryonic space is toward the top of figure in a, and left in b and c. No leak was seen in the embryo infected with 504 mRNA (a). Biotin molecules clearly penetrated through the tight junctions and labeled the intercellular spaces between the apical cells of embryo injected with 266 mRNA (b). However, there was no detectable leak in the embryo injected with both 504 and 266 mRNA (c). Bar, 15 μm.
Targeting of Exogenous Chicken Occludin to Tight Junctions in Early Xenopus Embryos
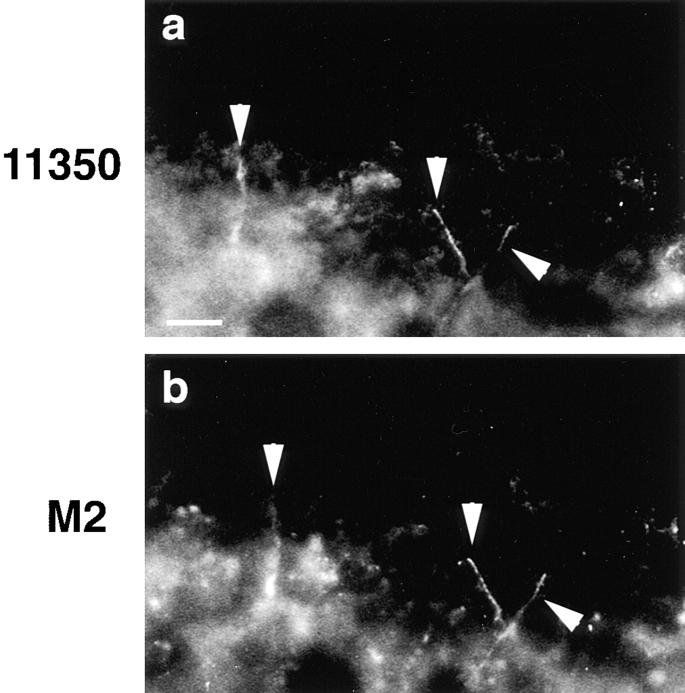
Full-length occludin, as well as all truncated mutants, could be localized to tight junctions in early Xenopus embryos except for the soluble CT. Fig. 5 shows an example of exogenous truncated occludin protein colocalized with endogenous full-length Xenopus occludin. Frozen sections of embryos injected with construct 266 mRNA (the most COOH-terminally truncated one) were double stained with anti-occludin polyclonal antibody 11350 (Fig. 5 a) and anti-FLAG monoclonal antibody M2 (Fig. 5 b). In Fig. 5, the extraembryonic space is oriented toward the top of the figure and the blastocoele is oriented toward the bottom. As the apical regions of the blastomeres remained in the plane of section for variable distances, the linear tight junction staining (Fig. 5, a and b, arrowheads) was sampled such that the staining appeared as single or branching slender threads outlining those portions of the apical cellular surfaces contained in the section. Since the anti-occludin 11350 did not recognize 266 mutant occludin (Fig. 2 a, lane 266), the signal in Fig. 5 a came from only endogenous full-length occludin, while the signal in Fig. 5 b localized the most COOH-terminally truncated exogenous occludin. In addition, all expressed exogenous proteins contained cytoplasmic staining. The colocalization of anti-occludin (Fig. 5 a) and anti-FLAG (Fig. 5 b) demonstrated that a fraction of the expressed exogenous occludin had been correctly targeted to tight junctions.
Figure 5.
Double labeling of frozen sections of Xenopus embryos with anti-occludin 11350 (a) and anti-FLAG M2 (b). 4 nl (∼10 pg) of mutant 266 mRNA were microinjected into the anterior, dorsal blastomere of eight-cell stage embryo and incubated for 8 h at room temperature before freezing. The arrowheads indicate the colocalization of Xenopus full-length occludin and chicken truncated occludin at the junctional complexes. Bar, 10 μm.
While the anti-FLAG antibody revealed threadlike staining patterns with all constructs (data not shown), double labeling with anti-FLAG and anti-occludin to demonstrate colocalization was ambiguous since the anti-occludin reagent recognized both exogenous and endogenous molecules except for construct 266 (shown in Fig. 5). For this reason, exogenous occludin mutants were colocalized with another tight junction marker ZO-1. Fig. 6 shows immunofluorescence photographs of frozen sections of an embryo injected with construct 266 mRNA, double stained with anti–ZO-1, an independent marker for the position of the tight junction (Stevenson et al., 1986), and anti-FLAG M2. The staining revealed a colocalization of the most COOH-terminally truncated exogenous occludin with endogenous ZO-1, indicating the targeting of mutant occludin to the junctional complex, even though it lacked almost the entire COOH terminus, which contains the binding domain to ZO-1. The full-length and the other four COOH-terminally truncated chicken occludin proteins were all localized at tight junctions in a similar pattern as shown in Figs. 5 and 6 (data not shown). For construct CT, which encodes a soluble COOH-terminal peptide, the anti-FLAG signal was in the cytoplasm and was not detectable at the tight junction (data not shown). By sampling embryos at different developmental stages, FLAG-tagged chicken occludins were detectable at the tight junctions by 4 h after injection. By 20 h after injection, immunofluorescence signals for the FLAG epitope were significantly decreased, which may be due to the degradation or dilution of the injected mRNA.
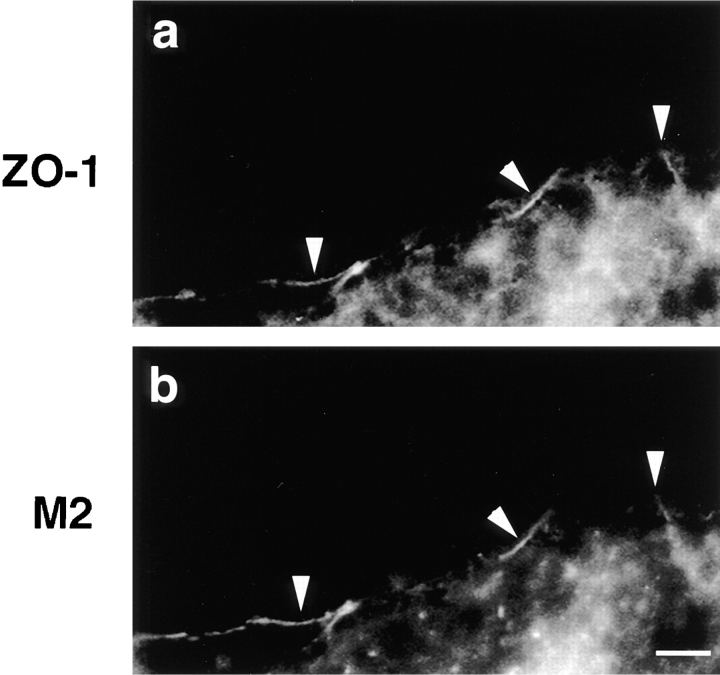
Figure 6.
Immunocolocalization of Xenopus ZO-1 with the protein directed by mutant 266, the most truncated occludin in Xenopus embryos. Frozen sections were made of embryos injected with mutant 266 mRNA and stained with either anti–ZO-1 (a) or with anti-FLAG M2 (b). The arrowheads indicate the colocalization of both proteins at the junctional complexes. Bar, 10 μm.
Immunoprecipitation Revealed Interactions between Endogenous Xenopus Occludin and Exogenous Chicken Occludin In Vivo
The fact that our polyclonal antiserum was unable to recognize construct 266, which lacked most of the cytoplasmic COOH terminus (see Fig. 2), offered an opportunity to investigate possible interactions between endogenous occludin and the mutant 266 by immunoprecipitation of detergent extracts. Embryos injected with mRNA from the construct 266 were detergent solubilized and immunoprecipitated with anti-occludin 11350 or anti-FLAG M2. After SDS-PAGE and electrophoretic transfer, the immunoprecipitates were then immunoblotted with anti-FLAG M2 or anti-occludin antibodies to probe for potential interactions between the native and truncated molecules. In Fig. 7, a and b, left lanes are immunoblots of immunoprecipitates from embryos injected with 266 mRNA; right lanes are samples from embryos without mRNA injection. Fig. 7 a (left lane) shows a 11350 immunoprecipitate blotted with anti-FLAG M2, and Fig. 7 b (left lane) shows an anti-FLAG immunoprecipitate blotted with 11350. In each case, immunoblotting reveals that each immunoprecipitate contains the alternate antigen, demonstrating that the endogenous and truncated occludins were both contained in a common detergent-soluble structure. Samples incubated with rabbit preimmune serum or with only protein A–Sepharose beads showed no specific signal. Similar coimmunoprecipitation results were obtained from oocytes injected with 266 mRNA (data not shown), indicating that the association of the mutant and endogenous occludin could occur in the absence of tight junction formation.
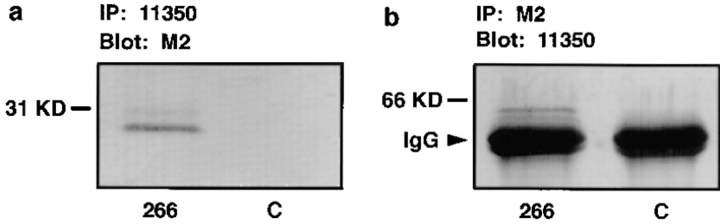
Figure 7.
The interaction of Xenopus occludin with chicken occludin in vivo assayed by immunoprecipitation. The embryos were microinjected with the most COOH-terminally truncated occludin mRNA (266; a and b, left lanes) or water (C; a and b, right lanes) and incubated at room temperature for 10 h. After homogenization, the samples were centrifuged at 100,000 g for 30 min at 4°C. The membrane pellet was solubilized in modified RIPA buffer (see Materials and Methods) and centrifuged at 100,000 g for 1 h at 4°C. The resulting supernatant was immunoprecipitated with anti-occludin 11350 (a) or anti-FLAG M2 (b) overnight at 4°C. Immunoprecipitates were immunoblotted with anti-FLAG M2 (a) or anti-occludin 11350 (b). A specific band corresponding to the chicken truncated occludin (compare with Fig. 2 b, lane 266) was present in a (left lane) after immunoprecipitated with anti-occludin 11350. The Xenopus full-length occludin was observed at b (left lane) after immunoprecipitated with anti–FLAG M2. The two thick bands in b were IgG heavy chain.
To demonstrate that the association of Xenopus occludin with chicken truncated occludin did not arise by subunit exchange between the detergent-solubilized forms of occludin during experimental manipulations, we performed an experiment in which radiolabeled, detergent-solubilized occludin from A6 cells was mixed and incubated in vitro with solubilized occludin from embryos (Fig. 8). After solubilization in detergent, the high speed supernatants from embryos injected with construct 266 mRNA were mixed with similarly solubilized [35S]methionine- labeled A6 cell lysates to test whether the radiolabeled occludin could be immunoprecipitated with the anti-FLAG antibody. The embryo and A6 detergent extracts were mixed, incubated, and then immunoprecipitated with either anti-occludin antibody (Fig. 8, left lane), anti-FLAG M2 (Fig. 8, center lane), or rabbit preimmune serum (Fig. 8, right lane). The immunoprecipitates were examined by SDS-PAGE and autoradiography. The 35S-occludin band was detectable only in the left lane (arrowhead), not in the middle or right lanes, indicating that radiolabeled occludin did not exchange between solubilized structures in detergent solutions. The top band in all three lanes was nonspecific. Therefore, the interaction of Xenopus full-length occludin with chicken truncated occludin was not an artifact of subunit exchange under detergent-solubilization conditions.
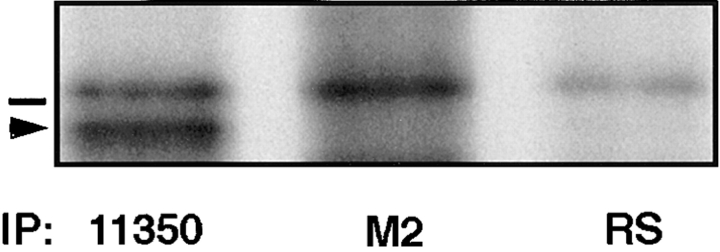
Figure 8.
Coimmunoprecipitation of the mutant with endogenous occludin was not the result of exchange of monomers between detergent-solubilized oligomeric assemblies. Confluent A6 cell monolayers were metabolically labeled with 150 μCi/ml [35S]methionine/cysteine for 20 h at 27°C, solubilized under nondenaturing conditions, and then mixed with similarly detergent-solubilized supernatant from Xenopus embryos injected with 266 construct mRNA at the two-cell stage and incubated for 10 h at room temperature. After immunoprecipitation with anti-occludin 11350 (left lane), anti-FLAG M2 (middle lane), or preimmune rabbit serum (right lane), samples were separated on 12% SDS-PAGE and autoradiographed. 35S-labeled occludin could not be immunoprecipitated with anti-FLAG M2 (middle lane), demonstrating that mixing of the mutant with wild-type molecules after extraction did not result in detergent-mediated exchange of protein monomers. The bar above the arrowhead indicates the molecular weight marker 66 kD.
Discussion
We demonstrate in the present study that expression of occludin mutants in the early Xenopus embryo resulted in the disruption of the transepithelial permeability barrier to solutes. Embryos injected with mRNAs encoding full-length occludin or the soluble occludin COOH terminus remained impermeable to a biotin tracer. Removal of the ultimate 18 amino acids of the occludin molecule was not sufficient to cause the leaky phenotype; only mutants which were truncated by 119 amino acids or more had the disruptive activity. The leaky phenotype could be rescued by coinjection of full-length and truncated occludin mRNA, indicating that the disruption of the permeability seal was not a nonspecific effect of exogenous protein expression. We also showed that the full-length and a nested set of five COOH-terminally truncated occludins were all localized at tight junctions as defined by colocalization with endogenous occludin and ZO-1. By immunoprecipitating detergent-solubilized membranes under nondenaturing conditions, we report here for the first time that exogenous truncated chicken occludin interacted with endogenous Xenopus occludin in vivo to form an oligomer during the tight junction assembly.
The mechanism by which the full-length occludin rescued the tight junction phenotype was not clear. The activity of mutant 266 was sensitive to a dilution of 1:50 but not 1:10, suggesting a competitive inhibition of the endogenous molecules. However, the oligomeric state of occludin is not known, nor is the number of copies of occludin required per oligomer to disrupt activity. Since chicken occludin may have different binding constants for amphibian molecules, it is not possible to predict the stoichiometries required for inhibition. The rescue data do demonstrate that the effects of the truncated molecules were not due to cytotoxicity or other nonspecific effects, since coexpression of full-length occludin protein can rescue the mutant phenotype.
In our studies, the FLAG signal could be detected in the tight junctions in frozen sections 4 h after RNA injection. This is consistent with the results obtained by McCarthy et al. (1996), who found chicken occludin staining in tight junctions of MDCK cells 4 h after addition of IPTG to the Lac-inducible system. Electron microscopy of Xenopus blastomeres expressing truncated occludins showed no evidence of structural perturbation of the tight junctions (data not shown), which was consistent with previously published studies in MDCK cells (Balda et al., 1996). Injected embryonic blastomeres were lineage marked by coinjection of 15 nm colloidal gold together with the mutant occludin 266 mRNA. We observed no cell death either grossly (Paul et al., 1995), or by electron microscopy (data not shown), indicating that the truncated proteins were not cytotoxic, and that the induced paracellular leaks could be tolerated by the developing embryos.
A previous study showed that chicken occludin with 120 amino acids deleted from the COOH terminus failed to localize at tight junctions in transfected MDBK cells (Furuse et al., 1994). In contrast, Balda et al. (1996) reported that in transfected MDCK cells, a chicken occludin lacking almost the entire COOH-terminal domain was efficiently transported to tight junctions. Their data indicate that this truncated occludin causes a discontinuous junctional distribution of transfected and endogenous occludin. Our results were partially consistent with Balda et al. (1996) in that COOH-terminally truncated occludin could be delivered to the tight junctions. We have not observed the discontinuous distribution pattern of mutant and endogenous occludin in Xenopus embryos. This difference could be due to the differences in the constructs used for study or in the expression systems used.
Protein oligomerization is a common step in the assembly of integral membrane proteins in cells, which usually occurs in the endoplasmic reticulum (Hurtley and Helenius, 1989) but may sometimes occur in the trans-Golgi (Musil and Goodenough, 1993). In the present study, our experimental data provide evidence that occludin formed an oligomeric complex in vivo during tight junction assembly, a finding consistent with the resolution of tight junction freeze-fracture strands as closely apposed 6–10-nm particles (Raviola et al., 1980). An intact COOH terminus on all copies of occludin in the oligomer is not required for this oligomerization process, since a truncated occludin will cooligomerize with full-length. While the intracellular location is not known, occludin oligomerization occurred in oocytes before tight junction assembly. In addition to its association with endogenous occludin, truncated occludin colocalized with ZO-1 by double immunofluorescence labeling, although it lacked a binding site for ZO-1 (Furuse et al., 1994). Taken together, these data indicate that the oligomerization of occludin occurred before the assembly of the tight junction, and that the presence of full-length endogenous occludin in the oligomeric assemblies provided the required signals for both ZO-1 binding and membrane targeting.
Acknowledgments
We would like to thank Drs. J. Goliger, J. Jiang, and A. Simon for their technical support and the members of the Paul/Goodenough laboratory for critical reading of the manuscript.
This work was supported by grant No. GM18974 from the National Institutes of Health.
Abbreviations used in this paper
- CT
COOH terminus
- GST
glutathione-S-transferase
- IPTG
isopropyl-β-d-thiogalactoside
- NHS-LC-biotin
sulfosuccinimidyl isopropyl-6-(biotinamido) Hexanoate
- TER
transepithelial resistance
- ZO
zonula occludens
Footnotes
Please address all correspondence to Daniel A. Goodenough, Department of Cell Biology, Harvard Medical School, 240 Longwood Avenue, Boston, MA 02115. Tel.: (617) 432-1652. Fax: (617) 432-2955. e-mail: dgoody@warren.med.harvard.edu
References
- Ando-Akatsuka Y, Saitou M, Hirase T, Kishi M, Sakakibara A, Itoh M, Yonemura S, Furuse M, Tsukita S. Interspecies diversity of the occludin sequence: cDNA cloning of human, mouse, dog, and rat-kangaroo homologues. J Cell Biol. 1996;133:43–47. doi: 10.1083/jcb.133.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balda MS, Whitney JA, Flores C, Gonzalez S, Cereijido M, Matter K. Functional dissociation of paracellular permeability and transepithelial electrical resistance and disruption of the apical-basolateral intramembrane diffusion barrier by expression of a mutant tight junction membrane protein. J Cell Biol. 1996;134:1031–1049. doi: 10.1083/jcb.134.4.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cereijido M, Ponce A, Gonzalez-Mariscal L. Tight junctions and apical/basolateral polarity. J Membr Biol. 1989;110:1–9. [Google Scholar]
- Citi S, Sabanay H, Jakes R, Geiger B, Kendrick-Jones J. Cingulin, a new peripheral component of tight junctions. Nature (Lond) 1988;333:272–276. doi: 10.1038/333272a0. [DOI] [PubMed] [Google Scholar]
- Claude P, Goodenough DA. Fracture faces of zonulae occludentes from “tight” and “leaky” epithelia. J Cell Biol. 1973;58:390–400. doi: 10.1083/jcb.58.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond J. The epithelial junction: bridge, gate, and fence. Physiologist. 1977;20:10–18. [PubMed] [Google Scholar]
- Duffey ME, Hainau B, Ho S, Bentzel CJ. Regulation of epithelial tight junction permeability by cyclic AMP. Nature (Lond) 1981;294:451–453. doi: 10.1038/294451a0. [DOI] [PubMed] [Google Scholar]
- Farquhar MG, Palade GE. Junctional complexes in various epithelia. J Cell Biol. 1963;17:375–412. doi: 10.1083/jcb.17.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto K. Freeze-fracture replica electron microscopy combined with SDS digestion for cytochemical labeling of integral membrane proteins. Application to the immunogold labeling of intercellular junctional complexes. J Cell Sci. 1995;108:3443–3449. doi: 10.1242/jcs.108.11.3443. [DOI] [PubMed] [Google Scholar]
- Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S, Tsukita S. Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol. 1993;123:1777–1788. doi: 10.1083/jcb.123.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse M, Itoh M, Hirase T, Nagafuchi A, Yonemura S, Tsukita S, Tsukita S. Direct association of occludin with ZO-1 and its possible involvement in the localization of occludin at tight junctions. J Cell Biol. 1994;127:1617–1626. doi: 10.1083/jcb.127.6.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodenough DA, Paul DL, Jesaitis LA. Topological distribution of two connexin32 antigenic sites in intact and split rodent hepatocyte gap junctions. J Cell Biol. 1988;107:1817–1824. doi: 10.1083/jcb.107.5.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumbiner B. Generation and maintenance of epithelial cell polarity. Curr Opin Cell Biol. 1990;2:881–887. doi: 10.1016/0955-0674(90)90087-u. [DOI] [PubMed] [Google Scholar]
- Gumbiner B, Lowenkopf T, Apatira D. Identification of a 160-kDa polypeptide that binds to the tight junction protein ZO-1. Proc Natl Acad Sci USA. 1991;88:3460–3464. doi: 10.1073/pnas.88.8.3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertzberg EL, Gilula NB. Isolation and characterization of gap junctions from rat liver. J Biol Chem. 1979;254:2138–2147. [PubMed] [Google Scholar]
- Hurtley SM, Helenius A. Protein oligomerization in the endoplasmic reticulum. Annu Rev Cell Biol. 1989;5:277–307. doi: 10.1146/annurev.cb.05.110189.001425. [DOI] [PubMed] [Google Scholar]
- Jesaitis LA, Goodenough DA. Molecular characterization and tissue distribution of ZO-2, a tight junction protein homologous to ZO-1 and the Drosophiladiscs-large tumor suppressor protein. J Cell Biol. 1994;124:949–961. doi: 10.1083/jcb.124.6.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachar B, Pinto da Silva P. Rapid massive assembly of tight junction strands. Science (Wash DC) 1981;213:541–544. doi: 10.1126/science.7244652. [DOI] [PubMed] [Google Scholar]
- Keon BH, Schafer S, Kuhn C, Grund C, Franke WW. Symplekin, a novel type of tight junction plaque protein. J Cell Biol. 1996;134:1003–1018. doi: 10.1083/jcb.134.4.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg PA, Melton DA. Functional messenger RNAs are produced by SP6 in vitro transcription of clones cDNAs. Nucleic Acids Res. 1984;12:7057–7070. doi: 10.1093/nar/12.18.7057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madara JL, Pappenheimer JR. Structural basis for physiological regulation of paracellular pathways in intestinal epithelia. J Membr Biol. 1987;100:149–164. doi: 10.1007/BF02209147. [DOI] [PubMed] [Google Scholar]
- Mazariegos MR, Tice LW, Hand AR. Alteration of tight junctional permeability in the rat parotid gland after isoproterenol stimulation. J Cell Biol. 1984;98:1865–1877. doi: 10.1083/jcb.98.5.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy KM, Skare IB, Stankewich MC, Furuse M, Tsukita S, Rogers RA, Lynch RD, Schneeberger EE. Occludin is a functional component of the tight junction. J Cell Sci. 1996;109:2287–2298. doi: 10.1242/jcs.109.9.2287. [DOI] [PubMed] [Google Scholar]
- Milks LC, Conyers GP, Cramer EB. The effect of neutrophil migration on epithelial permeability. J Cell Biol. 1986;103:2729–2738. doi: 10.1083/jcb.103.6.2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musil LS, Goodenough DA. Multisubunit assembly of an integral plasma membrane channel protein, gap junction connexin43, occurs after exit from the ER. Cell. 1993;74:1065–1077. doi: 10.1016/0092-8674(93)90728-9. [DOI] [PubMed] [Google Scholar]
- Pappenheimer JR. Physiological regulation of transepithelial impedance in the intestinal mucosa of rats and hamsters. J Membr Biol. 1987;100:137–148. doi: 10.1007/BF02209146. [DOI] [PubMed] [Google Scholar]
- Pappenheimer JR. Paracellular intestinal absorption of glucose, creatinine, and mannitol in normal animals: relation to body size. Am J Physiol. 1990;259:290–299. doi: 10.1152/ajpgi.1990.259.2.G290. [DOI] [PubMed] [Google Scholar]
- Pappenheimer JR, Reiss KZ. Contribution of solvent drag through intercellular junctions to absorption of nutrients by the small intestine of the rat. J Membr Biol. 1987;100:123–136. doi: 10.1007/BF02209145. [DOI] [PubMed] [Google Scholar]
- Paul DL, Yu K, Bruzzone R, Gimlich RL, Goodenough DA. Expression of a dominant negative inhibitor of intercellular communication in the early Xenopusembryo causes delamination and extrusion of cells. Development (Camb) 1995;121:371–381. doi: 10.1242/dev.121.2.371. [DOI] [PubMed] [Google Scholar]
- Raviola E, Goodenough DA, Raviola G. Structure of rapidly frozen gap junctions. J Cell Biol. 1980;87:273–279. doi: 10.1083/jcb.87.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuss L. Ion transport across gallbladder epithelium. Physiol Rev. 1989;69:503–545. doi: 10.1152/physrev.1989.69.2.503. [DOI] [PubMed] [Google Scholar]
- Schneeberger EE, Lynch RD. Structure, function, and regulation of cellular tight junctions. Am J Physiol. 1992;262:647–661. doi: 10.1152/ajplung.1992.262.6.L647. [DOI] [PubMed] [Google Scholar]
- Slack C, Warner AE. Intracellular and intercellular potentials in the early amphibian embryo. J Physiol. 1973;232:313–330. doi: 10.1113/jphysiol.1973.sp010272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DB, Johnson KS. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene (Amst) 1988;67:31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Stevenson BR, Siliciano JD, Mooseker MS, Goodenough DA. Identification of ZO-1: a high molecular weight polypeptide associated with the tight junction (zonula occludens)in a variety of epithelia. J Cell Biol. 1986;103:755–766. doi: 10.1083/jcb.103.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenson KI, Jordan JR, Beyer EC, Paul DL. Formation of gap junctions by expression of connexins in Xenopusoocyte pairs. Cell. 1989;57:145–155. doi: 10.1016/0092-8674(89)90180-3. [DOI] [PubMed] [Google Scholar]
- van Meer G, Gumbiner B, Simons K. The tight junction does not allow lipid molecules to diffuse from one epithelial cell to the next. Nature (Lond) 1986;322:639–641. doi: 10.1038/322639a0. [DOI] [PubMed] [Google Scholar]
- Wong V, Gumbiner BM. A synthetic peptide corresponding to the extracellular domain of occludin perturbs the tight junction permeability barrier. J Cell Biol. 1997;136:399–409. doi: 10.1083/jcb.136.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahraoui A, Joberty G, Arpin M, Fontaine JJ, Hellio R, Tavitian A, Louvard D. A small rab GTPase is distributed in cytoplasmic vesicles in nonpolarized cells but colocalizes with the tight junction marker ZO-1 in polarized epithelial cells. J Cell Biol. 1994;124:101–115. doi: 10.1083/jcb.124.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong YT, Saitoh T, Minase T, Sawada N, Enomoto K, Mori M. Monoclonal antibody 7H6 reacts with a novel tight junction-associated protein distinct from ZO-1, cingulin and ZO-2. J Cell Biol. 1993;120:477–483. doi: 10.1083/jcb.120.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]