Abstract
Syntaxins are membrane proteins involved in vesicle trafficking and are required for the release of neurotransmitter at nerve terminals. The presence of syntaxins on target membranes has been hypothesized to confer specificity to targeting and fusion via interactions with complementary vesicle-associated proteins, the synaptobrevins or VAMPS. We have mutagenized syntaxin1 in Drosophila and have found that it links the mechanism of synaptic transmission to a distinct cell biological process: the cellularization of early embryos. This specialized form of cell division separates the 6,000 nuclei of the syncytial blastoderm into separate cells through the invagination of the surface membrane of the embryo. During this process, syntaxin1 protein is present on the newly forming lateral cell surfaces and invaginating cleavage furrows. This protein is derived both from maternal deposition of mRNA and protein and from early zygotic transcription. To analyze syntaxin1's role in early development, female germ line mosaics mutant for syntaxin1 expression were generated by mitotic recombination to reduce the maternal contribution. Visualizing the actin cytoskeleton and glycosylated surface proteins reveals that embryos with insufficient syntaxin1 have large acellular patches. The patches do not appear until cellularization begins, and the process fails entirely within these regions. These results provide genetic evidence that membrane trafficking is required for the cellularization of the syncytial blastoderm. We propose that the invagination of the surface membrane proceeds by the fusion of intracellular membrane vesicles with the surface. This reaction uses the same syntaxin1 protein as is required for neurotransmitter secretion at synapses. Thus, a single syntaxin can participate in trafficking steps that are functionally as distinct as synaptic transmission and cell division.
The movement of membranes within a cell via transport vesicles is necessary for many cellular events, ranging from intracellular transport and constitutive secretion to the tightly regulated secretion of transmitter at nerve terminals (Bennett and Scheller, 1993). The molecular mechanisms underlying all such vesicle trafficking appear to be analogous, involving specific protein-mediated interactions between the transport vesicle and the acceptor membrane (Rothman and Wieland, 1996).
Syntaxins and related proteins that are collectively referred to as t-SNAREs reside on target membranes and are hypothesized to serve as address labels that identify a membrane compartment (for review see Sollner and Rothman, 1994). According to this hypothesis, the specificity of vesicular targeting arises from the interaction of a t-SNARE with its counterpart v-SNARE on the transport vesicle. In yeast, homologues of syntaxins are necessary for ER to Golgi (Hardwick and Pelham, 1992), Golgi to plasma membrane (Aalto et al., 1993), and vacuolar trafficking (Piper et al., 1994).
In nerve terminals, syntaxins are present on the presynaptic membrane and are known to interact with other proteins implicated in vesicular release (Bennett et al., 1992; for review see Sudhof, 1995). The counterpart v-SNARE for syntaxin is the synaptic vesicle protein synaptobrevin, also called VAMP, which has been shown to bind syntaxin (Calakos et al., 1994). Another vesicular protein, synaptotagmin, also binds to syntaxin (Kee and Scheller, 1996), as does the plasma membrane protein SNAP-25 and the cytosolic protein nsec1 (Pevsner et al., 1994). Additional cytosolic factors including NSF and α, β, and γ Snap can also be found in a larger complex containing syntaxin (Sollner et al., 1993a ,b).
Syntaxin's essential role in synaptic transmission has been shown using both neurotoxins and genetic experiments. Fusion of synaptic vesicles with the terminal membrane can be blocked by the syntaxin-specific protease botulinum toxin C1 (Blasi et al., 1993; Schiavo et al., 1995). A Drosophila homologue referred to as syntaxin1 (syx)1 was recently cloned (Cerezo et al., 1995; Parfitt et al., 1995; Schulze et al., 1995). Mutations in this gene are homozygous lethal, with severe alleles dying as late embryos. The loss of syx abolishes synaptic transmission; release could not be evoked by either electrical stimulation or black widow spider venom, and spontaneous vesicle fusions were absent (Broadie et al., 1995; Schulze et al., 1995). Other secretion phenotypes, such as a soft cuticle and undigested yolk, were also reported in the syntaxin1 mutants (Schulze et al., 1995). Interestingly, the genetic removal of syntaxin1 did not disrupt the ability of vesicles to be targeted to and morphologically docked at the nerve terminal membrane (Broadie et al., 1995). Thus, although this protein is clearly essential for synaptic transmission, its precise role in the targeting and fusion of vesicles remains uncertain.
While analyzing transcripts from the syntaxin1 gene, we observed the presence of syntaxin1 message at the earliest stages of Drosophila development, in embryos <3 h old (Parfitt et al., 1995). The presence of transcript at these times, when no neurons have differentiated and the cuticle has not yet been secreted, suggested a new and distinct role for syntaxin1 in development. A potential maternal contribution of mRNA is suggested by the transcript analysis, and therefore, the importance of syntaxin1 in early development may have been underestimated. As described below, we have obtained evidence that syntaxin1 functions in a specialized form of cytokinesis, the cellularization of the syncytial blastoderm, and mutations in this protein provide an opportunity to study the significance and mechanism of membrane addition in cellularization.
The cellularization of Drosophila embryos involves extensive membrane rearrangements. Before cellularization, the Drosophila embryo undergoes 13 synchronized cycles of nuclear divisions that are not accompanied by cytokinesis (Foe and Alberts, 1983; for comprehensive review see Foe et al., 1993). By 1.5 h after egg deposition (AED), the nuclei that will form the somatic tissues of the fly have migrated out to the cortex of the embryo to form the syncytial blastoderm. Cellularization occurs between 2 and 3 h AED (during the 14th mitotic cycle) as membranes from the surface of the embryo grow inward and segregate each of the 6,000 nuclei into individual cells. This process is known to involve the rearrangement of several cytoskeletal and cytoskeleton-associated proteins. Mutations in the genes encoding these proteins can interfere with cellularization (for review see Schejter and Wieschaus, 1993; Theurkauf, 1994) and have been isolated in screens for both maternal effect and zygotic developmental mutations.
The formation of the 6,000 cells has been estimated to require a 23-fold increase in plasma membrane area compared to that of the syncytial embryo (Foe et al., 1993). The source of the additional membrane has been uncertain. Two possibilities have been considered: the resorbtion of microvilli on the outer surface of the embryo and the addition of membranes from intracellular pools (Fullilove and Jacobson, 1971; Sanders, 1975; Turner and Mahowald, 1976; Loncar and Singer, 1995). The presence of transcripts for a t-SNARE such as syntaxin1 in early embryos suggested an opportunity to address the role of vesicle trafficking and membrane addition during cellularization and to investigate a novel function of syntaxin1.
We have examined the distribution of syntaxin1 in the early embryo and have generated homozygous mutant clones in the female germ line to reduce the maternal deposition of syntaxin1. Embryos derived from such clones showed defects in cellularization if the embryos were also zygotically mutant for syntaxin1. The results indicate that syntaxin1 is essential for proper cellularization as well as neurotransmitter release (Schulze et al., 1995). Vesicle trafficking is therefore a crucial component of membrane addition during cellularization, and this example of cytokinesis appears to share an underlying molecular mechanism with other forms of vesicle transport. The data also illustrate that two different vesicle classes involved in separate cell biological processes can employ the same t-SNARE, syntaxin1.
Materials and Methods
Western Blotting
Adult heads or embryos from timed collections were homogenized in 2× Laemmli sample buffer (0.0625 M Tris, pH 6.8, 4% SDS, 10% glycerol) and boiled for 2 min. Protein extracts were separated by SDS-PAGE using 12% acrylamide gels and transferred to Immobilon membranes (Millipore Corp., Bedford, MA). The blots were blocked overnight at 4°C in PBS (140 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.8 mM KH2PO4, pH 7.3) with 5% milk, 1% BSA, 0.1% Tween-20, and 2% normal goat serum. They were then probed with monoclonal antibody 8C3 at 1:10 dilution (kind gift of Dr. Seymour Benzer, California Institute of Technology, Pasadena, CA) and developed with the enhanced chemiluminescence kit, using a secondary antibody of goat anti–mouse IgG at 1:20,000 (Amersham Corp., Arlington Heights, FL). All washes and dilutions were done in PBS, 0.1% Tween 20. Protein concentrations were assayed using the BCA protein assay kit (Pierce, Rockford, IL). Protein loading was also assayed by reprobing the blot shown in Fig. 3 with a rabbit polyclonal antibody against zest-white3 (gift of Roel Nusse, Stanford University, Stanford, CA).
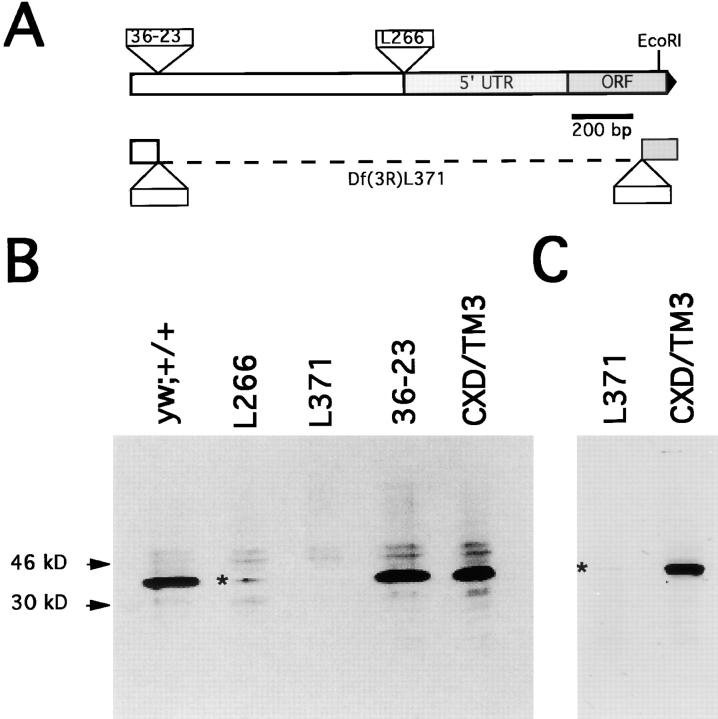
Figure 3.
(A) P-element insertions in the syntaxin1 transcription unit. The P36-23 P-element insertion is located ∼1,500 bp upstream of the syntaxin1 open reading frame and is homozygous viable. This insertion was mobilized to generate a series of P-element insertions that disrupt the syntaxin1 transcription unit and are homozygous lethal. The allele syx L266 carries both the original P36-23 insertion and a second insertion in or immediately adjacent to the 5′ untranslated sequence. syx L371 has two P-elements: the original P36-23 and a second, within the syntaxin1 open reading frame, interrupting the codon for amino acid K95. The intervening sequence is deleted as determined by Southern blotting, PCR, and plasmid rescue of the insertions. (B) Syntaxin1 protein in mutant embryos. In late stage embryos homozygous for the parent chromosome P36-23, the protein is present at normal levels, by comparison to control strains (CXD/TM3 or yw). The protein is dramatically reduced in syx L266/syx L266 and syx L371/syx L371 alleles. The syx L266/syx L266 level is higher than that of syx L371/ syx L371; this allele therefore produces a small amount of full-length protein. Full-length syntaxin1 is present in syx L371/syx L371 embryos because of the remaining maternal contribution and can be seen on longer exposures of the gel in B (not shown) or on gels with additional protein layered per lane (C). Homozygous mutant embryos from heterozygous parents were selected based on their failure to hatch after 27–33 h for both the syx L266 and the syx L371 alleles. Age-matched embryos that failed to hatch from CXD/TM3 heterozygous parents were used as a control. Homozygous P36-23 and yw embryos were collected at 24 h. 60 μg of protein was layered per lane in B, corresponding to ∼25 embryos. The protein extracted from 45 embryos was loaded in each lane of C.
Immunocytochemistry and Embryo Staining
For the localization of syntaxin1 during cellularization, wild-type embryos were collected overnight, dechorionated in 50% bleach for 2 min, added to methanol containing 25 mM EGTA and heptane, and shaken vigorously. The embryos were then allowed to stand for 3 h at room temperature, and those that lost the vitelline (those that sank) were collected for staining and rehydrated in PBT (PBS with 0.1% Triton X-100, 1% BSA, and 2% normal goat serum). The 8C3 monoclonal antibody was diluted 1:5 in PBT and incubated with the embryos overnight at 4°C. A Texas red– conjugated goat anti–mouse IgG secondary antibody was used for detection at a 1:200 dilution. The specificity of the syntaxin1 staining pattern was determined in side-by-side comparisons with and without 8C3 and by comparison to armadillo and engrailed monoclonal antibodies provided by Dr. Roel Nusse. The same staining pattern was also obtained using an FITC-conjugated secondary.
Staining for anillin was performed using a rabbit polyclonal antibody against anillin (kind gift of Dr. Chris Field, University of California, San Francisco, CA). For double labeling with phalloidin, embryos were prepared as described below, and the antibody was applied at a 1:1,000 to 1:3,000 dilution in PBT. This was visualized with an FITC goat anti–rabbit secondary antibody at 1:200 dilution. In double labels with 8C3, embryos were prepared as above. Both primary antibodies were applied at once, but the antianillin was diluted to 1:12,000, and the FITC-conjugated anti– rabbit secondary antibody was used at 1:500.
Staining with rhodamine- or FITC-conjugated phalloidin and Hoechst 33258 was performed according to published protocols (Ashburner, 1989). In brief, embryos were devitellinized in 50% bleach and fixed in 8% formaldehyde in PBS and heptane for 45 min. The embryos were then stuck to double-sided tape and immersed in 4 or 8% formaldehyde in PBS. The vitelline membranes were removed by hand. The embryos were then stained in phalloidin for 15 min, rinsed, and stained in Hoechst 33258 (1 μg/ml) for 4 min. For concanavalinA (conA) labeling, FITC-conjugated conA at 200 μg/ml was included with the phalloidin. During conA labeling, no detergents were applied. For antianillin and phalloidin double labeling, the embryos were moved from 4% formaldehyde in PBS to PBT and stained as described above. The phalloidin was included with the secondary antibody. Phalloidin, conA, and Hoechst 33258 were all obtained from Molecular Probes (Eugene, OR).
Staining whole-mount embryos for β-galactosidase activity using X-gal was performed according to Ashburner (1989). Ovaries were dissected from adult females of matched age for each genotype. The staining protocols used are the same as those used for embryos.
P-element Mutagenesis
A molecular screen for P-element insertions based on Zinsmaier et al. (1994) was performed. In brief, P-elements (P[w +]) marked with w + and carrying lacZ and the pUC sequences oriC and ampr were mobilized from a w − X chromosome (Bier et al., 1989) by crossing to flies with a TMS, Sb, Δ2-3 third chromosome, which provides a constant source of transposase. Males carrying both the X chromosome bearing the P-elements and the TMS chromosome were then mated to yw females. Male progeny of this cross with w + eye color carried novel insertions on the autosomes or Y. Pools of 50 males without the TMS chromosome and carrying novel P[w +] insertions were crossed to 200 yw females. Embryos were collected overnight from each of these pools. The embryos were then homogenized, and the genomic DNA was purified (Kaiser and Goodwin, 1990). Plasmids were rescued from the genomic DNA by digesting with EcoRI, which cuts in the P-element upstream of the pUC sequences and again in the flanking genomic DNA. These fragments were then diluted and recircularized with T4 DNA ligase. The resulting plasmids were then used to transform Electromax DH10B Escherichia coli (GIBCO BRL, Gaithersburg, MD), which were plated on LB agar plates containing 100 μg/ml ampicillin. For each pool, DNA from the resulting transformants was purified, digested with EcoRI, and screened on Southern blots by probing with the syntaxin1 cDNA. A hybridization signal indicated that a male in the pool carried a P-element insertion that flanked the syntaxin1 gene. Individual males from positive pools were then used to establish stocks, which were analyzed individually to isolate the chromosome carrying the insertion. Three insertions 1.5, 2.5, and 9 kb upstream of the syntaxin1 open reading frame were isolated from the 4,000 males screened.
To generate insertions in the syntaxin1 gene itself, the closest of these insertions, P36-23, which is homozygous viable, was remobilized. Such a “local hop screen” takes advantage of the observation that P-elements preferentially reinsert near their original site upon remobilization (Tower et al., 1993). From the offspring of flies carrying both the P36-23 insertion and the TMS balancer, males with new insertions were identified by darker eye color and used to establish stocks. These males were then recovered and analyzed by PCR using primers to the 31-bp inverted repeat of the P-element (O'Hare and Rubin, 1983) and to the syntaxin1 open reading frame to determine the location of the new insertion. Under standard conditions (1.5 mM MgCl2, using Tfl polymerase [Epicentre Technologies, Madison, WI]), amplification from the P36-23 insertion to the EcoRI site in the syx open reading frame was routinely possible. New insertions closer to the open reading frame were therefore easily identified using the same primers. From 1,100 flies, 11 bore insertions less than 1.5 kb from the open reading frame. All of these were homozygous lethal. Two of these insertions, syx L371 and syx L266, were used in the present study.
To characterize the molecular defects in the alleles syx L371 and syx L266, we used a combination of approaches. These included sequencing the genomic DNA surrounding the insertion site as recovered in the rescued plasmid, Southern blots of genomic DNA, and PCR between the P-element and coding regions of the syntaxin1 gene.
Generation of Germ Line Clones
The FLP/FRT, ovo D1 system was used to generate female germ line clones mutant for syntaxin1, which is located at 95E on chromosome 3R. In brief, the FLP recombinase system from yeast 2 μ plasmids as transferred to Drosophila (Golic and Lindquist, 1989) was used to induce mitotic recombinations. FLP recombinase on the X chromosome was driven by the heat shock promoter to induce recombination at FRT sequences inserted near the centromere, at 82B (Xu and Rubin, 1993). The production of germ line clones was made easier by the introduction of two copies of ovo D1, a dominant mutation that causes the female germ line to degenerate, onto chromosome 3R (Chou et al., 1993). Females were constructed that are heterozygous for syx alleles over ovo D1, both with 82B FRT sequences, and heat shock FLP on the X. The only oocytes produced in such a female must come from a recombinant germ line clone that is homozygous for syx alleles and lacking ovo D1 because both heterozygous and homozygous ovo D1-bearing clones degenerate. Clones were successfully induced by heat shocking at 37°C for 1 h each day for 3 d during the third instar larval or early pupal stages.
Fly Strains
All the strains necessary for the generation of germ line clones may be obtained from the Bloomington stock center (Bloomington, IN). Those used include: w; P{[w +]82B-FRT} P[w +]90E as the source of the 82BFRT chromosome onto which the syx alleles were recombined, yw P{[ry +] hsFLP}; Dr/TM3 as the X chromosome source of heat shock–driven transposase, and w; P{[w +]82BFRT} P{[w +]ovo D1}3R1 P{[w +]ovo D1}3R2/ru h st β-Tub85DD ss e/TM3 as the ovo D1 source used for negative selection in the female germ line. The TM3, lacZ chromosome used carries a P[ry, deformed-lacZ] construct and was kindly provided by David Bilder and Matthew Scott (Stanford University). This construct expresses lacZ in the anterior segments of the embryo. The P-element–laden X-chromosome used in the generation of insertions near syx was obtained from Konrad Zinsmaier (then with Seymour Benzer, Cal. Tech.). The TMS balancer was obtained from the Bloomington stock center.
Results
Syntaxin1 Is Present in the Early Embryo
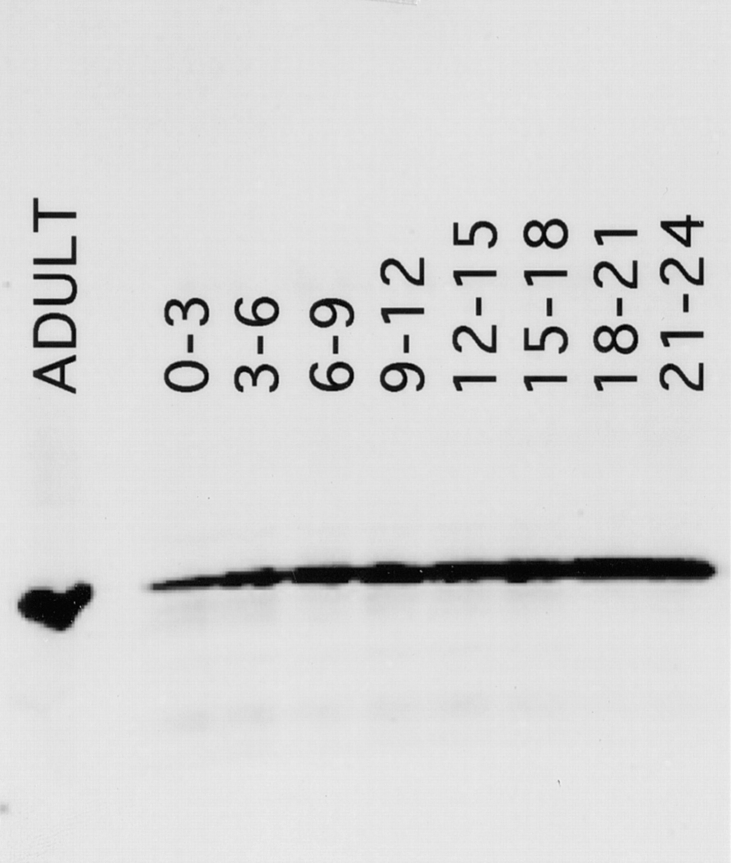
Previously reported mRNA analysis suggested that syntaxin1 mRNA is present in the early embryo, possibly because of maternal deposition into the oocyte (Parfitt et al., 1995; Schulze et al., 1995). We have extended this finding by examining syntaxin1 protein on Western blots of extracts from timed embryo collections (Fig. 1). The monoclonal antibody 8C3 detects syntaxin1 in 0–3-h-old embryos. This period precedes the development of the nervous system and corresponds to the time during which cellularization occurs and zygotic transcription begins (2 h AED; Foe et al., 1993). The amount of syntaxin1 per embryo increases steadily thereafter until approximately the time when the nervous system matures. At all stages, the embryonic syntaxin1 protein comigrates with the neuronal syntaxin1 observed in adult heads. Thus, although the size of syntaxin1 transcripts varies during development (Parfitt et al., 1995), the protein product appears identical at all stages. This finding is consistent with the reported lack of introns in the open reading frame of the gene (Schulze et al., 1995).
Figure 1.
Syntaxin1 levels during embryogenesis. Protein was extracted from timed embryo collections of the ages indicated (hours AED) and from adult heads and separated by SDS-PAGE. Per lane, the protein extracted from 30 embryos or one half of an adult head was loaded. The gels were blotted onto Immobilon membranes and were then probed with the monoclonal antibody 8C3. Syntaxin1 is present in 0–3-h embryos, the stage during which cellularization occurs, and levels increase as the nervous system matures. In both embryos and adults, the protein has a molecular mass of 35 kD, as predicted.
Syntaxin1 Localization in the Early Embryo
The presence of syntaxin1 at the onset of embryogenesis led us to hypothesize that this gene plays a role in early development. For an indication of its function, we examined the distribution of the protein by immunofluorescence and confocal microscopy. With the onset of cellularization during cycle 14, syntaxin1 immunoreactivity is observed on the newly forming lateral cell membranes as soon as they appear (Fig. 2, A and B). The immunoreactivity is present along the entire length of the lateral membrane, from the embryo's surface to the tip of the cleavage furrow (Fig. 2, B and C). The position of the tip of the furrow was determined either by Nomarski viewing of the embryos or by double labeling with an antibody against anillin, a cytoskeletal-associated protein that is present at the leading tip (Field and Alberts, 1995). If membrane addition during cellularization occurs by means of vesicle fusion, the lateral membranes and cleavage furrows would constitute the probable acceptor membranes for the fusion events. This localization of syntaxin1 is therefore consistent with a role in the cellularization of the embryos.
Figure 2.
Syntaxin1 localization by antibody staining and confocal microscopy in whole-mount embryos. (A and B) Embryos in early stages of cellularization. As the invaginating cleavage furrows form, syntaxin1 is localized to the newly formed furrows seen in superficial (A) or deep (B) confocal sections. (C) Syntaxin1 distribution as cellularization nears completion. When the cleavage furrows have progressed well beyond the nuclei, syntaxin1 is present along the length of the lateral membrane. Antisyntaxin immunostaining was performed with monoclonal antibody 8C3. The signal was visualized with a Texas red–conjugated secondary (see Materials and Methods). Bar, 10 μm.
Before cellularization, we did not discern syntaxin1 immunoreactivity on the surface of the embryo at a level that was above background. It is possible that some protein was present on the surface at this stage, but it is unlikely that most of the protein that later is visible on the lateral membranes derives from a surface pool. We surmise, therefore, that much of the syntaxin1 is added to the lateral membranes as they form. This hypothesis is consistent with genetic data, presented below, that zygotically transcribed syntaxin1 can contribute significantly to cellularization.
In later embryos, the syntaxin1 staining is strongest in synaptic regions of the nervous system, but significantly, the protein is still present on axons, cell bodies, and nonneuronal structures as well (data not shown).
The Generation of P-element Insertions in syx
To mutate syx, we performed a genetic screen that produced a collection of P-element insertions near the gene. The closest of these insertions, P36-23, was remobilized to generate 11 syntaxin1 alleles (see Materials and Methods) (Fig. 3 A). The P36-23 insertion does not alter the quantity of syntaxin1 per embryo, compared to either yw or CXD/ TM3 control embryos (Fig. 3 B). syx L371 and syx L266, two severe alleles that were derived from P36-23, were used in the present study. In syx L371, two P-elements in tandem flank a deletion of ∼1,700 bp that removes a 5′ untranslated region and the first one third of the open reading frame (through lysine 95). We consider syx L371 to be a null allele because it is missing the highly conserved H1 and H2 domains (Kee et al., 1995) and because the next available ATG for translation initiation is at amino acid 134, nearly half way through the open reading frame. While a truncated form of this protein may be produced but not recognized by our antibody, genetic evidence presented below does not indicate any dominant effects of this allele: the observed phenotypes are recessive and rescued by a syntaxin1 cDNA transgene. In syx L266, in addition to the original P36-23 insertion, a second P-element is inserted within a few bases of the 5′ most untranslated cDNA sequence we have obtained.
Examination of the protein expressed by these alleles is consistent with the molecular and genetic data that syx L266 is a hypomorphic allele still making wild-type protein. syx L266 homozygotes contain more protein than the null (syx L371) embryos (Fig. 3 B). Nevertheless, embryos homozygous for syx L371 contain detectable levels of full-length protein seen on longer exposures of Fig. 3 B (not shown) or on blots in which more protein is loaded (Fig. 3 C). Since this allele is incapable of making full-length protein, this signal can be attributed to maternally deposited mRNA and protein. The homozygous syx − embryos in Fig. 3, B and C, were selected based on their failure to hatch after 27 to 33 h after egg deposition. The maternally derived protein had therefore persisted throughout embryonic development.
Syntaxin1 Nulls Appear to Be Cell Lethal
To eliminate the maternally deposited syntaxin1, homozygous mutant clones in the female germ line were made by mitotic recombination with the ovo D1, FLP/FRT recombinase system (see Materials and Methods). Using this system, the only oocytes produced are from the homozygous syntaxin1-mutant clones in the female ovary, while any ovo D1-expressing ovarioles, the gamete-producing structures of the ovary, fail to mature (Fig. 4 A). This approach was applied to both syx L266 and syx L371 alleles, as well as to P36-23, the parent chromosome, as a control. Both P36-23 and syx L266 produce viable clones in the ovaries. For both lines, 80% of the females examined had mature ovarioles (Fig. 4, B and C). In contrast, females with syx L371 clones fail to produce any oocytes, and the ovaries of these females are rudimentary, like those of ovo D1 females (Fig. 4 D) (Spradling, 1993). The failure of the ovarioles to mature and the hypertrophy of the ovaries suggests that this allele causes cell lethality and therefore degeneration of the syntaxin1 null cells in a background of rudimentary ovarioles produced by the ovo D1 tissues. This interpretation is supported by the absence of any ovarioles clearly lacking syntaxin1 protein based upon staining with the 8C3 antibody. Syntaxin1 is present on the membranes of nurse cells in the early germarium, before stage 8 when the oocyte begins to enlarge (not shown and Schulze and Bellen, 1996). The degeneration or rudimentary nature of the syx L371 clones may be overcome by the expression of a syntaxin1 transgene on the second chromosome (Fig. 4, E and F). Females carrying this transgene develop mature ovarioles that produce viable oocytes (Fig. 4 E), while the ovaries of siblings that carry a balancer second chromosome (lacking the transgene) fail to develop (Fig. 4 F). Thus, the cell lethality of the syx L371 chromosome is entirely attributable to the mutation in syntaxin1. Moreover, since the effect is rescued by the expression of a wild-type transgene and is not seen in syx L371 heterozygotes, the cell lethal phenotype is not due to a dominant effect of the syx L371 allele.
Figure 4.

Ovaries from mitotic recombinations in syx/ovo D1 heterozygotes. ovo D1 is a dominant mutation that arrests ovariole development before stage 8. Examples of these rudimentary ovarioles are marked throughout with arrowheads and can be seen in A. Recombination in a P36-23/ovo D1 fly produces P36-23 homozygous clones in which follicles reach maturity and oocytes are produced (B, and marked with arrows throughout) alongside ovo D1-arrested ovaries. Mature ovarioles are also seen in syx L266 clones (C) but were not seen in syxL371 clones (D). syx L371 is therefore either a rudimentary ovary mutant comparable to ovo D1, or syx L371 is cell lethal, leaving only ovo D1 follicles. Mature oocytes are produced from syx L371 ovary clones that express syx on a second chromosome transgene (E). The genotype of these flies is HS-FLP; HS-GAL4:UAS-syx; syx L371-82BFRT/ovo D1-82BFRT. Siblings of these flies that do not carry the transgene show no developed ovarioles (F). All of the ovaries shown were stained with FITC-conjugated phalloidin to allow visualization of the ovarioles. Bar, 200 μm.
Missing bristles were also observed in females with homozygous syx L371 clones, suggesting that the complete absence of syntaxin1 was lethal in other tissues as well (data not shown). This finding is also consistent with the recent observations of others (Schulze and Bellen, 1996). To circumvent the cell lethality, our analysis of syntaxin1's role in development, therefore, had to be done using the hypomorphic syx L266 allele.
Developmental Defects Require Both Maternal and Zygotically Derived Syntaxin1 to Be Mutant
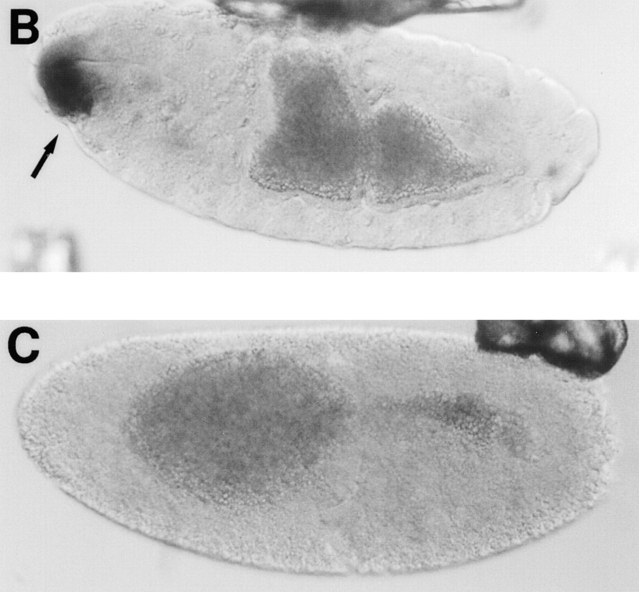
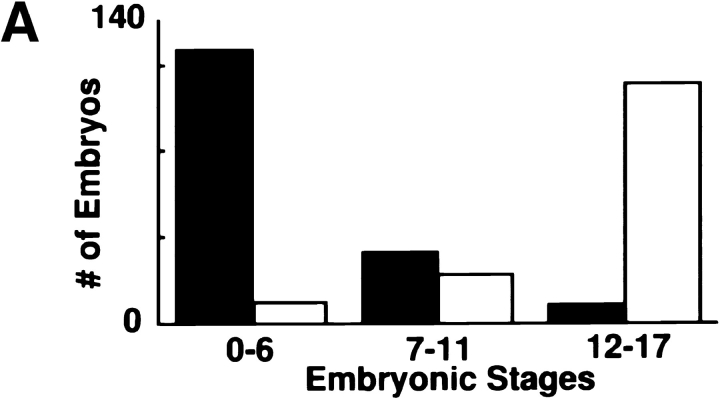
syx L266 clones in the mother's germline provide sufficient syntaxin1 to overcome the apparent cell lethality, but not enough to sustain normal embryogenesis. With syx L266 and the ovo D1, FLP/FRT system described above, we collected embryos that all had reduced maternally deposited syntaxin1 because of the homozygous syx L266 mutation in the maternal germline. Frequently, over 50% of eggs deposited by these mothers were unfertilized. This may be due to defects in the ability of the mutant ovarioles to properly form an oocyte, even though the ovarioles are outwardly normal. Nevertheless, many of the eggs from these clones were fertilized and could thus be used to examine the early developmental role of syntaxin1. Females carrying the homozygous syx L266 clones were crossed to syx L371/TM3, lacZ males, so that the offspring that received the syx + balancer chromosome (TM3, lacZ) from the father could be distinguished from those that received the mutant chromosome by staining for lacZ. Fertilized embryos were allowed to develop for 14–17 h. Normally developing embryos would progress to stages 16 or 17 in this interval and would have extensively differentiated internal structures (Campos- Ortega and Hartenstein, 1985). The embryos from the above cross, however, fell into two categories: (a) viable, normally developing embryos and (b) embryos that arrested very early, usually before germ band extension (Fig. 5 A). The fate of the embryo depended on the paternal chromosome it had received; the viable class bore the syx +, TM3lacZ + paternal chromosome (Fig. 5 B), while the defective embryos had received the syx L371 chromosome. A representative syx L266/syx L371 (lacZ −) embryo is shown in Fig. 5 C. The embryo is arrested in its development; no internal organs can be distinguished, and the embryo appears not to have undergone proper germ band extension. The anterior distribution of yolk suggests that some morphogenetic movements similar to those of germ band extension had occurred. Those few lacZ − embryos that did progress as far as germ band retraction typically arrested before stage 16. The lacZ positive and negative embryos appeared in the expected Mendelian ratio (144 lacZ-positive, 169-lacZ negative embryos scored). These results indicate that syntaxin1 expression from the embryo's paternal chromosome is sufficient to rescue the phenotype. In parallel control experiments, all embryos derived from clones of the P36-23 control chromosome were fertilized and developed normally, regardless of the paternal chromosome inherited (94 lacZ-negative and 91 lacZ-positive embryos scored).
Figure 5.
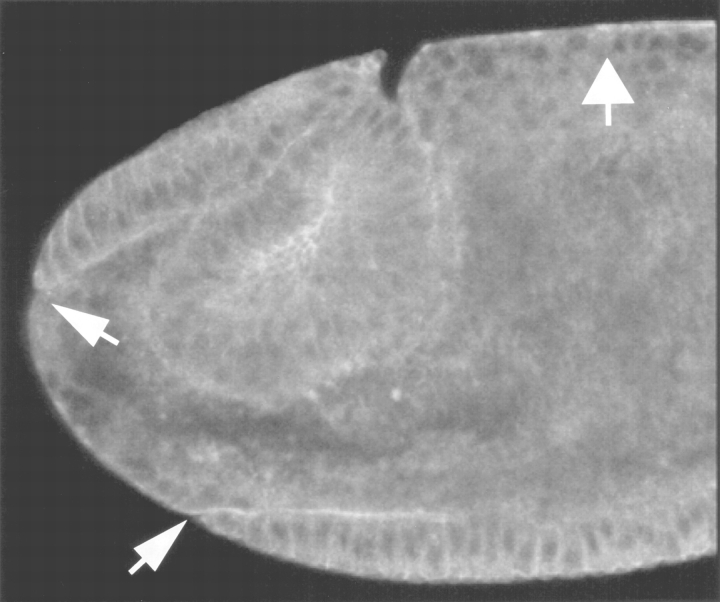
Abnormalities in developing embryos are manifest only in those that are maternally and zygotically mutant for syntaxin1. (A) The early lethality of embryos from syx L266/syx L266 maternal germ line clones is dependent on inheriting a syx L371 chromosome from the father. Females bearing homozygous syx L266 germ line clones were crossed to syx L371/TM3, lacZ males. 14–17-h-old embryos were collected, examined to determine if they had successfully progressed through development, and stained with X-gal to determine which embryos received the paternal syx + chromosome (white bars) and which had received the syx L371 chromosome (black bars). (B) Embryos that received the syx + paternal chromosome (lacZ positive, arrow) developed generally normally, with the majority reaching the appropriate stages (16–17) (144 embryos examined). (C) Those embryos with the syx L371 paternal chromosome (lacZ negative) arrested at very early stages, typically at or before germ band extension (169 embryos examined). As shown, the position of the yolk often suggested that some morphogenetic movements had been attempted, but normal germ band extension had not occurred and there had been no subsequent elaboration of internal organs. Anterior is to the left in both pictures. The dark, grainy material in the center of each embryo is the yolk.
Mutant Embryos Show Large Acellular Patches
To understand the phenotype of embryos such as the one shown in Fig. 5 C at the cellular level, we analyzed a number of cellular components, including the nucleus, cytoskeleton, and surface membrane. During mitotic cycle 14, embryos derived from P36-23 germ line clones cellularize normally (Fig. 6, A and B). The nuclei, stained with Hoechst 33258, are enveloped by cell membranes that invaginate inwards from the embryo's surface, and filamentous actin, stained with rhodamine-conjugated phalloidin, becomes associated with the plasma membrane of each cell. However, in embryos derived from syx L266 germ line clones, large acellular patches are apparent in half of the embryos. Based upon the results shown in Fig. 5, we conclude that these embryos correspond to those inheriting the paternal syx L371 chromosome. Embryos with comparably sized patches were obtained when females with syx L266 germ line clones were crossed to either syx L371/TM3 or syx L266/TM3 males. This result indicates the defect is not specific for the syx L371 paternal chromosome but is instead the result of a combination of reduced maternal and zygotic syntaxin1. Embryos from the P36-23 clones never showed defects such as this. Within these aberrant patches, the actin cytoskeleton that lines lateral membranes is completely absent (Fig. 6 C), and the nuclei are disorganized and begin to fall away from the surface of the embryo (Fig. 6 D). The regions of disorganized nuclei and of absent lateral actin correspond exactly (Fig. 6, C and D, large arrows). In addition to large patches, occasional smaller defects are also seen (Fig. 6 C, small arrow).
Figure 6.
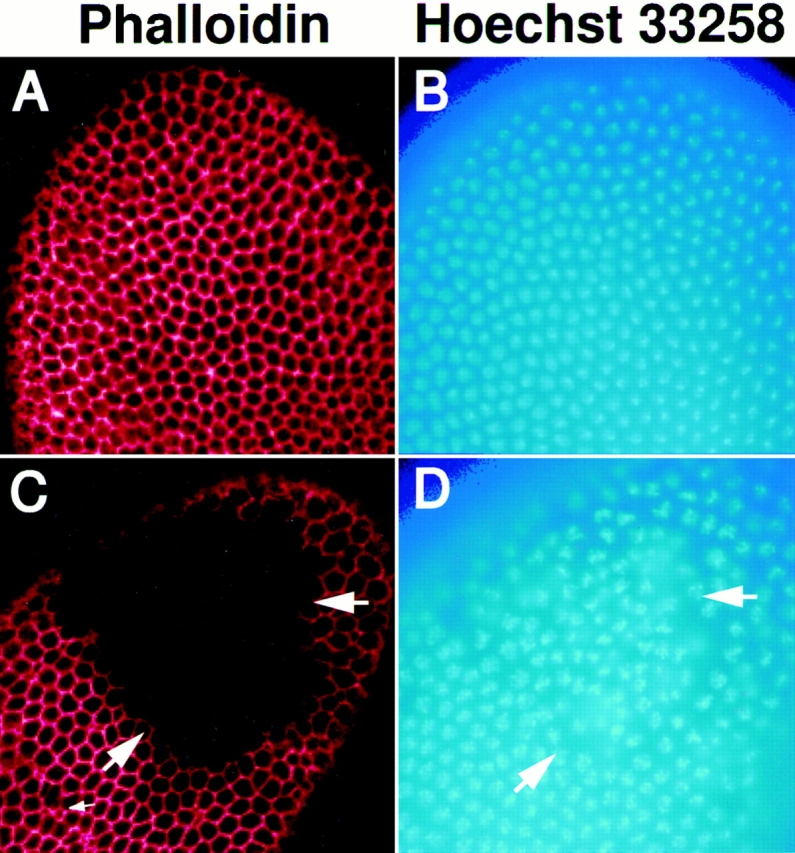
The disruption of cellularization by mutations in syntaxin1 can be seen in the distribution of nuclei and filamentous actin. Females with germ line clones homozygous for either syx L266 (C and D) or the P36-23 control chromosome (A and B) were crossed to syx L371/ TM3, lacZ males and examined with Hoechst 33258 to visualize nuclei (blue) and with rhodamine phalloidin to visualize actin (red). During cellularization, embryos from P36-23 clones (A and B) continue to develop normally; however, half of the embryos from syx L266 clones (C and D) show large acellular patches in which the nuclei were disorganized (D) and the network of filamentous actin was completely absent (C). The disorganization of the nuclei and cytoskeleton corresponded exactly (large arrows). In addition, small defects are also sometimes seen (small arrow, C). Rhodamine phalloidin images were gathered by confocal microscopy.
The defects are also seen in the morphology of the surface membrane in the regions that appeared to be acellular. conA was used to stain glycosylated surface membrane proteins. On the surface of a wild-type embryo, the conA and phalloidin staining overlap and reveal the honeycomb pattern of developing cell membranes (Fig. 7, A and B). In patches on an embryo from a syx L266 germ line clone, neither the cytoskeleton nor the surface membrane give any indication that membranes are forming to segregate each nucleus (Fig. 7, C and D). Therefore, it appears that these patches are truly acellular and represent areas where no membrane invagination is occurring.
Figure 7.
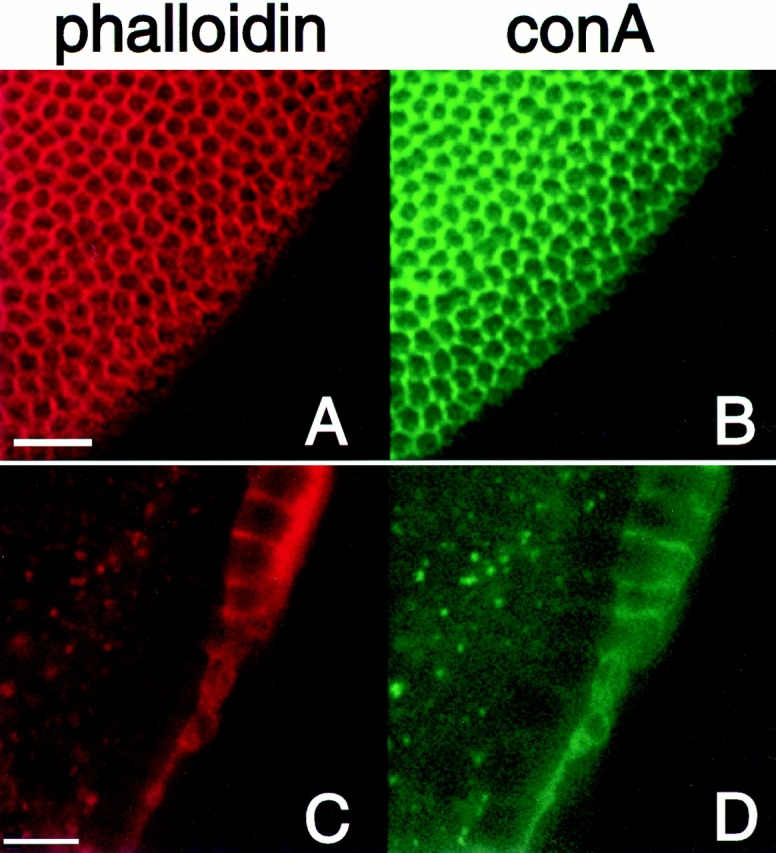
The defects in cellularization also affect surface membrane. Embryos from syx L266 clones were stained with rhodamine-conjugated phalloidin (red) and FITC-conjugated concanavalinA (green) to mark the actin cytoskeleton and the surface membrane, respectively. On the embryo's surface, the two markers label the honeycomb of newly forming cells (A and B). In a deeper section of the same embryo, a large acellular patch was present. Both markers fail to label anything within an acellular patch (C and D), indicating a failure of both the cytoskeleton to form and the surface membrane to invaginate in these areas. Bars: (A and B) 20 μm; (C and D) 10 μm.
syxL266 Defects Do Not Appear until Cellularization Begins
Embryos from homozygous syx L266 ovarian clones were examined more closely to determine the time of onset of developmental abnormalities in these mutants. Because the paternal chromosome was able to rescue the phenotype, the abnormalities we observed must be due to a lack of syntaxin1 at a stage after the onset of transcription from the zygotic genome. Zygotic transcription does not begin until the mitotic cycles 10–13—the cycles immediately before cellularization—and thus a requirement of syntaxin1 at this or a later step was indicated.
To determine the time of onset of defects in syx L266/ syx L371 embryos, mothers bearing syx L266/syx L266 germ lines were crossed to syx L371/TM3, lacZ males as above and stained with Hoechst 33258 to visualize the nuclei and with rhodamine-conjugated phalloidin to stain the F actin. In normal embryogenesis, the nuclei migrate outwards so as to reach the periphery in time for the 10th nuclear division. During the syncytial blastoderm stage, mitotic cycles 10–13, they form a cortex of nuclei, and a cap of F actin resides above each nucleus (for review see Foe et al., 1993). During metaphase of cycles 10–13, shallow membrane furrows have been observed that form between the nuclei and have associated F actin; the furrows may play a role in preventing chromosome loss during the nuclear divisions (Sullivan et al., 1993b ). In the mutant embryos, we therefore examined the position of the nuclei and the frequency at which they aberrantly fell away from the surface; we looked as well for the presence of the actin caps and metaphase furrows in cycles 10–13. Development progressed normally in embryos during these stages despite the lack of syntaxin 1. The nuclei migrated successfully to the periphery (Fig. 8, A and C) and actin caps corresponding to the underlying nuclei were always present and organized (Fig. 8, C and D). In addition, metaphase furrows form and appear to be similar to those in embryos from control (P36-23) germ line clones (Fig. 8, E and F). Only very rarely (in ∼1% of the embryos examined) were there severe defects during these stages.
Figure 8.

Defects due to insufficient syntaxin do not appear before cellularization. The nuclei (A and B) migrate to the surface, and actin caps form above them (C and D) in syx L266/syx L371 embryos derived from homozygous syx L266 germline clones (B and D), just as they do in control embryos derived from P36-23 clones (A and C). The formation of metaphase furrows also progresses normally during mitotic cycles 10–13. The furrows of embryos in mitotic cycle 12 from both P36-23 (E) and syx L266 clones (F) are shown. Furrows and caps are visualized with rhodamine-conjugated phalloidin, and nuclei are stained with Hoechst 33258. In both genotypes, minor irregularities in actin are seen. These regions correspond to nuclei that have fallen away from the cortex (not shown). The frequency of these defects was not significantly different between embryos from P36-23 and syx L266 clones, and was similar to previously reported results for wild type (see text). Bars, 20 μm.
In embryos from both P36-23 and syx L266 clones, small irregularities in the metaphase furrows are occasionally seen (Fig. 8, E and F), and in most cases these irregularities correspond to individual nuclei dropping back towards the center of the embryo. These normal imperfections in early development did not occur at a significantly higher rate nor were they significantly larger in size in embryos from mutant clones compared to controls (Table I). The values are comparable to the reported rate of 0.3% of the nuclei being lost in any given cycle (Sullivan et al., 1993a ). Therefore, it would appear that despite the requirement of syntaxin1 for cell viability and the large disruptions of cellularization observed at later stages, embryos that are from syx L266 clones and lacking in paternally derived syntaxin1 are nonetheless capable of sustaining normal development through cycle 13.
Table I.
Precellularization Defects Are Not Enhanced in syx
| Cycles 10 and 11 | Cycles 12 and 13 | |||||||
|---|---|---|---|---|---|---|---|---|
| No. of defective regions | No. of nuclei per region | No. of defective regions | No. of nuclei per region | |||||
| p36-23 | 1.2 ± 1.0 | 1.4 ± 0.6 | 5.3 ± 3.1 | 1.7 ± 0.8 | ||||
| clones | n = 15 | n = 20 | ||||||
| syx L266 | 1.5 ± 0.9 | 1.9 ± 0.8 | 5.4 ± 3.2 | 2.1 ± 1.1 | ||||
| clones | n = 10 | n = 11 | ||||||
Embryos were analyzed for the number of nuclei dropping away from the cortex of the syncytial blastoderm, either early in this stage (cycles 10 and 11) or late (cycles 12 and 13). The embryos were derived from females carrying germ line clones of homozygous P36-23 or syx L266 mated to syx L371/TM3 males. Nuclei were visualized with Hoechst 33258. The number of areas in which nuclei were dropping away and the number of nuclei in each area were determined and expressed as averages per embryo ± SD; n, the number of embryos examined.
Embryos with Insufficient Syntaxin1 Show Regions with Complete Disruption of Cellularization
As soon as embryos reach the 14th mitotic cycle and begin to cellularize, mutant regions appear. These acellular regions are variable in size, ranging from just a few percent of the embryo's surface to almost the entire surface. There was no apparent correlation between the size of the acellular patch and the stage of cellularization, i.e., the patches probably do not get larger as cellularization progresses. Also, the patches appeared on all surfaces of the embryo but were most common on the central, dorsal area. We examined these areas closely to determine just which stages of cellularization were disrupted. The normal progression through to the formation of actin caps and metaphase furrows argued that a gross disorganization of the surface of the embryo probably did not cause the failure to cellularize. As an additional way to look at the organization of the surface before cellularization, we used antisera to the cytoskeleton-associated protein anillin. Anillin, which is found at sites of membrane constriction, is recruited early to the sites at which the membranes grow inwards (Field and Alberts, 1995). Fig. 9, A–D, shows a cycle 14 embryo that is ∼75% acellular and is in the very early stages of cellularization. In patches on the embryo's surface, the cytoskeleton, as judged by phalloidin staining, is severely disrupted (Fig. 9 A). In these patches, however, some anillin localization persists and is reminiscent of the hexagonal array seen in cellularized parts of the embryo (Fig. 9 B), suggesting that the surface of these regions of the embryos is not completely disorganized. In cross section, it is evident that membrane invagination has only progressed about 2 μm in the cellularizing regions of the embryo (Fig. 9, C and D). The cytoskeleton has formed on the apical surface and lateral extent of cells (Fig. 9 C). The anillin is present in the advancing front of the cleavage furrows (Fig. 9 D) as in wild type (Field and Alberts, 1995). However, in the acellular region at the left side of these panels, no furrows are apparent at all.
Figure 9.
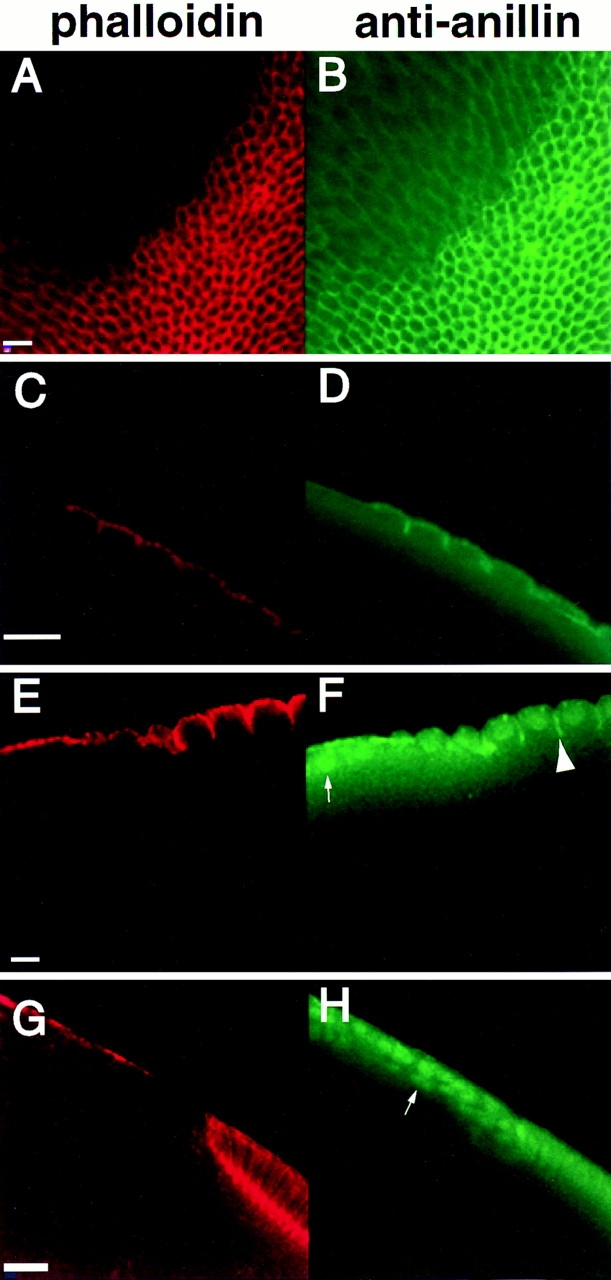
Defects arise at the onset of cellularization and persist throughout in embryos from syx L266 clones. As soon as membrane invagination begins in mitotic cycle 14, defects in the actin cytoskeleton are apparent (A and C). Anillin still shows a hexagonal grid early in cellularization (B), although it does not appear enriched in areas lacking F actin (D). The acellular patch begins near the left edge of C and D. By the midpoint of cellularization, defective regions still show no actin organization (E). Anillin is also disorganized in these regions, and shows a stronger nuclear localization than usual (small arrow, F). Anillin still localizes to the invaginating furrow in those regions undergoing cellularization (large arrowhead F). By the very late stages of cellularization, cytoskeletal organization is still completely lacking in defective regions (G). Anillin is almost completely localized to the nuclei in these regions (arrow, H). Bars: (A and B, C and D, and E and F) 10 μm; (G and H) 20 μm.
Later in cellularization, embryos from syx L266 female germ line clones continue to show patches with a complete lack of cells. Fig. 9, E–H, show two mutant embryos. In the first, cellularization has progressed so that the invaginating membranes have approximately reached the bottom of the nuclei (Fig. 9, E and F). This embryo has a large acellular patch at the anterior (left) side. Polymerized actin is present in this stage but lines the surface of the embryo and gives no indication that any in-growth of membranes between the nuclei has occurred (Fig. 9 E). The anillin, which at this stage should be concentrated at the leading tip of the furrow, is disorganized and largely in the nuclei in the acellular region (Fig. 9 F, small arrow). Anillin typically goes to the nucleus, but only when cellularization is complete and the confluence with the yolk is entirely severed (Field and Alberts, 1995). In the cellularizing areas, anillin also appears to be somewhat less localized to the tips of the furrows (Fig. 9 F, large arrow).
The second embryo shown in Fig. 9, G and H, is at a yet later stage of cellularization, when the membranes have progressed to nearly their full extent. In the acellular region, no suggestion of cellularization can be seen (Fig. 9 G). Anillin again shows less localization in the cellularized areas and a nuclear localization in the acellular areas (Fig. 9 H). Nuclei can also be seen accumulating below the cortex of the embryo in this region.
Cellularization has been described as proceeding in two phases. The first phase is slow and probably involves the addition of membrane to the surface. The second phase is rapid and may use the resorbtion of microvilli from the apical surface as its source of membrane (Foe et al., 1993). The defects seen in embryos from syx L266 clones persist throughout cellularization and no partial cellularization is seen, suggesting that neither phase of cellularization is progressing. The effect on the second, fast phase may be a direct result of syntaxin1's involvement in that phase or may be an indirect effect of the loss of the first, slow phase. As a result of having insufficient syntaxin1, however, regions consistently arise in which cellularization appears to fail completely.
Gastrulation Can Proceed in Improperly Cellularized Embryos
The cell migrations associated with gastrulation occur in the cellularized areas of defective embryos. Fig. 10 shows an embryo in which the posterior and dorsal surfaces are acellular (arrows). However, the caudal region has undergone involution and successfully migrated dorsally. Therefore, those cells that are formed are capable of undergoing significant developmental alterations. Development then arrests at approximately this stage in most of the defective embryos (Fig. 5).
Figure 10.
Gastrulation can occur in embryos with acellular regions. The posterior end of an embryo stained with rhodamine-conjugated phalloidin is shown. The extent of cellularization on the posterior end is indicated by arrows. In addition, the entire dorsal surface is acellular (wide arrow). However, caudal cells have involuted and migrated dorsally, as would normally occur during gastrulation. Such embryos typically arrest at approximately this stage.
Discussion
Proper cellularization of the embryo requires syntaxin1, a protein that is known to be important for membrane trafficking. Though this protein appears to be essential for the viability of many cells (Fig. 4; Schulze and Bellen, 1996), a direct role in cellularization is indicated by our analysis of the hypomorphic allele syx L266. Ovary cells homozygous for this allele are viable and give rise to embryos that can develop normally until the moments when membrane in-growth should occur. A model for the formation of the cell membranes can be proposed that incorporates both the genetic and cytochemical information on syntaxin1 and the existing wealth of morphological descriptions of cellularization (Fullilove and Jacobson, 1971; Sanders, 1975; Turner and Mahowald, 1976; Loncar and Singer, 1995). Most recently, an electron microscopic study (Loncar and Singer, 1995) observed vesicles that were lined up in front of the invaginating cleavage furrow between the nuclei of the syncytium. The addition of these vesicles is likely to be a major source of the membranes that advance the furrow and divide the newly forming cells. This process requires the t-SNARE syntaxin1. As in synaptic transmission, the syntaxin1 is present on the target membrane and is concentrated at sites where fusion occurs, the invaginating cleavage furrows. Syntaxin1, however, was not exclusively localized to a specific region of the furrow. This may mean that vesicles are added along its length, or it may simply reflect a lack of precision in syntaxin1 localization. This widespread distribution of syntaxin1 is similar to the situation in neurons, where syntaxin1 is not exclusively at the synapse but is also present along axons (Garcia et al., 1995; Sesack and Snyder, 1995).
Because the amount of syntaxin1 on the embryo's surface appears to increase during cellularization and because zygotically transcribed syntaxin1 contributes to this process, additional syntaxin1 must be added with the fusing vesicles. Therefore, vesicles may be able to fuse with one another, in addition to fusing with the surface membrane, and this pattern of growth has been suggested (Loncar and Singer, 1995). Though we have not seen syntaxin1 in advance of the furrow, it may be below our detection limit. Syntaxin is known to reside on synaptic vesicles as well as on the plasma membrane at synapses (Walch-Solimena et al., 1995).
Residual cellularization was observed in the syntaxin1-deficient embryos. This cellularization may have been mediated by the small amount of wild-type protein made by syx L266. Resorbtion of apical microvilli may serve as another source of membrane in normal furrow formation and may contribute to the residual cellularization that occurs outside of the defective patches. The acellular areas, however, did not have partial furrows; perhaps since microvillar resorbtion is thought to follow vesicular membrane addition, the resorbtion was blocked in these regions and could not contribute to furrow formation.
A noteworthy feature of the defect is its all or nothing nature: abrupt transitions were seen between properly cellularized and completely acellular areas. Though the patchiness of the phenotype is not yet understood, two possibilities can be suggested. First, regions of the surface that have a little syntaxin1 at the start of cellularization may be competent to receive additional syntaxin1 via vesicle fusion and thereby progress successfully at the expense of regions lacking syntaxin1. Alternatively, a failure in cellularization in a small area may expand into a larger patch because of an interdependence of neighboring cells on membrane and cytoskeletal interactions.
The onset of the phenotype coincides with the onset of cellularization. The formation of metaphase furrows in the mitotic cycle before cellularization may proceed by a different, syntaxin1-independent mechanism, e.g., the rearrangement of microvilli or other features of the surface. Alternatively, the demands placed on membrane trafficking may not be as great at the earlier stages, and the small amount of syntaxin1 present in syx L266 may be sufficient to meet the demand. Metaphase furrows are neither as deep as cleavage furrows nor are there as many of them since there are fewer nuclei at the surface during earlier cycles. Therefore, the initial phase of cellularization at cycle 14 probably represents the first time membrane trafficking is severely taxed during development, and this demand causes defects to arise when insufficient amounts of syntaxin1 are present.
The requirement for a syntaxin1 protein during cellularization is consistent with recent findings in other systems. Membrane fusions may be important for cell divisions in Arabidopsis thalliana as well, where a distant homologue of syntaxin, KNOLLE, has been implicated in cytokinesis (Lukowitz et al., 1996). One explanation of the apparent cell lethality of syx L371 is that syntaxin1 is required for cell divisions in many Drosophila tissues in addition to the syncytial blastoderm. In its absence, these tissues would be unable to form. Thus, membrane addition via vesicle trafficking during cytokinesis may be essential for the formation of new cells (Rothman and Warren, 1994). The apparent cell lethality may also reflect a requirement of syntaxin1 in constitutive membrane trafficking to the plasma membrane in all cells.
The existence of both neuronal and nonneuronal functions for the syntaxin1 gene was previously indicated by subtle abnormalities of the cuticle, gut, and trachea in zygotic null mutants (Schulze et al., 1995) and is confirmed by our present study. In synapses, syntaxin acts with a cognate v-SNARE called VAMP or synaptobrevin. Current models of SNARE function require such an interaction to occur in the early embryo as well. Interestingly, however, the embryo and the synapse probably employ two different v-SNAREs to pair with the same syntaxin1 protein. We have previously found a neuron-specific synaptobrevin isoform (n-syb) (DiAntonio et al., 1993) that is the likely mediator of synaptic transmission (Sweeney et al., 1995). We have also shown that a different, widely expressed gene (syb) (Sudhof et al., 1989) is present in 0–3-h embryos (Chin et al., 1993). The use of distinct synaptobrevins may reflect the distinct regulatory control of these different trafficking events. It also indicates that the specificity of vesicle fusion cannot rely entirely on the interaction of syntaxin with synaptobrevin. This lack of specificity becomes particularly problematic if regulated neurosecretion and constitutive trafficking require the same t-SNARE in the same cell, despite the fact that their vesicles must be directed to distinct domains on the cell surface. Other proteins, perhaps in the SNARE complex, may contribute to the specificity or fine-tune it. For instance, syntaxin1 may complex with one SNAP-25 homologue in the early embryo, allowing it to bind with syb, while it complexes with another SNAP-25 in the nerve terminal, allowing it to bind n-syb.
Previous studies have indicated that docking of synaptic vesicles at appropriate sites persists in syx mutants (Broadie et al., 1995), and these studies have also shown that syntaxin is not localized only to active zones (Garcia et al., 1995; Sesack et al., 1995). Our present observations suggest that a single isoform of syntaxin can mediate fusions by many different classes of transport vesicle. Though syntaxin may indeed play a role in docking and targeting, additional proteins must be invoked to support docking in its absence and to refine the specificity of targeting. On the other hand, a posttargeting role in exocytosis, such as a role in fusion itself, is consistent with our current findings and strongly implicated by the earlier genetic analysis (Broadie et al., 1995; Schulze et al., 1995).
In addition to syntaxin1, the cellularization of early Drosophila embryos will undoubtedly involve many other proteins to mediate and direct the addition of membrane to the cell surface. Although the cytoskeletal rearrangements that accompany the membrane changes have been the object of considerable study (for reviews see Schejter and Wieschaus, 1993; Theurkauf, 1994), the genetic analysis of the membrane addition remains largely unexplored. Many of the components, like syntaxin1, may be provided by both maternal contributions and zygotic transcription. Once cellularization is complete, the blastoderm must differentiate into a polarized tissue (Wodarz et al., 1995) that selectively secretes cuticle proteins and signaling molecules from distinct domains. Thus, the formation of the cellular blastoderm may also provide a valuable genetic system in which to investigate the development of vectorial membrane trafficking.
Acknowledgments
The authors are grateful to Irene Inman for outstanding technical assistance, to Daria Siekhaus for participating in the screen that isolated the original syx P-insertions, and to Dr. Chris Field (University of California, San Francisco, CA) for the anillin antibody and her comments on the data. We also thank Drs. Konrad Zinsmaier (University of Pennsylvania, Philadelphia, PA) and Seymour Benzer (California Institute of Technology, Pasadena, CA) for providing monoclonal 8C3. Confocal microscopy was performed at the Cell Sciences Imaging Facility at Stanford, and we thank Dr. Susan Palmieri for her help.
This work was supported by a Silvio Conti Center for Neuroscience Award from the National Institute of Mental Health (NIMH) and by the Muscular Dystrophy Association. R.W. Burgess was an NIMH predoctoral fellow. D.L. Deitcher was a National Institute of Neurological Disorders and Stroke postdoctoral fellow.
Abbreviations used in this paper
- AED
after egg deposition
- conA
concanavalinA
- syx
syntaxin1
Footnotes
Address all correspondence to Thomas L. Schwarz, Department of Molecular and Cellular Physiology, Stanford University, Stanford, CA 94305-5426. Tel.: (415) 725-7770. Fax: (415) 725-8021. E-mail: tschwarz@leland.stanford.edu
References
- Aalto MK, Ronne H, Keranen S. Yeast syntaxins Sso1p and Sso2p belong to a family of related membrane proteins that function in vesicular transport. EMBO (Eur Mol Biol Organ) J. 1993;12:4095–4104. doi: 10.1002/j.1460-2075.1993.tb06093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner, M. 1989. Drosophila, A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. 434 pp.
- Bennett MK, Scheller RH. The molecular machinery for secretion is conserved from yeast to neurons. Proc Natl Acad Sci USA. 1993;90:2559–2563. doi: 10.1073/pnas.90.7.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett MK, Calakos N, Scheller RH. Syntaxin: a synaptic protein implicated in docking of synaptic vesicles at presynaptic active zones. Science (Wash DC) 1992;257:255–259. doi: 10.1126/science.1321498. [DOI] [PubMed] [Google Scholar]
- Bier E, Vaessin H, Shepherd S, Lee K, McCall K, Barbel S, Ackerman L, Carretto R, Uemura T, Grell E, et al. Searching for pattern and mutation in the Drosophila genome with a P-lacZvector. Genes Dev. 1989;3:1273–1287. doi: 10.1101/gad.3.9.1273. [DOI] [PubMed] [Google Scholar]
- Blasi J, Chapman ER, Yamasaki S, Binz T, Niemann H, Jahn R. Botulinum neurotoxin C1 blocks neurotransmitter release by means of cleaving HPC-1/syntaxin. EMBO (Eur Mol Biol Organ) J. 1993;12:4821–4828. doi: 10.1002/j.1460-2075.1993.tb06171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadie K, Prokop A, Bellen HJ, O'Kane CJ, Schulze KL, Sweeney ST. Syntaxin and synaptobrevin function downstream of vesicle docking in Drosophila. . Neuron. 1995;15:663–673. doi: 10.1016/0896-6273(95)90154-x. [DOI] [PubMed] [Google Scholar]
- Calakos N, Bennett MK, Peterson KE, Scheller RH. Protein-protein interactions contributing to the specificity of intracellular vesicular transport. Science (Wash DC) 1994;263:1146–1149. doi: 10.1126/science.8108733. [DOI] [PubMed] [Google Scholar]
- Campos-Ortega, J.A., and V. Hartenstein. 1985. The Embryonic Development of Drosophila melanogaster. Springer-Verlag, Berlin. 227 pp.
- Cerezo JR, Jimenez F, Moya F. Characterization and gene cloning of Drosophila syntaxin 1 (Dsynt1): the fruit fly homologue of rat syntaxin 1. Mol Brain Res. 1995;29:245–252. doi: 10.1016/0169-328x(94)00254-c. [DOI] [PubMed] [Google Scholar]
- Chin AC, Burgess RW, Wong BR, Schwarz TL, Scheller RH. Differential expression of transcripts from syb, a Drosophila melanogastergene encoding VAMP (synaptobrevin) that is abundant in non-neuronal cells. Gene. 1993;131:175–181. doi: 10.1016/0378-1119(93)90291-a. [DOI] [PubMed] [Google Scholar]
- Chou T-B, Noll E, Perrimon N. Autosomal P[ovoD1] dominant female-sterile insertions in Drosophilaand their use in generating germ-line chimeras. 1993. Development (Camb) 1993;199:1359–1369. doi: 10.1242/dev.119.4.1359. [DOI] [PubMed] [Google Scholar]
- DiAntonio A, Burgess RW, Chin AC, Deitcher DL, Scheller RH, Schwarz TL. Identification and characterization of Drosophilagenes for synaptic vesicle proteins. J Neurosci. 1993;13:4924–4935. doi: 10.1523/JNEUROSCI.13-11-04924.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field CM, Alberts BM. Anillin, a contractile ring protein that cycles from the nucleus to the cell cortex. J Cell Biol. 1995;131:165–178. doi: 10.1083/jcb.131.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foe VE, Alberts BM. Studies of nuclear and cytoplasmic behaviour during the five mitotic cycles that precede gastrulation in Drosophilaembryogenesis. J Cell Sci. 1983;61:31–70. doi: 10.1242/jcs.61.1.31. [DOI] [PubMed] [Google Scholar]
- Foe, V.E., G.M. Odell, and B.A. Edgar. 1993. Mitosis and morphogenesis in the Drosophila embryo: point and counterpoint. In The Development of Drosophila melanogaster. M. Bate and A. Martinez-Arias, editors. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. 149–300.
- Fullilove SL, Jacobson AG. Nuclear elongation and cytokinesis in Drosophila montana. . Dev Biol. 1971;26:560–577. doi: 10.1016/0012-1606(71)90141-2. [DOI] [PubMed] [Google Scholar]
- Garcia EP, McPherson PS, Chilcote TJ, Takei K, DeCamilli P. rbSec1A and B colocalize with syntaxin 1 and SNAP-25 throughout the axon, but are not in a stable complex with syntaxin. J Cell Biol. 1995;129:105–120. doi: 10.1083/jcb.129.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golic KG, Lindquist S. The FLP recombinase of yeast catalyzes site-specific recombination in the Drosophilagenome. Cell. 1989;59:499–509. doi: 10.1016/0092-8674(89)90033-0. [DOI] [PubMed] [Google Scholar]
- Hardwick KG, Pelham HR. SED5 encodes a 39-kD integral membrane protein required for vesicular transport between the ER and the Golgi complex. J Cell Biol. 1992;119:513–521. doi: 10.1083/jcb.119.3.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser K, Goodwin SF. “Site-selected” transposon mutagenesis of Drosophila. . Proc Natl Acad Sci USA. 1990;87:1686–1690. doi: 10.1073/pnas.87.5.1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kee Y, Scheller RH. Localization of synaptotagmin-binding domains on syntaxin. J Neurosci. 1996;16:1975–1981. doi: 10.1523/JNEUROSCI.16-06-01975.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kee Y, Lin RC, Hsu S-C, Scheller RH. Distinct domains of syntaxin are required for synaptic vesicle fusion complex formation and dissociation. Neuron. 1995;14:991–998. doi: 10.1016/0896-6273(95)90337-2. [DOI] [PubMed] [Google Scholar]
- Loncar D, Singer SJ. Cell membrane formation during the cellularization of the syncytial blastoderm of Drosophila. . Proc Natl Acad Sci USA. 1995;92:2199–2203. doi: 10.1073/pnas.92.6.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukowitz W, Mayer U, Jurgens G. Cytokinesis in the Arabidopsis embryo involves the syntaxin-related KNOLLE gene product. Cell. 1996;84:61–71. doi: 10.1016/s0092-8674(00)80993-9. [DOI] [PubMed] [Google Scholar]
- O'Hare K, Rubin GM. Structures of P transposable elements and their sites of insertion and excision in the Drosophila melanogastergenome. Cell. 1983;34:25–35. doi: 10.1016/0092-8674(83)90133-2. [DOI] [PubMed] [Google Scholar]
- Parfitt, K.D., N. Reist, J. Li, R.W. Burgess, D. Deitcher, A. DiAntonio, and T.L. Schwarz. 1995. Drosophila genetics and the functions of synaptic proteins. Cold Spring Harbor Symp. Quant. Biol. In press. [DOI] [PubMed]
- Pevsner J, Hsu S-C, Scheller RH. N-Sec1: a neuronal-specific syntaxin-binding protein. Proc Natl Acad Sci USA. 1994;91:1445–1449. doi: 10.1073/pnas.91.4.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper RC, Whitters EA, Stevens TH. Yeast Vps45p is a sec1p-like protein required for the consumption of vacuole-targeted, post Golgi transport vesicles. Eur J Cell Biol. 1994;65:305–318. [PubMed] [Google Scholar]
- Rothman JE, Warren G. Implications of the SNARE hypothesis for intracellular membrane topology and dynamics. Curr Biol. 1994;4:220–233. doi: 10.1016/s0960-9822(00)00051-8. [DOI] [PubMed] [Google Scholar]
- Rothman JE, Wieland FT. Protein sorting by transport vesicles. Science (Wash DC) 1996;272:227–234. doi: 10.1126/science.272.5259.227. [DOI] [PubMed] [Google Scholar]
- Sanders EJ. Aspects of furrow membrane formation in the cleaving Drosophilaembryo. Cell Tiss Res. 1975;156:463–474. doi: 10.1007/BF00225106. [DOI] [PubMed] [Google Scholar]
- Schejter ED, Wieschaus E. Functional elements of the cytoskeleton in the early Drosophilaembryo. Annu Rev Cell Biol. 1993;9:67–99. doi: 10.1146/annurev.cb.09.110193.000435. [DOI] [PubMed] [Google Scholar]
- Schiavo G, Shone CC, Bennett MK, Scheller RH, Montecucco C. Botulinum neurotoxin type C cleaves a single Lys-Ala bond within the carboxyl-terminal region of syntaxins. J Biol Chem. 1995;270:10566–10570. doi: 10.1074/jbc.270.18.10566. [DOI] [PubMed] [Google Scholar]
- Schulze KL, Bellen HJ. Drosophila syntaxinis required for cell viability and may function in membrane formation and stabilization. Genetics. 1996;144:1713–1724. doi: 10.1093/genetics/144.4.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze KL, Broadie K, Perin MS, Bellen HJ. Genetic and electrophysiological studies of Drosophilasyntaxin-1A demonstrate its role in non-neuronal secretion and neurotransmission. Cell. 1995;80:311–320. doi: 10.1016/0092-8674(95)90414-x. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Snyder CL. Cellular and subcellular localization of syntaxin-like immunoreactivity in the rat striatum and cortex. Neuroscience. 1995;67:993–1007. doi: 10.1016/0306-4522(95)00087-y. [DOI] [PubMed] [Google Scholar]
- Sollner T, Rothman JE. Neurotransmission: harnessing fusion machinery at the synapse. Trends Neurosci. 1994;17:344–348. doi: 10.1016/0166-2236(94)90178-3. [DOI] [PubMed] [Google Scholar]
- Sollner T, Bennett MK, Whiteheart SW, Scheller RH, Rothman JE. A protein assembly-disassembly pathway in vitro that may correspond to sequential steps of vesicle docking, activation and fusion. Cell. 1993a;75:409–418. doi: 10.1016/0092-8674(93)90376-2. [DOI] [PubMed] [Google Scholar]
- Sollner TSW, Whiteheart M, Brunner H, Erdjuman-Bromage H, Geromanos S, Tempst P, Rothman JE. SNAP receptors implicated in vesicle targeting and fusion. Nature (Lond) 1993b;362:318–324. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- Spradling, A. 1993. Developmental genetics of oogenesis. In The Development of Drosophila melanogaster. M. Bate and A. Martinez-Arias, editors. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. 149–300.
- Sudhof TC. The synaptic vesicle cycle: a cascade of protein-protein interactions. Nature (Lond) 1995;375:645–653. doi: 10.1038/375645a0. [DOI] [PubMed] [Google Scholar]
- Sudhof TC, Baumert M, Perin MS, Jahn R. A synaptic vesicle membrane protein is conserved from mammals to Drosophila. . Neuron. 1989;2:1475–1481. doi: 10.1016/0896-6273(89)90193-1. [DOI] [PubMed] [Google Scholar]
- Sullivan W, Daily DR, Fogarty P, Yook KJ, Pimpinelli S. Delays in anaphase initiation occur in individual nuclei of the syncytial Drosophilaembryo. Mol Biol Cell. 1993a;4:885–896. doi: 10.1091/mbc.4.9.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan W, Fogarty P, Theurkauf W. Mutations affecting the cytoskeletal organization of syncytial Drosophilaembryos. Development (Camb) 1993b;118:1245–1254. doi: 10.1242/dev.118.4.1245. [DOI] [PubMed] [Google Scholar]
- Sweeney ST, Broadie K, Keane J, Niemann H, O'Kane CJ. Targeted expression of tetanus toxin light chain in Drosophilaspecifically eliminates synaptic transmission and causes behavioral defects. Neuron. 1995;14:341–351. doi: 10.1016/0896-6273(95)90290-2. [DOI] [PubMed] [Google Scholar]
- Theurkauf WE. Actin cytoskeleton. Through the bottleneck. Curr Biol. 1994;4:76–78. doi: 10.1016/s0960-9822(00)00019-1. [DOI] [PubMed] [Google Scholar]
- Tower J, Karpen GH, Craig N, Spradling AC. Preferential transposition of DrosophilaP elements to nearby chromosomal sites. Genetics. 1993;133:347–359. doi: 10.1093/genetics/133.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner FR, Mahowald AP. Scanning electron microscopy of Drosophilaembryogenesis. I. The structure of the egg envelope and the formation of the cellular blastoderm. Dev Biol. 1976;50:95–108. doi: 10.1016/0012-1606(76)90070-1. [DOI] [PubMed] [Google Scholar]
- Walch-Solimena C, Blasi J, Edelmann L, Chapman ER, von Mollard GF, Jahn R. The t-SNAREs syntaxin1 and SNAP-25 are present on organelles that participate in synaptic vesicle recycling. J Cell Biol. 1995;128:637–645. doi: 10.1083/jcb.128.4.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wodarz A, Hinz U, Engelbert M, Knust E. Expression of crumbs confers apical character on plasma membrane domains of ectodermal epithelia of Drosophila. . Cell. 1995;82:67–76. doi: 10.1016/0092-8674(95)90053-5. [DOI] [PubMed] [Google Scholar]
- Xu T, Rubin GM. Analysis of genetic mosaics in developing and adult Drosophilatissues. Development (Camb) 1993;117:1223–1237. doi: 10.1242/dev.117.4.1223. [DOI] [PubMed] [Google Scholar]
- Zinsmaier KE, Eberle KK, Buchner E, Walter N, Benzer S. Paralysis and early death in cysteine string protein mutants of Drosophila. . Science (Wash DC) 1994;263:977–980. doi: 10.1126/science.8310297. [DOI] [PubMed] [Google Scholar]