Abstract
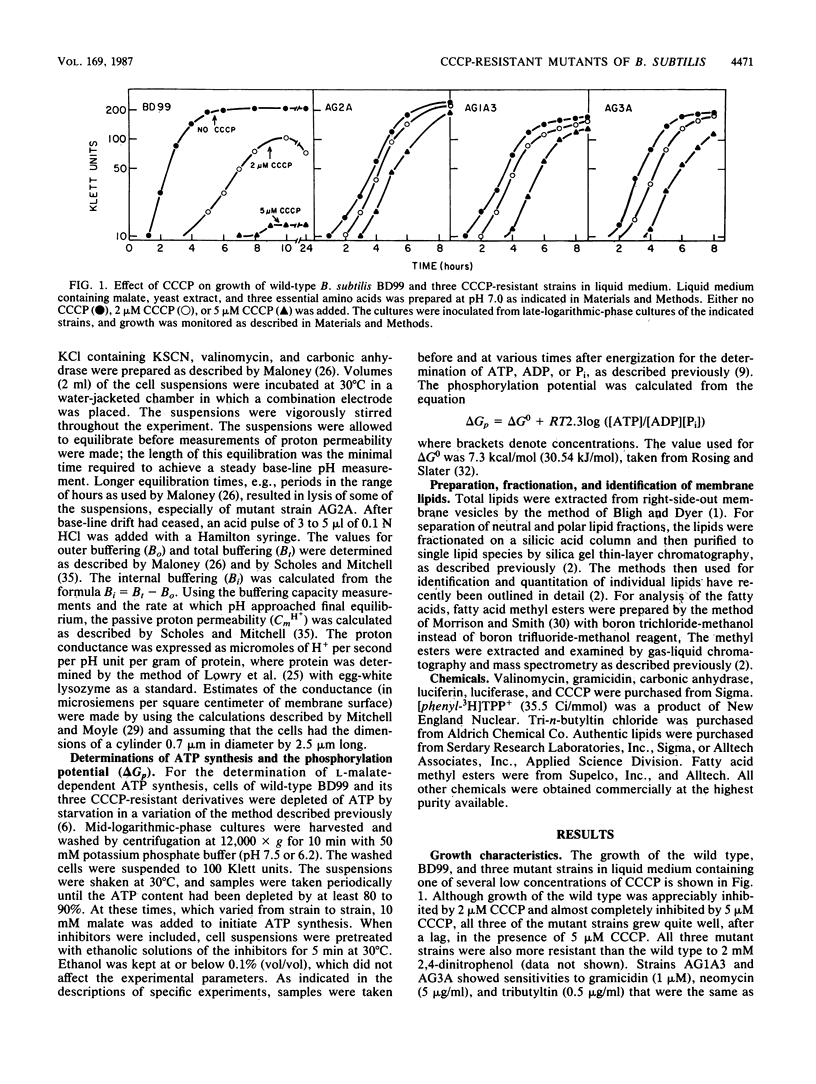
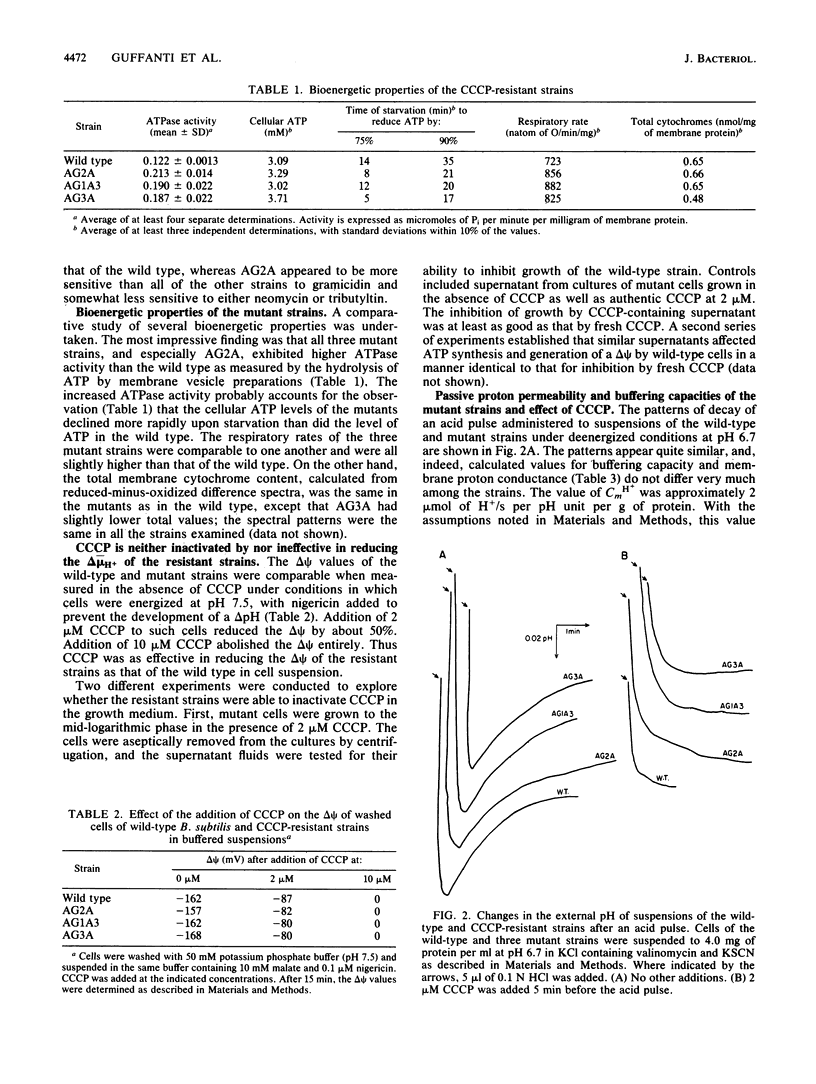
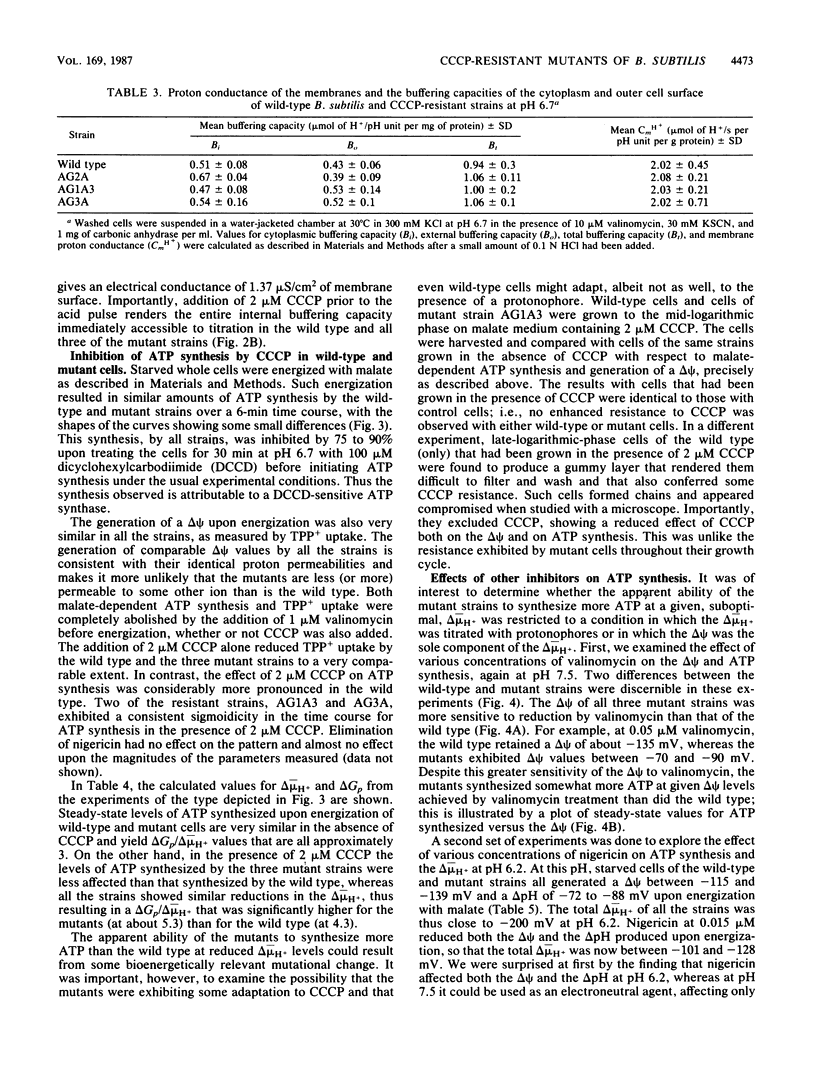
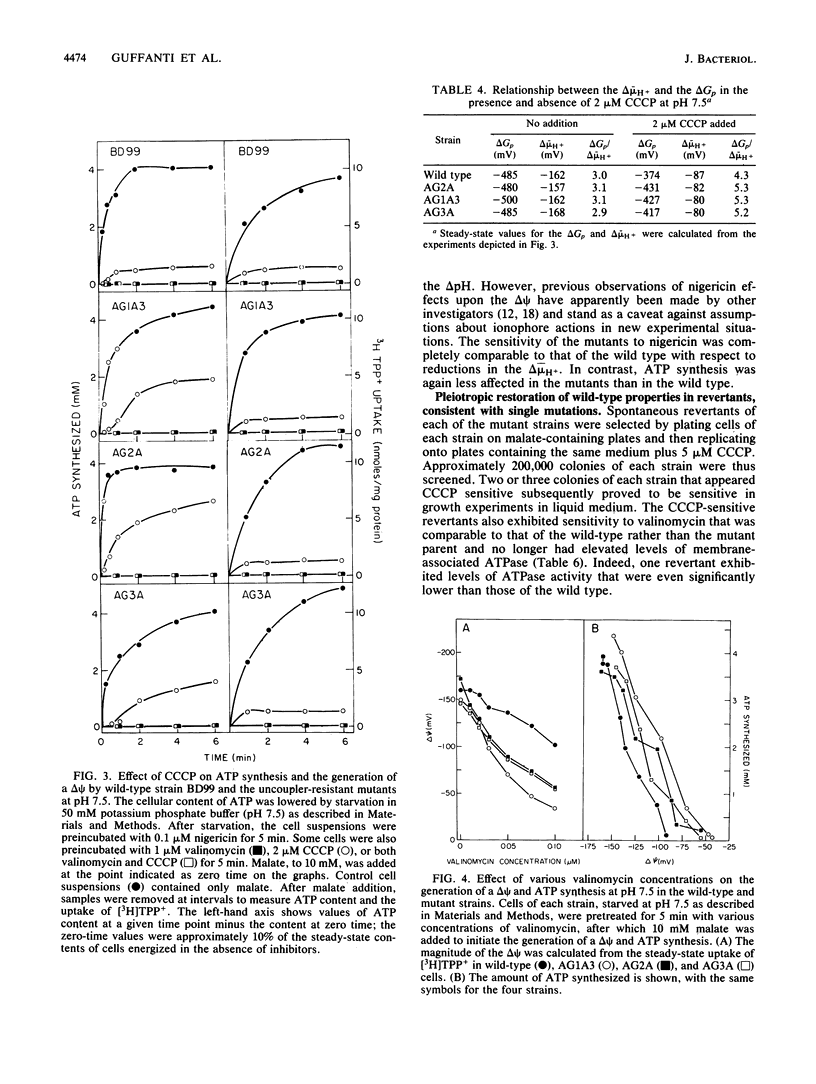
Three mutant strains of Bacillus subtilis were isolated on the basis of their ability to grow in the presence of 5 microM carbonyl cyanide m-chlorophenylhydrazone (CCCP). The mutants (AG2A, AG1A3, and AG3A) were also resistant to 2,4-dinitrophenol, and AG2A exhibited resistance to tributyltin and neomycin. The mutants all exhibited (i) elevated levels of membrane ATPase activity relative to the wild type; (ii) slightly elevated respiratory rates, with the cytochrome contents of the membranes being the same as or slightly lower than those of the wild type; (3) a passive membrane permeability to protons that was indistinguishable from that of the wild type in the absence of CCCP and that was increased by addition of CCCP to the same extent as observed with the wild type; and (4) an enhanced sensitivity to valinomycin with respect to the ability of the ionophore to reduce the transmembrane electrical potential. Finally and importantly, starved whole cells of all the mutants synthesized more ATP than the wild type did upon energization in the presence of any one of several agents that lowered the proton motive force. Studies of revertants indicated that the phenotype resulted from a single mutation. Since a mutation in the coupling membrane might produce such pleiotropic effects, an analysis of the membrane lipids was undertaken with preparations made from cells grown in the absence of CCCP. The membrane lipids of the uncoupler-resistant strains differed from those of the wild type in having reduced amounts of monounsaturated C16 fatty acids and increased ratios of iso/anteiso branches on the C15 fatty acids. Correlations between protonophore resistance and the membrane lipid compositions of the wild type, mutants, and revertants were most consistent with the hypothesis that a reduction in the content of monounsaturated C16 fatty acids in the membrane phospholipids is related, perhaps casually, to the ability to synthesize ATP at low bulk transmembrane electrochemical gradients of protons.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Clejan S., Krulwich T. A., Mondrus K. R., Seto-Young D. Membrane lipid composition of obligately and facultatively alkalophilic strains of Bacillus spp. J Bacteriol. 1986 Oct;168(1):334–340. doi: 10.1128/jb.168.1.334-340.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker S. J., Lang D. R. Membrane bioenergetic parameters in uncoupler-resistant mutants of Bacillus megaterium. J Biol Chem. 1978 Oct 10;253(19):6738–6743. [PubMed] [Google Scholar]
- Decker S. J., Lang D. R. Mutants of Bacillus megaterium resistant to uncouplers of oxidative phosphorylation. J Biol Chem. 1977 Sep 10;252(17):5936–5938. [PubMed] [Google Scholar]
- Guffanti A. A., Blumenfeld H., Krulwich T. A. ATP synthesis by an uncoupler-resistant mutant of Bacillus megaterium. J Biol Chem. 1981 Aug 25;256(16):8416–8421. [PubMed] [Google Scholar]
- Guffanti A. A., Chiu E., Krulwich T. A. Failure of an alkalophilic bacterium to synthesize ATP in response to a valinomycin-induced potassium diffusion potential at high pH. Arch Biochem Biophys. 1985 Jun;239(2):327–333. doi: 10.1016/0003-9861(85)90695-2. [DOI] [PubMed] [Google Scholar]
- Guffanti A. A., Fuchs R. T., Krulwich T. A. Oxidative phosphorylation by isolated membrane vesicles from Bacillus megaterium and its uncoupler-resistant mutant derivative. J Biol Chem. 1983 Jan 10;258(1):35–37. [PubMed] [Google Scholar]
- Guffanti A. A., Fuchs R. T., Schneier M., Chiu E., Krulwich T. A. A transmembrane electrical potential generated by respiration is not equivalent to a diffusion potential of the same magnitude for ATP synthesis by Bacillus firmus RAB. J Biol Chem. 1984 Mar 10;259(5):2971–2975. [PubMed] [Google Scholar]
- Hanstein W. G., Hatefi Y. Characterization and localization of mitochondrial uncoupler binding sites with an uncoupler capable of photoaffinity labeling. J Biol Chem. 1974 Mar 10;249(5):1356–1362. [PubMed] [Google Scholar]
- Hanstein W. G. Uncoupling of oxidative phosphorylation. Biochim Biophys Acta. 1976 Sep 27;456(2):129–148. doi: 10.1016/0304-4173(76)90010-0. [DOI] [PubMed] [Google Scholar]
- Harrington C. R., Baddiley J. Synthesis of peptidoglycan and teichoic acid in Bacillus subtilis: role of the electrochemical proton gradient. J Bacteriol. 1984 Sep;159(3):925–933. doi: 10.1128/jb.159.3.925-933.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring F. G., Krisman A., Sedgwick E. G., Bragg P. D. Electron spin resonance studies of lipid fluidity changes in membranes of an uncoupler-resistant mutant of Escherichia coli. Biochim Biophys Acta. 1985 Oct 10;819(2):231–240. doi: 10.1016/0005-2736(85)90178-6. [DOI] [PubMed] [Google Scholar]
- Hicks D. B., Krulwich T. A. The membrane ATPase of alkalophilic Bacillus firmus RAB is an F1-type ATPase. J Biol Chem. 1986 Sep 25;261(27):12896–12902. [PubMed] [Google Scholar]
- Hopfer U., Lehninger A. L., Thompson T. E. Protonic conductance across phospholipid bilayer membranes induced by uncoupling agents for oxidative phosphorylation. Proc Natl Acad Sci U S A. 1968 Feb;59(2):484–490. doi: 10.1073/pnas.59.2.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M., Ohnishi Y. Isolation of Escherichia coli mutants which are resistant to an inhibitor of H+-ATPase, tributyltin and also to uncouplers of oxidative phosphorylation. FEBS Lett. 1981 Dec 28;136(2):225–230. doi: 10.1016/0014-5793(81)80623-0. [DOI] [PubMed] [Google Scholar]
- Ito M., Ohnishi Y., Itoh S., Nishimura M. Carbonyl cyanide-m-chlorophenyl hydrazone-resistant Escherichia coli mutant that exhibits a temperature-sensitive unc phenotype. J Bacteriol. 1983 Jan;153(1):310–315. doi: 10.1128/jb.153.1.310-315.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolliffe L. K., Doyle R. J., Streips U. N. The energized membrane and cellular autolysis in Bacillus subtilis. Cell. 1981 Sep;25(3):753–763. doi: 10.1016/0092-8674(81)90183-5. [DOI] [PubMed] [Google Scholar]
- Katre N. V., Wilson D. F. A specific uncoupler-binding protein in Tetrahymena pyriformis and Paracoccus denitrificans. Biochim Biophys Acta. 1980 Dec 3;593(2):224–229. doi: 10.1016/0005-2728(80)90060-2. [DOI] [PubMed] [Google Scholar]
- Krulwich T. A., Agus R., Schneier M., Guffanti A. A. Buffering capacity of bacilli that grow at different pH ranges. J Bacteriol. 1985 May;162(2):768–772. doi: 10.1128/jb.162.2.768-772.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krulwich T. A., Clejan S., Falk L. H., Guffanti A. A. Incorporation of specific exogenous fatty acids into membrane lipids modulates protonophore resistance in Bacillus subtilis. J Bacteriol. 1987 Oct;169(10):4479–4485. doi: 10.1128/jb.169.10.4479-4485.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lewis R. J., Belkina S., Krulwich T. A. Alkalophiles have much higher cytochrome contents than conventional bacteria and than their own non-alkalophilic mutant derivatives. Biochem Biophys Res Commun. 1980 Jul 31;95(2):857–863. doi: 10.1016/0006-291x(80)90866-9. [DOI] [PubMed] [Google Scholar]
- MITCHELL P. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature. 1961 Jul 8;191:144–148. doi: 10.1038/191144a0. [DOI] [PubMed] [Google Scholar]
- MORRISON W. R., SMITH L. M. PREPARATION OF FATTY ACID METHYL ESTERS AND DIMETHYLACETALS FROM LIPIDS WITH BORON FLUORIDE--METHANOL. J Lipid Res. 1964 Oct;5:600–608. [PubMed] [Google Scholar]
- Maloney P. C. Membrane H+ conductance of Streptococcus lactis. J Bacteriol. 1979 Oct;140(1):197–205. doi: 10.1128/jb.140.1.197-205.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin S. G., Dilger J. P. Transport of protons across membranes by weak acids. Physiol Rev. 1980 Jul;60(3):825–863. doi: 10.1152/physrev.1980.60.3.825. [DOI] [PubMed] [Google Scholar]
- Mitchell P., Moyle J. Acid-base titration across the membrane system of rat-liver mitochondria. Catalysis by uncouplers. Biochem J. 1967 Aug;104(2):588–600. doi: 10.1042/bj1040588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padan E., Rottenberg H. Respiratory control and the proton electrochemical gradient in mitochondria. Eur J Biochem. 1973 Dec 17;40(2):431–437. doi: 10.1111/j.1432-1033.1973.tb03212.x. [DOI] [PubMed] [Google Scholar]
- Rosing J., Slater E. C. The value of G degrees for the hydrolysis of ATP. Biochim Biophys Acta. 1972 May 25;267(2):275–290. doi: 10.1016/0005-2728(72)90116-8. [DOI] [PubMed] [Google Scholar]
- Rottenberg H., Hashimoto K. Fatty acid uncoupling of oxidative phosphorylation in rat liver mitochondria. Biochemistry. 1986 Apr 8;25(7):1747–1755. doi: 10.1021/bi00355a045. [DOI] [PubMed] [Google Scholar]
- Rottenberg H. The measurement of membrane potential and deltapH in cells, organelles, and vesicles. Methods Enzymol. 1979;55:547–569. doi: 10.1016/0076-6879(79)55066-6. [DOI] [PubMed] [Google Scholar]
- Scholes P., Mitchell P. Acid-base titration across the plasma membrane of Micrococcus denitrificans: factors affecting the effective proton conductance and the respiratory rate. J Bioenerg. 1970 Jun;1(1):61–72. doi: 10.1007/BF01516089. [DOI] [PubMed] [Google Scholar]
- Spizizen J. TRANSFORMATION OF BIOCHEMICALLY DEFICIENT STRAINS OF BACILLUS SUBTILIS BY DEOXYRIBONUCLEATE. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1072–1078. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerhoff H. V., Melandri B. A., Venturoli G., Azzone G. F., Kell D. B. A minimal hypothesis for membrane-linked free-energy transduction. The role of independent, small coupling units. Biochim Biophys Acta. 1984 Dec 17;768(3-4):257–292. doi: 10.1016/0304-4173(84)90019-3. [DOI] [PubMed] [Google Scholar]
- Willecke K., Pardee A. B. Fatty acid-requiring mutant of bacillus subtilis defective in branched chain alpha-keto acid dehydrogenase. J Biol Chem. 1971 Sep 10;246(17):5264–5272. [PubMed] [Google Scholar]
- Wolfson P. J., Krulwich T. A. Inhibition of isocitrate lyase: the basis for inhibition of growth of two Arthrobacter species by pyruvate. J Bacteriol. 1972 Oct;112(1):356–364. doi: 10.1128/jb.112.1.356-364.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]



