Abstract
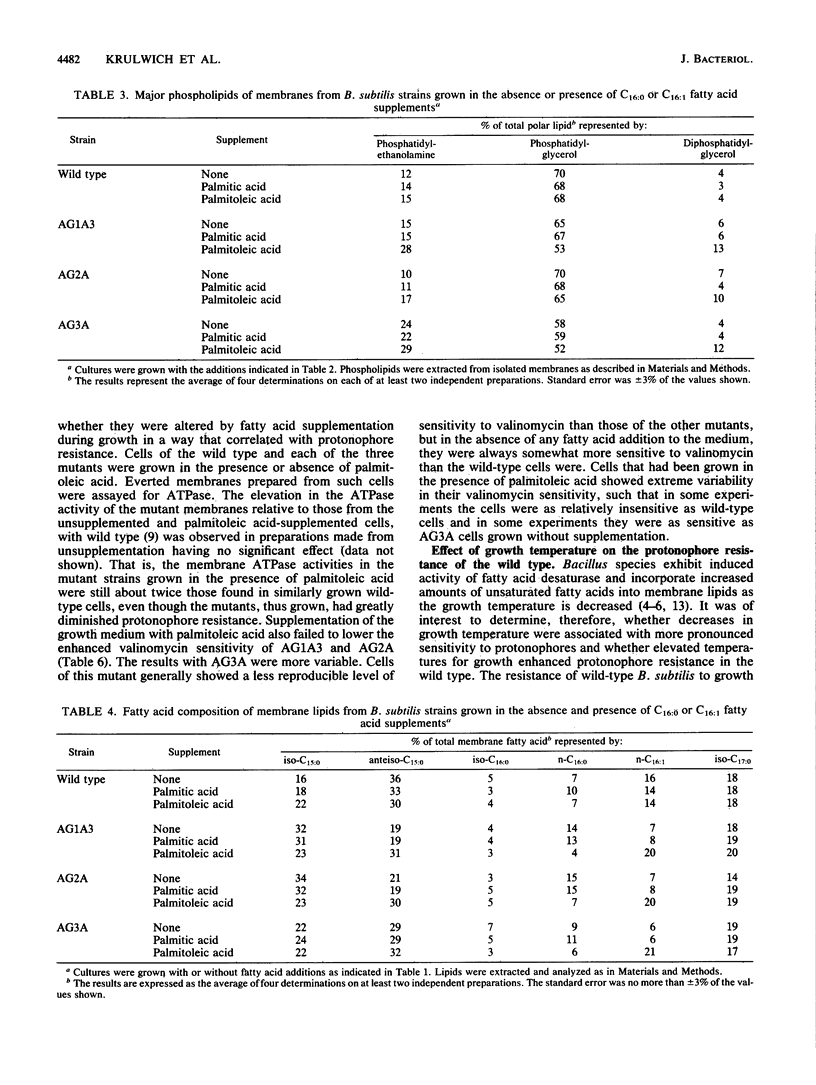
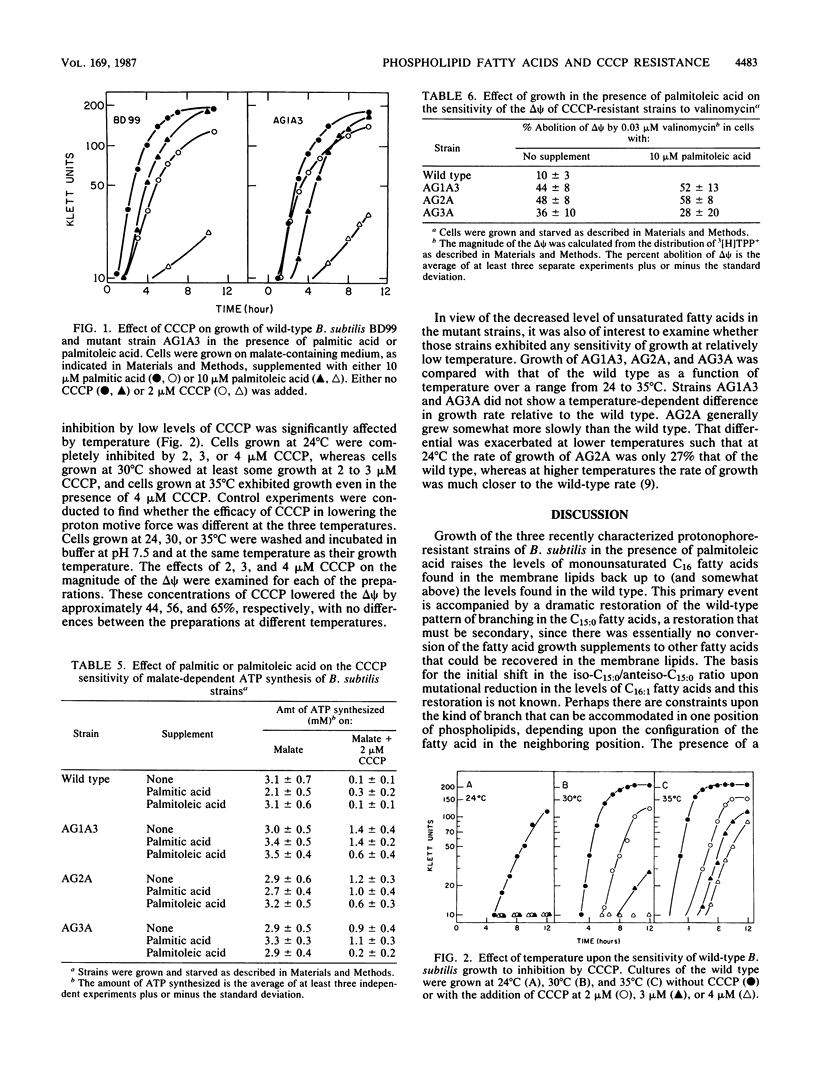
Attempts to manipulate the level of C16:1 fatty acids in membrane phospholipids were made by using Bacillus subtilis and its protonophore-resistant mutants to test the hypothesis that C16:1 fatty acid levels relate to the bioenergetic properties of the mutant strains. Growth of the three mutants in the presence of palmitoleic acid restored the level of C16:1 fatty acids in the membrane lipids to somewhat above those found in the wild type. The palmitoleic acid was preferentially incorporated into diphosphatidylglycerol (cardiolipin) and phosphatidylethanolamine and was associated with increased levels of these phospholipids. These membrane preparations showed no increase in the levels of free fatty acids. The increase in C16:1 fatty acids achieved by growth in the presence of palmitoleic acid was accompanied by secondary changes in membrane lipids as well as a pronounced diminution in the protonophore resistance of growth and ATP synthesis. Other membrane-associated properties that had been observed in these mutants, e.g., elevated ATPase levels, were not altered coordinately with protonophore resistance and C16:1 fatty acid levels. Growth of the wild type in the presence of palmitic acid caused a modest elevation of the C16:0 of the membrane lipids and a modest increase in the protonophore resistance of growth and ATP synthesis. Growth of the wild type at elevated temperatures, in the absence of fatty acid supplementation, also enhanced its resistance to protonophores. The results support the hypothesis that specific changes in membrane lipid composition underlie the bioenergetic changes associated with protonophore resistance.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clejan S., Krulwich T. A., Mondrus K. R., Seto-Young D. Membrane lipid composition of obligately and facultatively alkalophilic strains of Bacillus spp. J Bacteriol. 1986 Oct;168(1):334–340. doi: 10.1128/jb.168.1.334-340.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker S. J., Lang D. R. Membrane bioenergetic parameters in uncoupler-resistant mutants of Bacillus megaterium. J Biol Chem. 1978 Oct 10;253(19):6738–6743. [PubMed] [Google Scholar]
- Decker S. J., Lang D. R. Mutants of Bacillus megaterium resistant to uncouplers of oxidative phosphorylation. J Biol Chem. 1977 Sep 10;252(17):5936–5938. [PubMed] [Google Scholar]
- Fujii D. K., Fulco A. J. Biosynthesis of unsaturated fatty acids by bacilli. Hyperinduction and modulation of desaturase synthesis. J Biol Chem. 1977 Jun 10;252(11):3660–3670. [PubMed] [Google Scholar]
- Fulco A. J. Fatty acid metabolism in bacteria. Prog Lipid Res. 1983;22(2):133–160. doi: 10.1016/0163-7827(83)90005-x. [DOI] [PubMed] [Google Scholar]
- Fulco A. J. The biosynthesis of unsaturated fatty acids by bacilli. I. Temperature induction of the desaturation reaction. J Biol Chem. 1969 Feb 10;244(3):889–895. [PubMed] [Google Scholar]
- Guffanti A. A., Blumenfeld H., Krulwich T. A. ATP synthesis by an uncoupler-resistant mutant of Bacillus megaterium. J Biol Chem. 1981 Aug 25;256(16):8416–8421. [PubMed] [Google Scholar]
- Guffanti A. A., Chiu E., Krulwich T. A. Failure of an alkalophilic bacterium to synthesize ATP in response to a valinomycin-induced potassium diffusion potential at high pH. Arch Biochem Biophys. 1985 Jun;239(2):327–333. doi: 10.1016/0003-9861(85)90695-2. [DOI] [PubMed] [Google Scholar]
- Guffanti A. A., Clejan S., Falk L. H., Hicks D. B., Krulwich T. A. Isolation and characterization of uncoupler-resistant mutants of Bacillus subtilis. J Bacteriol. 1987 Oct;169(10):4469–4478. doi: 10.1128/jb.169.10.4469-4478.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring F. G., Krisman A., Sedgwick E. G., Bragg P. D. Electron spin resonance studies of lipid fluidity changes in membranes of an uncoupler-resistant mutant of Escherichia coli. Biochim Biophys Acta. 1985 Oct 10;819(2):231–240. doi: 10.1016/0005-2736(85)90178-6. [DOI] [PubMed] [Google Scholar]
- Ito M., Ohnishi Y. Isolation of Escherichia coli mutants which are resistant to an inhibitor of H+-ATPase, tributyltin and also to uncouplers of oxidative phosphorylation. FEBS Lett. 1981 Dec 28;136(2):225–230. doi: 10.1016/0014-5793(81)80623-0. [DOI] [PubMed] [Google Scholar]
- Ito M., Ohnishi Y., Itoh S., Nishimura M. Carbonyl cyanide-m-chlorophenyl hydrazone-resistant Escherichia coli mutant that exhibits a temperature-sensitive unc phenotype. J Bacteriol. 1983 Jan;153(1):310–315. doi: 10.1128/jb.153.1.310-315.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneda T. Fatty acids of the genus Bacillus: an example of branched-chain preference. Bacteriol Rev. 1977 Jun;41(2):391–418. doi: 10.1128/br.41.2.391-418.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MITCHELL P. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature. 1961 Jul 8;191:144–148. doi: 10.1038/191144a0. [DOI] [PubMed] [Google Scholar]
- Quint J. F., Fulco A. J. The biosynthesis of unsaturated fatty acids by bacilli. V. In vivo substrate specificities of fatty acid desaturases. J Biol Chem. 1973 Oct 10;248(19):6885–6895. [PubMed] [Google Scholar]
- Rottenberg H., Hashimoto K. Fatty acid uncoupling of oxidative phosphorylation in rat liver mitochondria. Biochemistry. 1986 Apr 8;25(7):1747–1755. doi: 10.1021/bi00355a045. [DOI] [PubMed] [Google Scholar]
- Spizizen J. TRANSFORMATION OF BIOCHEMICALLY DEFICIENT STRAINS OF BACILLUS SUBTILIS BY DEOXYRIBONUCLEATE. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1072–1078. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong P. T., MacLennan D. H. Restoration by fatty acids of active transport in a lactose transport mutant of Escherichia coli. Can J Biochem. 1973 May;51(5):538–549. doi: 10.1139/o73-067. [DOI] [PubMed] [Google Scholar]



