Abstract
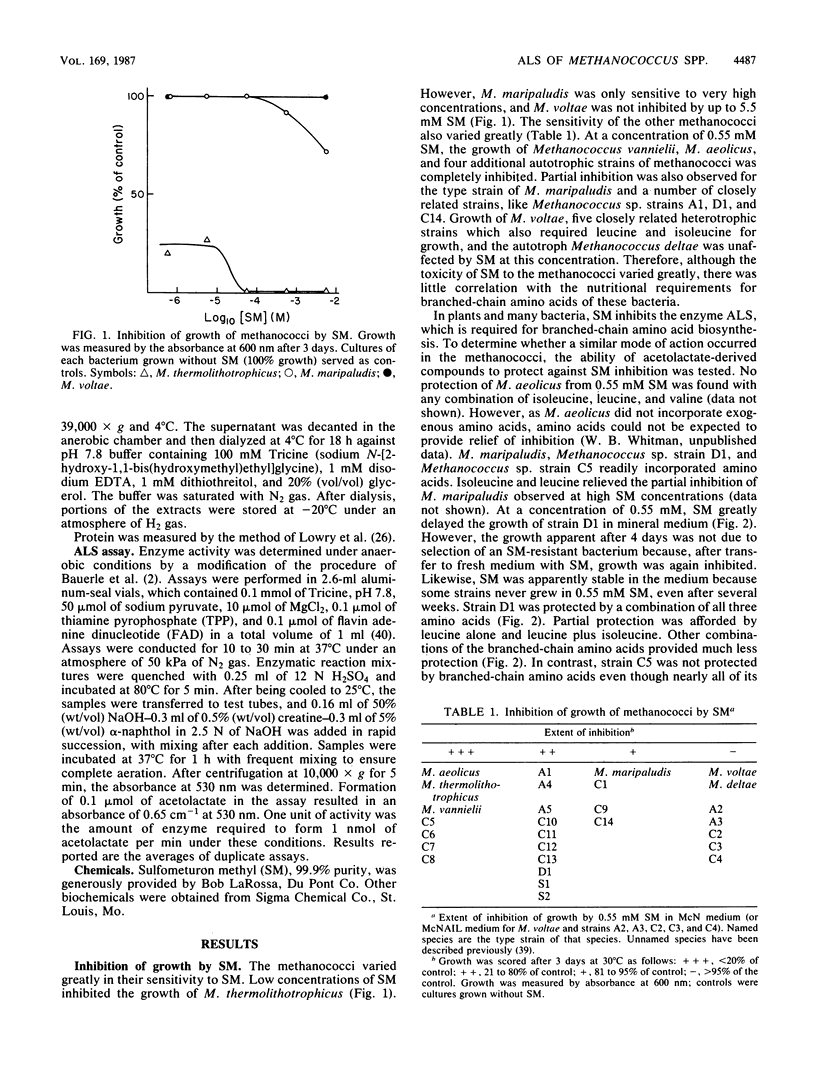
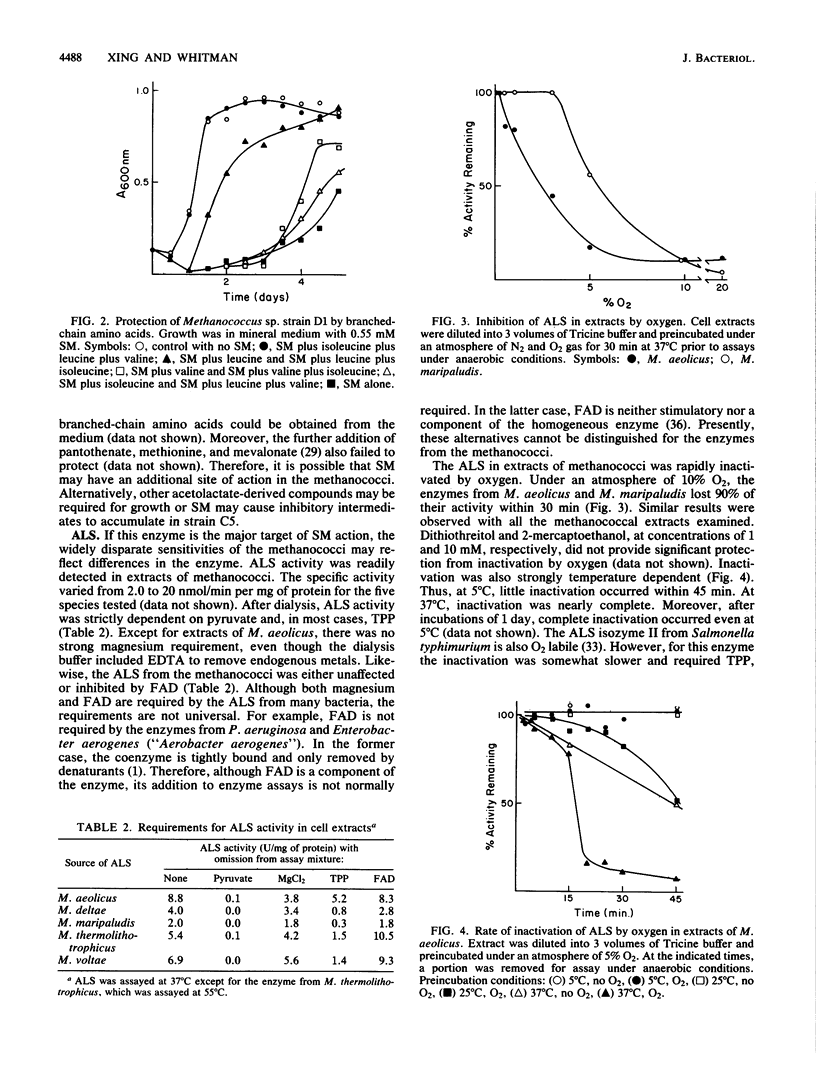
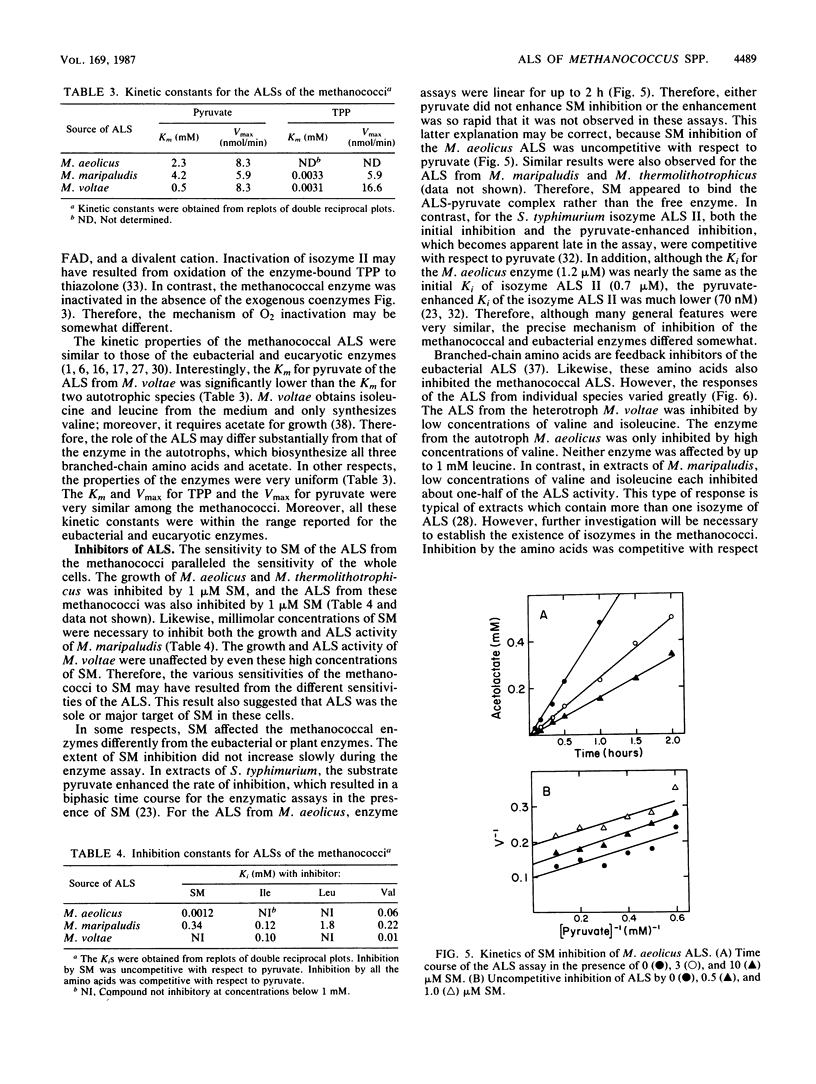
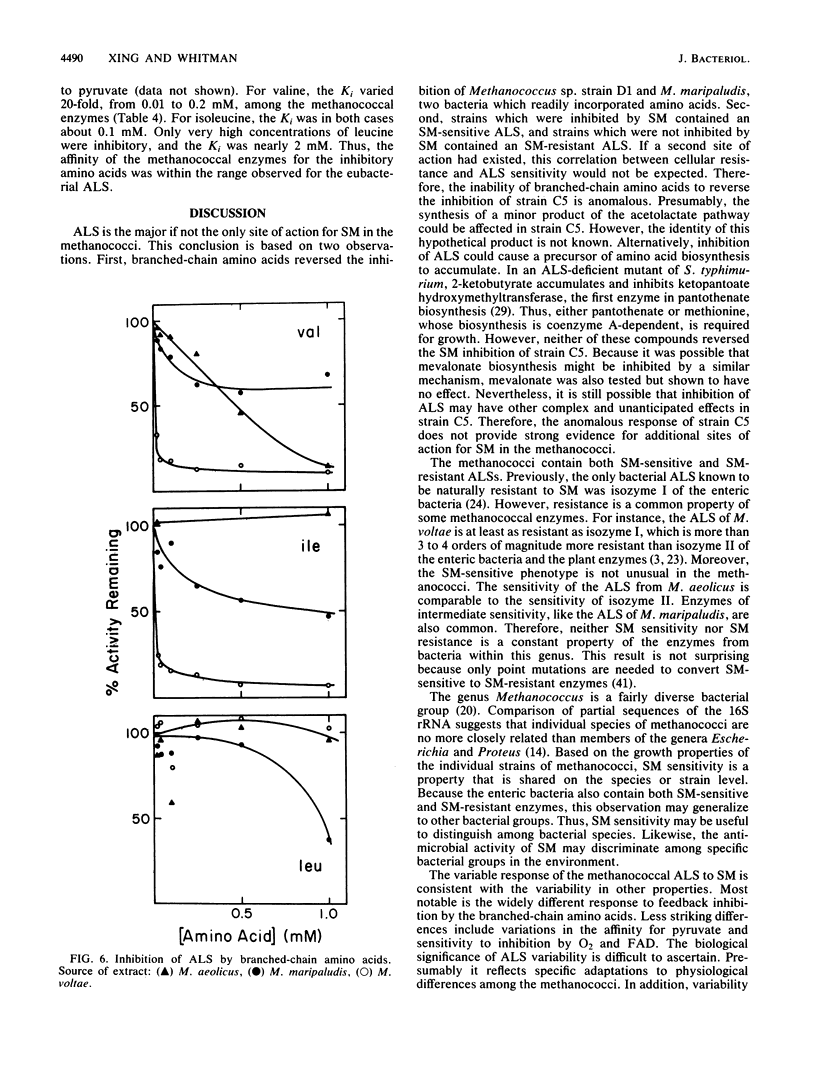
The herbicide sulfometuron methyl (SM) inhibited growth of some methanococci. Of 28 strains tested, the growth of 7 was completely inhibited by 0.55 mM SM. Growth of an additional 14 strains was partially inhibited, and the growth of 7 strains was unaffected by this concentration of SM. In some cases, the branched-chain amino acids protected growth. Growth inhibition was correlated with the Ki for SM of acetolactate synthase (ALS). For the enzymes from bacteria representative of the sensitive, partially resistant, and resistant methanococci (Methanococcus aeolicus, Methanococcus maripaludis, and Methanococcus voltae, respectively), the Ki for SM was 0.0012, 0.34, and greater than 1.0 mM, respectively. Inhibition was uncompetitive with respect to pyruvate. Based on these observations, ALS appeared to be the major if not the sole site of action of SM in the methanococci. The sensitivity of the ALS from these three methanococci to feedback inhibition by branched-chain amino acids was also quite different. Although all three were sensitive to feedback inhibition by valine, the Ki varied 20-fold, from 0.01 to 0.22 mM. Moreover, only the ALS from M. maripaludis was sensitive to inhibition by leucine, and the Ki was 1.8 mM. The Ki for isoleucine for the ALS from both M. maripaludis and M. voltae was about 0.1 mM. The ALS from M. aeolicus was not inhibited by isoleucine. In other respects, the ALSs from the methanococci were very similar. After dialysis, thiamine pyrophosphate but not FAD and Mg2+ was required for maximal activity, and they were all rapidly inactivated by oxygen. Although the methanococcal ALSs exhibited diverse properties, the range of catalytic and regulatory properties closely resembled those of the eubacterial enzymes.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arfin S. M., Koziell D. A. Acetolactate synthase of Pseudomonas aeruginosa I. Purification and allosteric properties. Biochim Biophys Acta. 1973 Sep 15;321(1):348–355. doi: 10.1016/0005-2744(73)90089-2. [DOI] [PubMed] [Google Scholar]
- BAUERLE R. H., FRUENDLICH M., STORMER F. C., UMBARGER H. E. CONTROL OF ISOLEUCINE, VALINE AND LEUCINE BIOSYNTHESIS. II. ENDPRODUCT INHIBITION BY VALINE OF ACETOHYDROXY ACID SYNTHETASE IN SALMONELLA TYPHIMURIUM. Biochim Biophys Acta. 1964 Oct 23;92:142–149. [PubMed] [Google Scholar]
- Chaleff R. S., Mauvais C. J. Acetolactate synthase is the site of action of two sulfonylurea herbicides in higher plants. Science. 1984 Jun 29;224(4656):1443–1445. doi: 10.1126/science.224.4656.1443. [DOI] [PubMed] [Google Scholar]
- De Felice M., Levinthal M., Iaccarino M., Guardiola J. Growth inhibition as a consequence of antagonism between related amino acids: effect of valine in Escherichia coli K-12. Microbiol Rev. 1979 Mar;43(1):42–58. doi: 10.1128/mr.43.1.42-58.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekiel I., Jarrell K. F., Sprott G. D. Amino acid biosynthesis and sodium-dependent transport in Methanococcus voltae, as revealed by 13C NMR. Eur J Biochem. 1985 Jun 3;149(2):437–444. doi: 10.1111/j.1432-1033.1985.tb08944.x. [DOI] [PubMed] [Google Scholar]
- Ekiel I., Smith I. C., Sprott G. D. Biosynthetic pathways in Methanospirillum hungatei as determined by 13C nuclear magnetic resonance. J Bacteriol. 1983 Oct;156(1):316–326. doi: 10.1128/jb.156.1.316-326.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eoyang L., Silverman P. M. Purification and subunit composition of acetohydroxyacid synthase I from Escherichia coli K-12. J Bacteriol. 1984 Jan;157(1):184–189. doi: 10.1128/jb.157.1.184-189.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falco S. C., Dumas K. S., Livak K. J. Nucleotide sequence of the yeast ILV2 gene which encodes acetolactate synthase. Nucleic Acids Res. 1985 Jun 11;13(11):4011–4027. doi: 10.1093/nar/13.11.4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox G. E., Stackebrandt E., Hespell R. B., Gibson J., Maniloff J., Dyer T. A., Wolfe R. S., Balch W. E., Tanner R. S., Magrum L. J. The phylogeny of prokaryotes. Science. 1980 Jul 25;209(4455):457–463. doi: 10.1126/science.6771870. [DOI] [PubMed] [Google Scholar]
- Friden P., Donegan J., Mullen J., Tsui P., Freundlich M., Eoyang L., Weber R., Silverman P. M. The ilvB locus of Escherichia coli K-12 is an operon encoding both subunits of acetohydroxyacid synthase I. Nucleic Acids Res. 1985 Jun 11;13(11):3979–3993. doi: 10.1093/nar/13.11.3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glatzer L., Eakin E., Wagner R. P. Acetohydroxy acid synthetase with a pH optimum of 7.5 from Neurospora crassa mitochondria: characterization and partial purification. J Bacteriol. 1972 Oct;112(1):453–464. doi: 10.1128/jb.112.1.453-464.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimminger H., Umbarger H. E. Acetohydroxy acid synthase I of Escherichia coli: purification and properties. J Bacteriol. 1979 Feb;137(2):846–853. doi: 10.1128/jb.137.2.846-853.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen R. A. Biochemical pathways in prokaryotes can be traced backward through evolutionary time. Mol Biol Evol. 1985 Mar;2(2):92–108. doi: 10.1093/oxfordjournals.molbev.a040338. [DOI] [PubMed] [Google Scholar]
- Jones W. J., Nagle D. P., Jr, Whitman W. B. Methanogens and the diversity of archaebacteria. Microbiol Rev. 1987 Mar;51(1):135–177. doi: 10.1128/mr.51.1.135-177.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LaRossa R. A., Schloss J. V. The sulfonylurea herbicide sulfometuron methyl is an extremely potent and selective inhibitor of acetolactate synthase in Salmonella typhimurium. J Biol Chem. 1984 Jul 25;259(14):8753–8757. [PubMed] [Google Scholar]
- LaRossa R. A., Smulski D. R. ilvB-encoded acetolactate synthase is resistant to the herbicide sulfometuron methyl. J Bacteriol. 1984 Oct;160(1):391–394. doi: 10.1128/jb.160.1.391-394.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawther R. P., Calhoun D. H., Adams C. W., Hauser C. A., Gray J., Hatfield G. W. Molecular basis of valine resistance in Escherichia coli K-12. Proc Natl Acad Sci U S A. 1981 Feb;78(2):922–925. doi: 10.1073/pnas.78.2.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee P. T., Robichon-Szulmajster H. The regulation of isoleucine-valine biosynthesis in Saccharomyces cerevisiae. 3. Properties and regulation of the activity of acetohydroxyacid synthetase. Eur J Biochem. 1968 Feb;3(4):507–511. doi: 10.1111/j.1432-1033.1967.tb19560.x. [DOI] [PubMed] [Google Scholar]
- O'Neill J. P., Freundlich M. Two forms of biosynthetic acetohydroxy acid synthetase in Salmonella typhimurium. Biochem Biophys Res Commun. 1972 Jul 25;48(2):437–443. doi: 10.1016/s0006-291x(72)80070-6. [DOI] [PubMed] [Google Scholar]
- Primerano D. A., Burns R. O. Metabolic basis for the isoleucine, pantothenate or methionine requirement of ilvG strains of Salmonella typhimurium. J Bacteriol. 1982 Jun;150(3):1202–1211. doi: 10.1128/jb.150.3.1202-1211.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raimond J., Grelet N. Mécanisme de régulation de l' -acétohydroxyacide-synthétase chez Bacillus cereus. Biochimie. 1971;53(6):783–788. [PubMed] [Google Scholar]
- Ray T. B. Site of action of chlorsulfuron: inhibition of valine and isoleucine biosynthesis in plants. Plant Physiol. 1984 Jul;75(3):827–831. doi: 10.1104/pp.75.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schloss J. V., Van Dyk D. E., Vasta J. F., Kutny R. M. Purification and properties of Salmonella typhimurium acetolactate synthase isozyme II from Escherichia coli HB101/pDU9. Biochemistry. 1985 Aug 27;24(18):4952–4959. doi: 10.1021/bi00339a034. [DOI] [PubMed] [Google Scholar]
- Sprott G. D., Usher J. R. The electrochemical proton gradient and phenylalanine transport in Escherichia coli irradiated with near-ultraviolet light. Can J Microbiol. 1977 Dec;23(12):1683–1688. doi: 10.1139/m77-242. [DOI] [PubMed] [Google Scholar]
- Squires C. H., De Felice M., Devereux J., Calvo J. M. Molecular structure of ilvIH and its evolutionary relationship to ilvG in Escherichia coli K12. Nucleic Acids Res. 1983 Aug 11;11(15):5299–5313. doi: 10.1093/nar/11.15.5299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Störmer F. C. Isolation of crystalline pH 6 acetolactate-forming enzyme from Aerobacter aerogenes. J Biol Chem. 1967 Apr 25;242(8):1756–1759. [PubMed] [Google Scholar]
- Störmer F. C. The pH 6 acetolactate-forming enzyme from Aerobacter aerogenes. II. Evidence that it is not a flavoprotein. J Biol Chem. 1968 Jul 10;243(13):3740–3741. [PubMed] [Google Scholar]
- Umbarger H. E. Amino acid biosynthesis and its regulation. Annu Rev Biochem. 1978;47:532–606. doi: 10.1146/annurev.bi.47.070178.002533. [DOI] [PubMed] [Google Scholar]
- Whitman W. B., Ankwanda E., Wolfe R. S. Nutrition and carbon metabolism of Methanococcus voltae. J Bacteriol. 1982 Mar;149(3):852–863. doi: 10.1128/jb.149.3.852-863.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitman W. B., Wolfe R. S. Activation of the methylreductase system from Methanobacterium bryantii by corrins. J Bacteriol. 1985 Oct;164(1):165–172. doi: 10.1128/jb.164.1.165-172.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav N., McDevitt R. E., Benard S., Falco S. C. Single amino acid substitutions in the enzyme acetolactate synthase confer resistance to the herbicide sulfometuron methyl. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4418–4422. doi: 10.1073/pnas.83.12.4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Felice M., Lago C. T., Squires C. H., Calvo J. M. Acetohydroxy acid synthase isoenzymes of Escherichia coli K12 and Salmonella typhimurium. Ann Microbiol (Paris) 1982 Mar-Apr;133(2):251–256. [PubMed] [Google Scholar]



