Abstract
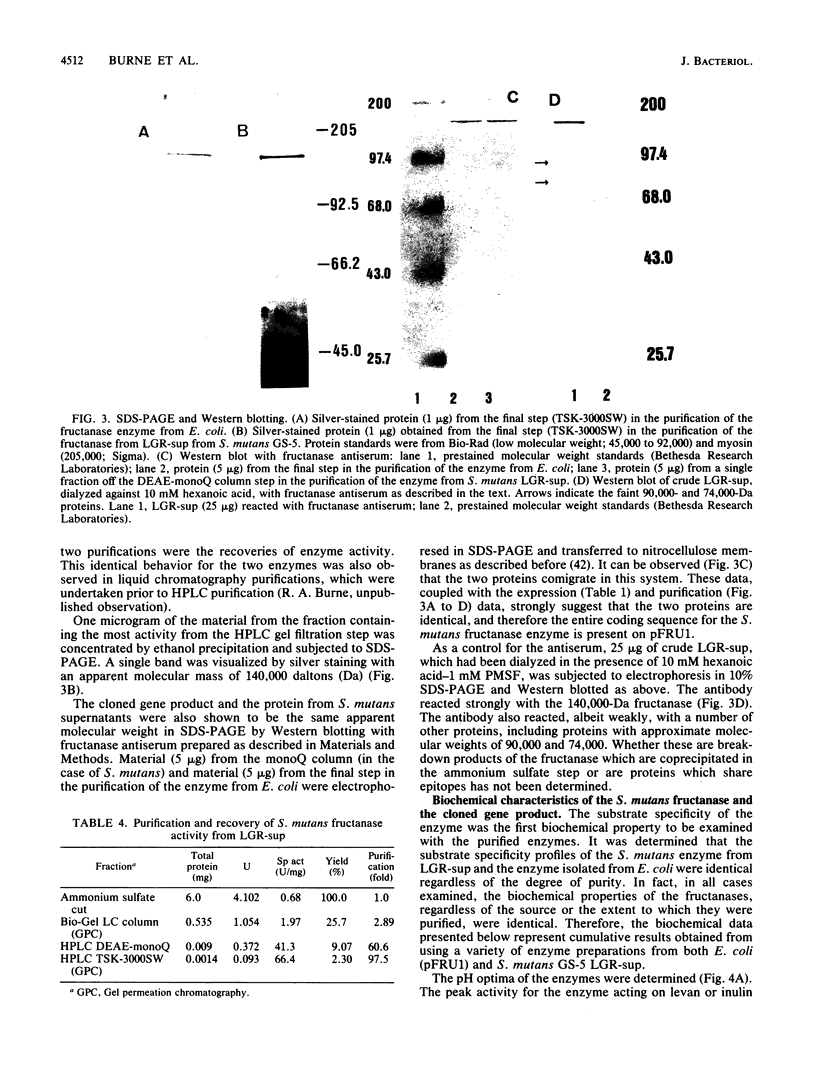
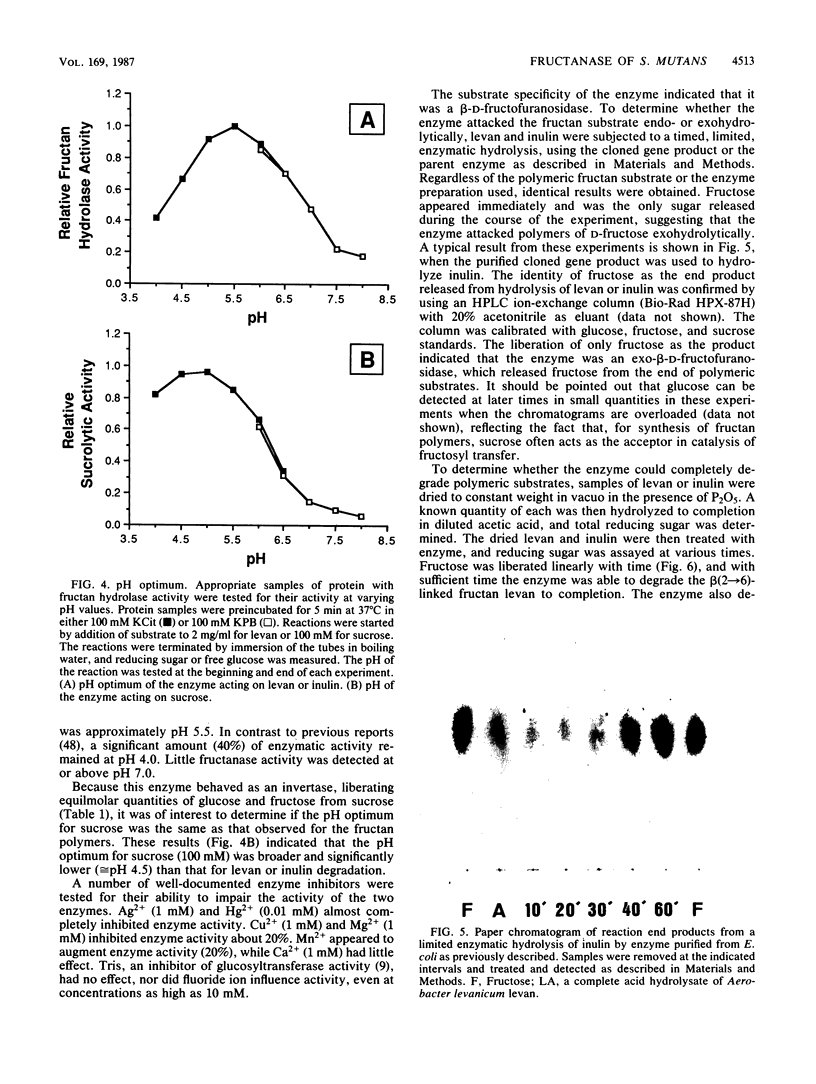
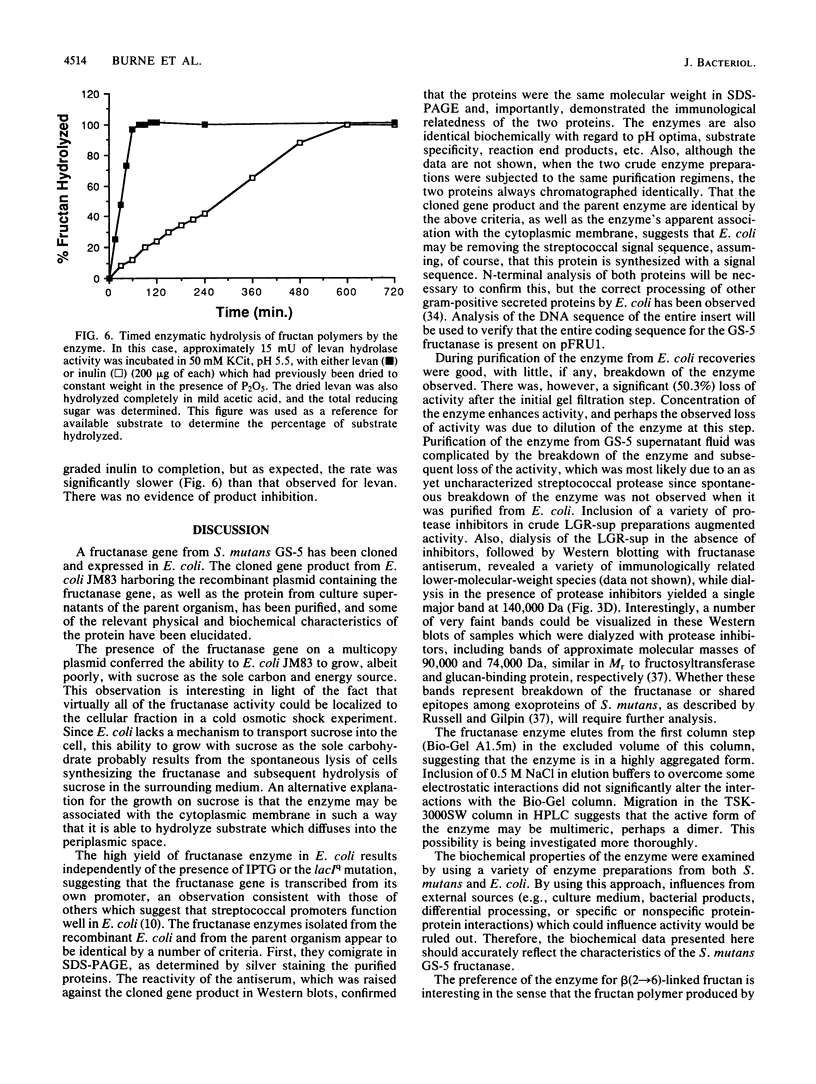
A genetic library of Streptococcus mutans GS-5, constructed in an Escherichia coli plasmid vector, was screened for cells which could utilize sucrose as the sole carbon and energy source. The recombinant plasmid pFRU1, containing a 4.2-kilobase pair insert of S. mutans DNA, was shown to confer this phenotype. Further characterization of the gene product encoded by pFRU1 revealed that the enzyme was a beta-D-fructosidase with the highest specificity for the beta (2----6)-linked fructan polymer levan. The enzyme could also hydrolyze inulin [beta (2----1)-linked fructan], sucrose, and raffinose with 34, 21, and 12%, respectively, of the activity observed for levan. The gene (designated fruA) appeared to be expressed under its own control in E. coli, as judged by the lack of influence on gene product activity of induction or repression of the beta-galactosidase promoter adjacent to the insertion site on the cloning vector. The protein was purified to homogeneity, as judged by silver staining of purified protein in denaturing and reducing conditions in polyacrylamide gels, from sonic lysate of E. coli, as well as from culture supernatants of S. mutans GS-5 grown in a chemostat at low dilution rate with fructose as the sole carbohydrate source. Both purified proteins had an apparent molecular mass of 140,000 daltons in sodium dodecyl sulfate-polyacrylamide gel electrophoresis, were immunologically related and comigrated in sodium dodecyl sulfate-polyacrylamide gel electrophoresis as determined by Western blotting with antisera raised against the cloned gene product, and were identical in all physical and biochemical properties tested. The pH optimum of the enzyme acting on fructan polymers was 5.5, with a significant amount of activity remaining at pH 4.0. The optimum pH for sucrose degradation was broader and lower, with a peak at approximately 4.5. Enzyme activity was inhibited almost completely by Hg2+ and Ag2+, inhibited partially by Cu2+, not inhibited by fluoride ion or Tris, and slightly stimulated by Mn2+ and Co2+. Fructan polymers were attacked exohydrolytically by the enzyme, fructose being the only product released. With sufficient time, both levan and inulin were degraded to completion, with no evidence of product inhibition.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoki H., Shiroza T., Hayakawa M., Sato S., Kuramitsu H. K. Cloning of a Streptococcus mutans glucosyltransferase gene coding for insoluble glucan synthesis. Infect Immun. 1986 Sep;53(3):587–594. doi: 10.1128/iai.53.3.587-594.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird J. K., Longyear V. M., Ellwood D. C. Water insoluble and soluble glucans produced by extracellular glycosyltransferases from Streptococcus mutans. Microbios. 1973 Sep-Oct;8(30):143–150. [PubMed] [Google Scholar]
- Birkhed D., Rosell K. G., Granath K. Structure of extracellular water-soluble polysaccharides synthesized from sucrose by oral strains of Streptococcus mutans, Streptococcus salivarius, Streptococcus sanguis and Actinomyces viscosus. Arch Oral Biol. 1979;24(1):53–61. doi: 10.1016/0003-9969(79)90175-4. [DOI] [PubMed] [Google Scholar]
- Burne R. A., Rubinfeld B., Bowen W. H., Yasbin R. E. Tight genetic linkage of a glucosyltransferase and dextranase of Streptococcus mutans GS-5. J Dent Res. 1986 Dec;65(12):1392–1401. doi: 10.1177/00220345860650120301. [DOI] [PubMed] [Google Scholar]
- Chassy B. M. A gentle method for the lysis of oral streptococci. Biochem Biophys Res Commun. 1976 Jan 26;68(2):603–608. doi: 10.1016/0006-291x(76)91188-8. [DOI] [PubMed] [Google Scholar]
- Chassy B. M., Bielawski R. M., Beall J. R., Porter E. V., Krichevsky M. I., Donkersloot J. A. Extracellular invertase in Streptococcus mutans and Streptococcus salivarius. Life Sci. 1974 Sep 15;15(6):1173–1180. doi: 10.1016/s0024-3205(74)80013-5. [DOI] [PubMed] [Google Scholar]
- Chassy B. M., Porter E. V. Initial characterization of sucrose-6-phosphate hydrolase from Streptococcus mutans and its apparent identity with intracellular invertase. Biochem Biophys Res Commun. 1979 Jul 12;89(1):307–314. doi: 10.1016/0006-291x(79)90979-3. [DOI] [PubMed] [Google Scholar]
- Ciardi J. E., Bowen W. H., Rölla G. The effect of antibacterial compounds on glucosyltransferase activity from Streptococcus mutans. Arch Oral Biol. 1978;23(4):301–305. doi: 10.1016/0003-9969(78)90023-7. [DOI] [PubMed] [Google Scholar]
- Curtiss R., 3rd Genetic analysis of Streptococcus mutans virulence. Curr Top Microbiol Immunol. 1985;118:253–277. doi: 10.1007/978-3-642-70586-1_14. [DOI] [PubMed] [Google Scholar]
- DaCosta T., Gibbons R. J. Hydrolysis of levan by human plaque streptococci. Arch Oral Biol. 1968 Jun;13(6):609–617. doi: 10.1016/0003-9969(68)90139-8. [DOI] [PubMed] [Google Scholar]
- Ebisu S., Kato K., Kotani S., Misaki A. Structural differences in fructans elaborated by streptococcus mutans and Strep. salivarius. J Biochem. 1975 Nov;78(5):879–887. doi: 10.1093/oxfordjournals.jbchem.a130993. [DOI] [PubMed] [Google Scholar]
- Ehrlich J., Stivala S. S., Bahary W. S., Garg S. K., Long L. W., Newbrun E. Levans: I. Fractionation, solution viscosity, and chemical analysis of levan produced by Streptococcus salivarius. J Dent Res. 1975 Mar-Apr;54(2):290–297. [PubMed] [Google Scholar]
- Godson G. N., Vapnek D. A simple method of preparing large amounts of phiX174 RF 1 supercoiled DNA. Biochim Biophys Acta. 1973 Apr 11;299(4):516–520. doi: 10.1016/0005-2787(73)90223-2. [DOI] [PubMed] [Google Scholar]
- Gold W., Preston F. B., Lache M. C., Blechman H. Production of levan and dextran in plaque in vivo. J Dent Res. 1974 Mar-Apr;53(2):442–446. doi: 10.1177/00220345740530024401. [DOI] [PubMed] [Google Scholar]
- Hamada S., Slade H. D. Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiol Rev. 1980 Jun;44(2):331–384. doi: 10.1128/mr.44.2.331-384.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy L., Jacques N. A., Forester H., Campbell L. K., Knox K. W., Wicken A. J. Effect of fructose and other carbohydrates on the surface properties, lipoteichoic acid production, and extracellular proteins of Streptococcus mutans Ingbritt grown in continuous culture. Infect Immun. 1981 Jan;31(1):78–87. doi: 10.1128/iai.31.1.78-87.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazelbauer G. L., Harayama S. Mutants in transmission of chemotactic signals from two independent receptors of E. coli. Cell. 1979 Mar;16(3):617–625. doi: 10.1016/0092-8674(79)90035-7. [DOI] [PubMed] [Google Scholar]
- Higuchi M., Iwami Y., Yamada T., Araya S. Levan synthesis and accumulation by human dental plaque. Arch Oral Biol. 1970 Jun;15(6):563–567. doi: 10.1016/0003-9969(70)90111-1. [DOI] [PubMed] [Google Scholar]
- Jacques N. J., Morrey-Jones J. G., Walker G. J. Inducible and constitutive formation of fructanase in batch and continuous cultures of Streptococcus mutans. J Gen Microbiol. 1985 Jul;131(7):1625–1633. doi: 10.1099/00221287-131-7-1625. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LUCHSINGER W. W., CORNESKY R. A. Reducing power by the dinitrosalicylic acid method. Anal Biochem. 1962 Oct;4:346–347. doi: 10.1016/0003-2697(62)90098-2. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mandel M., Higa A. Calcium-dependent bacteriophage DNA infection. J Mol Biol. 1970 Oct 14;53(1):159–162. doi: 10.1016/0022-2836(70)90051-3. [DOI] [PubMed] [Google Scholar]
- Manly R. S., Richardson D. T. Metabolism of levan by oral samples. J Dent Res. 1968 Nov-Dec;47(6):1080–1086. doi: 10.1177/00220345680470061301. [DOI] [PubMed] [Google Scholar]
- Marshall K., Weigel H. Evidence of multiple branching in the levan elaborated by Streptococcus salivarius strain 51. Carbohydr Res. 1980 Aug 15;83(2):321–326. doi: 10.1016/s0008-6215(00)84544-9. [DOI] [PubMed] [Google Scholar]
- Maynard M. T., Kuramitsu H. K. Purification and antigenic properties of intracellular invertase from Streptococcus mutans. Infect Immun. 1979 Mar;23(3):873–883. doi: 10.1128/iai.23.3.873-883.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa K., Takano T., Sohma A., Yamane K. Secretion activities of Bacillus subtilis alpha-amylase signal peptides of different lengths in Escherichia coli cells. Biochem Biophys Res Commun. 1986 Jan 29;134(2):624–631. doi: 10.1016/s0006-291x(86)80465-x. [DOI] [PubMed] [Google Scholar]
- Robrish S. A., Reid W., Krichevsky M. I. Distribution of enzymes forming polysaccharide from sucrose and the composition of extracellular polysaccharide synthesized by Streptococcus mutans. Appl Microbiol. 1972 Aug;24(2):184–190. doi: 10.1128/am.24.2.184-190.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHILO M., WOLMAN B. Activities of bacterial levans and of lipopolysaccharides in the processes of inflammation and infection. Br J Exp Pathol. 1958 Dec;39(6):652–660. [PMC free article] [PubMed] [Google Scholar]
- Stein D. C., Kopec L. K., Yasbin R. E., Young F. E. Characterization of Bacillus subtilis DSM704 and its production of 1-deoxynojirimycin. Appl Environ Microbiol. 1984 Aug;48(2):280–284. doi: 10.1128/aem.48.2.280-284.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N., Mizuno F., Takamori K. Purification and preliminary characterization of exo-beta-D-fructosidase in Streptococcus salivarius KTA-19. Infect Immun. 1985 Jan;47(1):271–276. doi: 10.1128/iai.47.1.271-276.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Walker G. J., Brown R. A., Taylor C. Activity of Streptococcus mutans alpha-D-glucosyltransferases released under various growth conditions. J Dent Res. 1984 Mar;63(3):397–400. doi: 10.1177/00220345840630030801. [DOI] [PubMed] [Google Scholar]
- Walker G. J., Hare M. D., Morrey-Jones J. G. Activity of fructanase in batch cultures of oral streptococci. Carbohydr Res. 1983 Feb 16;113(1):101–112. doi: 10.1016/0008-6215(83)88222-6. [DOI] [PubMed] [Google Scholar]
- Warner T. N., Miller C. H. Cell-associated levan of Actinomyces viscosus. Infect Immun. 1978 Feb;19(2):711–719. doi: 10.1128/iai.19.2.711-719.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- van Houte J., Jansen H. M. Levan degradation by streptococci isolated from human dental plaque. Arch Oral Biol. 1968 Jul;13(7):827–830. doi: 10.1016/0003-9969(68)90102-7. [DOI] [PubMed] [Google Scholar]