Abstract
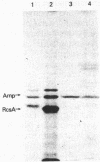
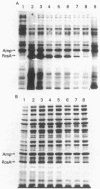
A primary determinant of pathogenicity in Erwinia stewartii is the production of extracellular polysaccharide (EPS). A single mutation can abolish both EPS synthesis and pathogenicity; both properties are restored by a single cosmid clone. Subcloning and insertion analysis have defined a single positive regulatory function which shares a number of similarities with the rcsA function of Escherichia coli K-12, a positive regulator for capsular polysaccharide synthesis. In E. stewartii, the gene promotes the transcription of at least two operons (cps) involved in EPS synthesis; we have previously demonstrated a similar function for rcsA in E. coli. Both genes code for proteins of 25 to 27 kilodaltons; both proteins are unstable in E. coli. The E. stewartii RcsA protein was stabilized in E. coli lon mutants, as the RcsA product from E. coli is. The E. stewartii function complemented E. coli rcsA mutants, and the E. coli RcsA function increased cps expression and restored virulence in E. stewartii mutants. Therefore, these two gram-negative organisms share a similar component of their regulatory circuitry for the control of capsular polysaccharide synthesis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen P., Hart C. A., Saunders J. R. Isolation from Klebsiella and characterization of two rcs genes that activate colanic acid capsular biosynthesis in Escherichia coli. J Gen Microbiol. 1987 Feb;133(2):331–340. doi: 10.1099/00221287-133-2-331. [DOI] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Bradshaw-Rouse J. J., Whatley M. H., Coplin D. L., Woods A., Sequeira L., Kelman A. Agglutination of Erwinia stewartii Strains with a Corn Agglutinin: Correlation with Extracellular Polysaccharide Production and Pathogenicity. Appl Environ Microbiol. 1981 Aug;42(2):344–350. doi: 10.1128/aem.42.2.344-350.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coplin D. L., Frederick R. D., Majerczak D. R., Haas E. S. Molecular cloning of virulence genes from Erwinia stewartii. J Bacteriol. 1986 Nov;168(2):619–623. doi: 10.1128/jb.168.2.619-623.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coplin D. L., Rowan R. G., Chisholm D. A., Whitmoyer R. E. Characterization of plasmids in Erwinia stewartii. Appl Environ Microbiol. 1981 Oct;42(4):599–604. doi: 10.1128/aem.42.4.599-604.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costerton J. W., Irvin R. T., Cheng K. J. The role of bacterial surface structures in pathogenesis. Crit Rev Microbiol. 1981;8(4):303–338. doi: 10.3109/10408418109085082. [DOI] [PubMed] [Google Scholar]
- Figurski D. H., Helinski D. R. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman S., Trisler P., Torres-Cabassa A. Regulation of capsular polysaccharide synthesis in Escherichia coli K-12: characterization of three regulatory genes. J Bacteriol. 1985 Jun;162(3):1111–1119. doi: 10.1128/jb.162.3.1111-1119.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanoff S. S., Riker A. J., Dettwiler H. A. STUDIES ON CULTURAL CHARACTERISTICS, PHYSIOLOGY AND PATHOGENICITY OF STRAIN TYPES OF PHYTOMONAS STEWARTI. J Bacteriol. 1938 Mar;35(3):235–253. doi: 10.1128/jb.35.3.235-253.1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobanputra R. S., Datta N. Trimethoprim R factors in enterobacteria from clinical specimens. J Med Microbiol. 1974 May;7(2):169–177. doi: 10.1099/00222615-7-2-169. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maurizi M. R., Trisler P., Gottesman S. Insertional mutagenesis of the lon gene in Escherichia coli: lon is dispensable. J Bacteriol. 1985 Dec;164(3):1124–1135. doi: 10.1128/jb.164.3.1124-1135.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruvkun G. B., Ausubel F. M. A general method for site-directed mutagenesis in prokaryotes. Nature. 1981 Jan 1;289(5793):85–88. doi: 10.1038/289085a0. [DOI] [PubMed] [Google Scholar]
- Stachel S. E., An G., Flores C., Nester E. W. A Tn3 lacZ transposon for the random generation of beta-galactosidase gene fusions: application to the analysis of gene expression in Agrobacterium. EMBO J. 1985 Apr;4(4):891–898. doi: 10.1002/j.1460-2075.1985.tb03715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland I. W. Microbial exopolysaccharides -- their role in microbial adhesion in aqueous systems. Crit Rev Microbiol. 1983;10(2):173–201. doi: 10.3109/10408418209113562. [DOI] [PubMed] [Google Scholar]
- Torres-Cabassa A. S., Gottesman S. Capsule synthesis in Escherichia coli K-12 is regulated by proteolysis. J Bacteriol. 1987 Mar;169(3):981–989. doi: 10.1128/jb.169.3.981-989.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trisler P., Gottesman S. lon transcriptional regulation of genes necessary for capsular polysaccharide synthesis in Escherichia coli K-12. J Bacteriol. 1984 Oct;160(1):184–191. doi: 10.1128/jb.160.1.184-191.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Way J. C., Davis M. A., Morisato D., Roberts D. E., Kleckner N. New Tn10 derivatives for transposon mutagenesis and for construction of lacZ operon fusions by transposition. Gene. 1984 Dec;32(3):369–379. doi: 10.1016/0378-1119(84)90012-x. [DOI] [PubMed] [Google Scholar]