Abstract
The dissociation, migration, and remodeling of epithelial monolayers induced by hepatocyte growth factor (HGF) entail modifications in cell adhesion and in the actin cytoskeleton through unknown mechanisms. Here we report that ezrin, a membrane–cytoskeleton linker, is crucial to HGF-mediated morphogenesis in a polarized kidney-derived epithelial cell line, LLC-PK1. Ezrin is a substrate for the tyrosine kinase HGF receptor both in vitro and in vivo. HGF stimulation causes enrichment of ezrin recovered in the detergent-insoluble cytoskeleton fraction. Overproduction of wild-type ezrin, by stable transfection in LLC-PK1 cells, enhances cell migration and tubulogenesis induced by HGF stimulation. Overproduction of a truncated variant of ezrin causes mislocalization of endogenous ezrin from microvilli into lateral surfaces. This is concomitant with altered cell shape, characterized by loss of microvilli and cell flattening. Moreover, the truncated variant of ezrin impairs the morphogenic and motogenic response to HGF, thus suggesting a dominant-negative mechanism of action. Site-directed mutagenesis of ezrin codons Y145 and Y353 to phenylalanine does not affect the localization of ezrin at microvilli, but perturbs the motogenic and morphogenic responses to HGF. These results provide evidence that ezrin displays activities that can control cell shape and signaling.
The so-called epithelial–mesenchymal transitions, such as migration in embryonic development, invasion, and metastasis, are important processes occurring in both physiological and pathological situations. Mesenchymal cells secrete hepatocyte growth factor (HGF)1, a glycoprotein which acts in a paracrine fashion to induce the proliferation, dissociation, motility (scattering), and invasiveness of epithelial cells (Comoglio and Vigna, 1995). HGF has also recently been shown to be a survival factor (Bardelli et al., 1996) and to protect cells from “anoikis,” a form of apoptosis induced by disruption of cell adhesion (Frisch and Francis, 1994; Amicone et al., 1997). Through the concerted activation of these complex biological responses, HGF directs the remodeling of epithelial cells grown in three-dimensional collagen gels in vitro (Montesano et al., 1991; Berdichevsky et al., 1994; Brinkmann et al., 1995; Soriano et al., 1995; Medico et al., 1996), promotes branching morphogenesis in mammary gland (Yang et al., 1995) and metanephric organ cultures (Woolf et al., 1995), and stimulates angiogenesis in vivo (Bussolino et al., 1992; Grant et al., 1993). It is also involved in development of the spinal cord during embryogenesis (Bronner-Fraser, 1995). Disruption of HGF or HGF-receptor genes by homologous recombination shows that they have a major function for placental, liver, and limb muscle development in vivo (Bladt et al., 1995; Schmidt et al., 1995; Uehara et al., 1995; Maina et al., 1996). Thus HGF also controls migration of myogenic precursor cells from the somites to the limb bud.
The diverse biological effects of HGF are transmitted through activation of its transmembrane receptor, the tyrosine kinase encoded by the c-met proto-oncogene (Bottaro et al., 1991; Naldini et al., 1991). In epithelial cells, the HGF-receptor is localized at the lateral surface (Prat et al., 1991a ; Crepaldi et al., 1994). The mechanisms by which HGF triggers cell motility clearly depend on the activation of multiple intracellular molecular pathways. Different molecules containing SH2 domains bind to the tyrosine-phosphorylated HGF-receptor (Graziani et al., 1991; Bardelli et al., 1992; Ponzetto et al., 1994; Pelicci et al., 1995). So far, the roles of Shc, Ras, PI3-kinase, and PLC-γ in the HGF-mediated motility signal have been established. Overproduction of Shc increases cell motility in response to HGF (Pelicci et al., 1995). The expression of a dominant-negative mutant Ras protein (Hartmann et al., 1994) and the microinjection of a neutralizing antibody for Ras (Ridley et al., 1995) block HGF-induced dissociation and scattering of MDCK epithelial cells. Drug inhibition studies suggest that PI3-kinase (Derman et al., 1996; Royal and Park, 1995) and phospholipase C-(PLC)-γ (Derman et al., 1996) activation are required for HGF-induced motility. More recently, an insulin receptor 1–like substrate (Gab1) has been identified to bind specifically to c-met by the yeast two-hybrid system. Its overproduction in MDCK cells is sufficient to induce scattering and tubulogenesis (Weidner et al., 1996). However, the mechanisms by which HGF triggers cell motility and coordinates the cell adhesion system with the actin cytoskeleton machinery are not known. Cytochalasin D treatment prevents MDCK scattering (Rosen et al., 1990), suggesting the involvement of the actin cytoskeleton machinery. The small GTP-binding proteins, Rac (Ridley et al., 1995) and Rho (Nishiyama et al., 1994), are involved in HGF-induced membrane ruffling. Several proteins, including β-catenin, plakoglobin (Shibamoto et al., 1994), and focal adhesion kinase (Matsumoto et al., 1994), implicated in the control of cell adhesion, are tyrosine phosphorylated after stimulation with HGF. Here we report that ezrin, a membrane–cytoskeleton linker, is a downstream target of the HGF-receptor. We show that ezrin is required for the motility and morphogenetic responses induced by HGF in the kidney-derived LLC-PK1 cell line.
Ezrin and the two closely related proteins radixin and moesin constitute the ezrin-radixin-moesin (ERM) protein family. The ERMs belong to a superfamily of proteins whose prototypes are talin and band 4.1, two proteins whose roles in membrane-cytoskeleton interaction are well documented (for review see Arpin et al., 1994; Tsukita et al., 1997). That ezrin too has a similar role is supported by the following: (a) it is primarily expressed in the microvilli and other actin-rich surface projections (Bretscher, 1983; Berryman et al., 1993); (b) it associates with various membrane proteins such as the hyaluronan receptor CD44 (Tsukita et al., 1994), ICAM-2 (Helander et al., 1996), and type II cAMP–dependent protein kinases (Dransfield et al., 1997); (c) its COOH-terminal domain is capable of direct association with F-actin in vitro (Turunen et al., 1994; Pestonjamasp et al., 1995) and in vivo (Algrain et al., 1993), while the complete protein binds poorly to F-actin in solution. The last observation suggests that the F-actin binding site could be masked in the wild-type protein, an observation confirmed when ezrin was found to be capable of intermolecular associations leading to homo- and hetero-oligomerization (Gary and Bretscher, 1993; Andreoli et al., 1994; Berryman et al., 1995; Bretscher et al., 1995). Moreover, it has been shown that in the native ezrin molecule, the F-actin binding site and the oligomerization domains are masked by intramolecular association between head and tail domains (Gary and Bretscher, 1995). Thus, it has been suggested that both interaction with F-actin and oligomerization contribute to the assembly of cell surface structures, like microvilli and membrane ruffles (Berryman et al., 1995). Regulatory signals (for example, phosphorylation) are likely to change the protein conformation and unmask the F-actin binding site and the oligomerization domains. To further support this model, it was shown that overproduction of the COOH-terminal half of ezrin alone displays morphogenic effects in transfected cells (Martin et al., 1995), and these effects can be inhibited by concomitant overproduction of the NH2-terminal half.
We report that ezrin is a downstream target of the HGF-receptor for the following reasons: (a) Ezrin is a substrate for the tyrosine kinase HGF-receptor (b) Overproduction of the wild-type ezrin enhances the motility response to HGF, as measured by the faster healing of a wound made in subconfluent cell monolayer, as well as cyst and tubule formation of LLC-PK1 cells grown in collagen gels. (c) Introduction of the NH2-terminal half of ezrin into LLC-PK1 cells induces the redistribution of endogenous ezrin from apical microvilli to lateral cell–cell contacts, and the loss of functional ezrin alters the cell shape and impairs the HGF-mediated cell migration and tubulogenesis. (d) Mutations of Y145 and Y353 to phenylalanine also alter the response to HGF. Thus, ezrin seems to play a critical role in coordinated control of cell shape, motility, and signaling.
Materials and Methods
Cells, Recombinant Proteins, and Antibodies
LLC-PK1 (CCL 101; American Type Culture Collection, Rockville, MD) and MDCK (CCL 34; American Type Culture Collection) cells were grown in DME growth medium (GIBCO BRL, Gaithersburg, MD) supplemented with 10% FCS and maintained at 37°C in 10% CO2. As a source of HGF we used either dia-filtered, human fibroblast MRC5-conditioned medium or purified recombinant HGF from the baculovirus expression system (Naldini et al., 1995), kindly provided by Dr. C. Stella, Institute for Cancer Research, University of Torino, Italy. The two preparations yielded 3 and 0.9 μg/ml purified protein, respectively (0.3 ng = 1 scatter unit). The glutathione-S-transferase–fused kinase domain of c-MET cDNA was cloned into baculovirus vector as described (Bardelli et al., 1992) and kindly provided by Dr. A. Bardelli, Institute for Cancer Research. PGEX-2T plasmids containing the cDNA coding for the full-length ezrin and for its NH2-terminal domain (amino acids 1–309) were constructed and used for the production of fusion proteins as previously described (Andreoli et al., 1994).
P5D4 mAb raised against the 11–amino acid COOH terminus of the vesicular stomatitis virus glycoprotein G (VSVG) was previously described (Kreis, 1986). The DO-24 mAb was raised against the extracellular domain of the HGF-receptor (Prat et al., 1991b ). Rabbit polyclonal anti-ezrin antibody was raised against the entire ezrin produced in bacteria and was previously described (Algrain et al., 1993). Rabbit polyclonal anti-phosphotyrosine and anti–ZO-1 antisera were purchased from Zymed Laboratories (South San Francisco, CA). Rabbit polyclonal anti– p62c-yes antiserum was from Santa Cruz Biotechnology (Santa Cruz, CA) and mAb anti–β-catenin was from Transduction Laboratories (Lexington, KY). Antisera against GST were produced by Dr. M Arpin.
DNA Constructs and Transfection
cDNA coding for either wild-type or NH2-terminal domain of human ezrin were fused to oligonucleotides encoding the 11–amino acid COOH terminus of the VSVG as described (Algrain et al., 1993). The fused cDNAs were then inserted into the expression vector pCB6, downstream from the cytomegalovirus promoter. For generating the plasmid-producing ezrin mutated on tyrosines 145 and 353, the following constructs were made: to make the F353 mutant, the two oligonucleotides, 5′ CGGAATTCCGGCTGCAGGACTTTGAGGAG 3′ and 5′ CGCGGATCCATTGTGGGTCCTCTTA 3′ (flanked with EcoRI and BamHI restriction sites, respectively), were used to amplify the fragment (nucleotides 1,125–1,730) using the Ampli Taq polymerase (Perkin-Elmer Corp., Norwalk, CT). This fragment was then subcloned into the Bluescript plasmid and checked by double-strand DNA sequencing using the T7 sequencing kit (Pharmacia Fine Chemicals, Piscataway, NJ). The PstI–PstI fragment corresponding to the sequence 1,131–1,197 and containing the mutated codon was inserted into the AvaI–AvaI fragment (nucleotides 1,002–1,698) in the plasmid psp64. The AvaI–AvaI fragment of the full-length ezrin cDNA in the plasmid psp64 was then replaced by the AvaI–AvaI fragment containing the mutated codon. To make the F145 mutant, a double amplification by PCR was performed with the Taq polymerase. A first amplification was made with the oligonucleotides 5′ ATCCATGCCGAAACCAATCAATGTCCGAGTTACCAC 3′ and 5′ AGAGCTGAGGAACCCAGACTT 3′. The fragment generated was used as a primer to perform a second amplification with the oligonucleotide 5′ GTTCCTGATTTCACTCCAAG 3′. The fragment obtained was inserted into the plasmid pCRTMII, using the TA cloning site (Invitrogen, Carlsbad, CA), and checked by the double-strand DNA sequencing. The plasmid was then digested by the unique restriction sites NcoI and HpaI corresponding to the sequence 108–777. This fragment with the mutated codon was then inserted in the ezrin cDNA containing the F353 mutant. This double mutant ezrin cDNA was then cloned in the expression vector pCB6 through the HindIII and XbaI restriction sites. Exponentially growing LLC-PK1 cells were seeded 24 h before DNA transfer on 10-cm tissue culture dishes. DNA transfer was performed following the procedure of Chen and Okayama (1987), and transfected cells were selected by growing in media containing 0.7 mg/ml G-418 for 2–3 wk. For each transfection, three/four clones which overproduced the transfected protein (as detected by immunoblot and immunofluorescence analysis) were selected for further study.
Indirect Immunofluorescence and Scanning Electron Microscopy
For indirect immunofluorescence studies, cells grown on coverslips were fixed with 3% paraformaldehyde, permeabilized with 0.5% Triton X-100, incubated first with primary antibodies, and then incubated with rhodamine- or fluorescein-conjugated secondary antibodies. Samples were viewed with Zeiss epifluorescence optics (Carl Zeiss, Inc., Thornwood, NY). For confocal laser scanner microscopy (CLSM), cells were seeded at 2.2 × 105/cm2 on 12-mm Transwells filters, grown for 4 d, and processed for indirect immunofluorescence. Affinity-purified polyclonal anti-ezrin and monoclonal P5D4 antibodies were added to both apical and basolateral compartments of the Transwell unit. Transwell filters were then incubated with rhodamine-coupled anti–rabbit, and fluorescein-linked anti–mouse IgG antibodies, mounted on glass slides in a solution of Mowiol (Calbiochem-Novabiochem Corp., La Jolla, CA) and viewed on a Leica CLSM (Vienna, Austria). For each X-Y section, the intensity of fluorescence was determined with arbitrary units 1–255. All readings were normalized to this scale. All X-Y sections were summed, and the intensities were averaged and represented with the color scale on two dimensions.
Scanning EM was carried out on cultures grown on coverslips to confluency. After fixation with 2% glutaraldehyde in cacodylate buffer, cultures were postfixed in 2% OsO4 aqueous solution and dehydrated in a graded series of ethanol incubation. Wet coverslips were transferred into Freon 113 and dried after substitution with liquid CO2 in a Balzers critical point drier (Balzers S.P.A., Milan, Italy). Dried cultures were coated with gold with a Polaron gold sputter coater (Polaron Instruments, Inc., Hatfield, PA). Samples were viewed with a scanning electron microscope.
Biological Assays
For the wound healing assay 1.5 × 106 cells were seeded into 35-mm plate wells and grown for 48 h. Cells were washed with DME, and a wound was marked in the confluent monolayer using a plastic pipet tip. Cells were then incubated for 15 h in the presence of 0.2% FCS, ±15 ng/ml HGF. After wounding, time-lapse video microscopy was performed with an Axiovert 135 inverted microscope (Carl Zeiss, Inc., Thornwood, NY) linked to a camera. Motility measurements of the margins of the wound were performed over time with a micrometer.
For the tubulogenesis assay in three-dimensional collagen gels, the trypsinized cells were suspended at a final concentration of 1 × 105 cells per ml in gelling solution, prepared as follows: 1 part DME 10× (GIBCO BRL), 1 part NaHCO3 (37 g/liter), and 1 part FCS all were mixed with 3.5 parts of a suspension of 3 × 105 cells per ml, and 3.5 parts of type I collagen at 5 mg/ml (Becton Dickinson, Bedford, MA) at room temperature. 100 μl of this mixture was seeded in a microtiter plate onto 100 μl of a first layer of collagen without cell suspension. After 5 min at 37°C, the gels were covered with cell culture medium ±30 ng/ml HGF. Photographs were taken with a light microscope (Leica) equipped with Nomarski interference optics. To analyze the structure of the tubules, cells were fixed with 4% paraformaldehyde, and embedded in 10% gelatin at 37°C. Small blocks were cut from the gel and frozen in liquid nitrogen in the presence of 2.5 M sucrose. Semithin sections were stained with toluidine blue and analyzed with a light microscope (Axiophot; Carl Zeiss, Inc.).
For the cell proliferation assay, the Amersham kit (Amersham Intl., Little Chalfont, UK) was used to measure incorporation of the thymidine analogue 5-bromo-2′-deoxyuridine (BrdU) into nascent DNA. Briefly, cells were plated in microtiter plate to reach confluence, starved in DME 0.2% FCS for 24 h, and then stimulated with 15 ng/ml of HGF. After 6 h, BrdU was added and left to incorporate for another 3 h. Incorporated BrdU was detected by a specific mAb and a peroxidase-conjugated secondary antibody. Incubation with chromogen peroxidase substrate yielded a soluble green dye with absorbance at 410 nm.
HGF Stimulation, Cell Lysis, and Immunoprecipitation
Cells were seeded at 1.5 × 104/cm2 on 24.5-mm Transwell filters, grown for 3 d in spent medium, and then stimulated with DME containing 25 mM Hepes, 100 μg/ml bacitracin, 0.05% BSA, with or without 120 ng/ml of HGF for 10 min at 37°C. Four Transwell filters were used for each immunoprecipitation. Cells were washed at 4°C and lysed in 20 mM Tris-HCl, pH 7.4, 150 mM NaCl, 0.1% SDS, 1% Triton X-100, 1% deoxycholic acid, sodium salt, 5 mM EDTA, 1 mM Na3VO4 (RIPA buffer), and inhibitors of proteases (2 mM PMSF [Sigma Chemical Co., Poole, UK], 50 μg/ ml pepstatin, 50 μg/ml chymostatin, and 10 μg/ml antipain [Chemicon Intl., Inc., Temecula, CA]) for 15 min on ice. Clarified cell extracts were rotated 2 h at 4°C with antibodies covalently linked to Sepharose protein A by cross-linking with dimethyl pimelimidate (Pierce Chemical Co., Rockford, IL). Beads were washed three times with lysis buffer and samples were eluted by boiling in Laemmli buffer containing 100 mM DTT. Eluted proteins were electrophoresed on 7% and 10% SDS-PAGE, transferred to nitrocellulose, and probed with specific primary and secondary antibodies. Immunoblots were developed with an enhanced chemiluminescence kit (ECL; Amersham Intl.), according to the instructions of manufacturer, and visualized on XOMAT AR films (Eastman Kodak Co., Rochester, NY).
Cell Fractionation into Detergent-soluble and -insoluble Fractions
Cells were seeded at 6 × 104/cm2 on 24.5-mm Transwell filters, grown for 3 d in spent medium, and then stimulated with 120 ng/ml of HGF for 10 min at 37°C. Cells were extracted on the filters for 40 s at room temperature with 300 μl of the extraction buffer MES [50 mM 2-(N-morpholino) ethane sulfonic acid, 3 mM EGTA, 5 mM MgCl2, 0.5% Triton X-100, pH 6.4]. Detergent-soluble fractions were precipitated for 3 h in 85% acetone at −20°C and the pellets were recovered after centrifugation for 10 min at 300 g at 4°C. These pellets and the detergent-insoluble material were resuspended in the same volume of Laemmli buffer before protein separation on a 7% SDS-PAGE and transfer to nitrocellulose. The blots were either probed with the polyclonal anti-ezrin antibody or with the monoclonal anti-tag antibody (P5D4), and were developed with an enhanced chemiluminescence kit. The bands were quantitated on a Bio-Profil station (Vilbert-Lourmat, Marne-La-Vallée, France). The protein levels were normalized to the sum of protein in the supernatant and pellet fractions.
In Vitro Kinase Assay
Sf9 cells expressing the recombinant GST-fused kinase domain of HGF-receptor (∼2 × 106 cells per point) were lysed 48 h after infection, in buffer A (10 mM Tris-HCl buffer, pH 7.5, 150 mM NaCl [TBS], 10% glycerol, 1% Triton X-100, 5 mM EDTA), and inhibitors of proteases (2 mM PMSF [Sigma Chemical Co.], 50 μg/ml pepstatin, 50 μg/ml chymostatin, and 10 μg/ml antipain [Chemicon Inc.]) for 15 min at 4°C. Clarified cell lysates were coupled to glutathione–Sepharose beads for 1 h at 4°C. The beads were washed two times with buffer A and once with buffer B (25 mM Hepes buffer, pH 7.2, 100 mM NaCl, 5 mM MgCl2, 0.1% Triton X-100). The in vitro kinase assay was performed in 100 μl of buffer B containing 0.1 mM Na3VO4, 0.1 mM ATP, with or without 12 μg ezrin or NH2-terminal domain of ezrin fused to GST for 5 min at 30°C. Beads were washed twice with TBS–10 mM EDTA; proteins were eluted by boiling in Laemmli buffer, electrophoresed on 10% SDS-PAGE, and Western blotted with anti-phosphotyrosine and anti-GST polyclonal antibodies.
Results
Isolation of LLC-PK1 Cells Overproducing Wild-Type Ezrin or NH2-terminal Domain of Ezrin
LLC-PK1 is a polarized epithelial cell line derived from the proximal tubules of pig kidney (Pfaller et al., 1990). It displays a well-developed brush border at the apical surface. We generated stable transfectants using the pCB6 eukaryotic expression vector, which carries cDNA constructs coding for wild-type ezrin or its NH2-terminal domain (amino acids 1–309), as previously described (Algrain et al., 1993). Both cDNAs were fused at the COOH terminus to oligonucleotides encoding the 11–amino acid COOH terminus of the VSVG, which allowed discrimination between the transfected and endogenous ezrins. The G418-resistant colonies were screened for transfected ezrin expression by anti-tag antibody reactivity in Western blot and immunofluorescence. Protein levels in the transfected cells were determined by quantitative immunoblotting of total cell extracts (0.1–2 μg), and then compared to that of endogenous ezrin in untransfected LLC-PK1 cells (Fig. 1). Clones E7 (transfected with wild-type ezrin) and N2 (transfected with the NH2-terminal domain of ezrin) produced high protein levels by Western blot analysis (10-fold and threefold the endogenous ezrin level, respectively), and relatively homogenous and strong staining by immunofluorescence with anti-tag antibody (Fig. 2). Four other independent clones with similar features analyzed during the study gave identical results as E7 and N2 cells. LLC-PK1 cells transfected with vector alone behaved like untransfected LLC-PK1 cells.
Figure 1.
Protein levels of endogenous and transfected ezrin. Serial dilutions of total cell extracts (0.1–2 μg) were run on a 7% SDS-PAGE under reducing conditions, and transferred to nitrocellulose membranes. The membranes were probed with polyclonal anti-ezrin antibody (lanes A–C) and monoclonal P5D4 antibody (lane D), followed by peroxidase-conjugated secondary antibody, and developed with chemiluminescence. (Lanes A–D) 1 μg of total cell extract. (Lane A) LLC-PK1 cells; (lane B) E7 cells; (lane C) FF1 cells (see p. 428); (lane D) N2 cells.
Figure 2.
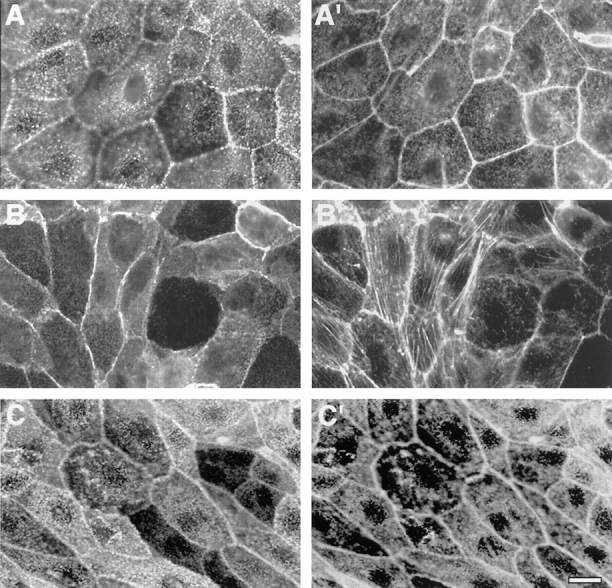
Cellular distribution of ezrin and its mutants in transfected LLC-PK1 cells by immunofluorescence analysis. (A and A′) Cells transfected with the cDNA encoding the epitope-tagged, full-length ezrin–E7 clone. (B and B′) Cells transfected with the cDNA encoding the epitope-tagged NH2-terminal domain of ezrin–N2 clone. (C and C′) Cells transfected with the cDNA encoding the epitope-tagged ezrin with mutations of Y145 and Y353 into phenylalanine–FF1 clone. Paraformaldehyde-fixed and detergent-permeabilized cells were double labeled with the anti-tag mAb P5D4 followed by incubation with FITC-conjugated secondary antibody (A–C), and rhodamine-coupled phalloidin (A′–C′). Bar, 7 μm.
Overproduction of the NH2-terminal Domain of Ezrin Prevents Microvilli Formation
The E7 cells displayed a phenotype similar to that of the parental cell line, while the N2 cells were quite different. When grown at low density, N2 cells were flattened and spread out; at confluency, they had a spindle-like cell shape (Fig. 2, B and B′). In immunofluorescence, the transfected NH2-terminal domain of ezrin was found in the cytosol and underneath the membranes, mainly concentrated along the lateral surface at cell–cell boundaries (Fig. 2). It did not colocalize with actin-stress fibers (Fig. 2, B and B′), as already reported for CV1 cells (Algrain et al., 1993). However, we observed an increase in the amount of stress fibers in the N2 cells (Fig. 2 B′). Three- dimensional analysis by CLSM of endogenous ezrin and transfected NH2-terminal domain of ezrin in N2 cells revealed the expression of both proteins to be reduced at the apical surface and now concentrated at lateral surfaces. Cells producing wild-type ezrin did not show comparable pattern (Fig. 3 A). The distribution of tight and adherens junction markers, such as ZO-1 and β-catenin, was unchanged (data not shown). Scanning EM of N2 cells showed that their microvilli were severely reduced in number and shorter in length (Fig. 3 B). Notably, a row of long microvilli was present at cell–cell contacts. On the contrary, untransfected LLC-PK1 cells showed a well-developed brush border.
Figure 3.


Dominant-negative effects of NH2-terminal domain of ezrin in LLC-PK1 cells. (A) E7 cells, (top) were labeled with the anti-tag mAb P5D4 (E); N2 cells (bottom) were double labeled with mAb P5D4 (N) and rabbit polyclonal anti–ezrin antibody (E), which does not recognize the NH2-terminal domain of ezrin. FITC-conjugated anti–mouse IgG and TRITC-conjugated anti–rabbit IgG secondary antibodies were used. Horizontal optical sections (insets) and three-dimensional analyses were obtained by CLSM. For each X–Y section, the intensity of fluorescence was determined with arbitrary units 1–255. All readings were normalized to this scale. All X–Y sections were summed, and the intensities were averaged and represented with the color scale on two dimensions. (B) LLC-PK1 cells (top) and N2 cells (bottom) were analyzed by scanning EM. Bar, 1 μm.
Altogether, these results suggest that the production of the NH2-terminal domain of ezrin in LLC-PK1 cells impedes the correct localization of endogenous ezrin by a dominant-negative mechanism. This is concomitant with impaired microvilli formation.
Ezrin Is a Substrate of HGF-Receptor
Ezrin is tyrosine phosphorylated upon stimulation with growth factors (Bretscher, 1989; Fazioli et al., 1993). We thus investigated if ezrin is a target for the tyrosine kinase activity of the HGF-receptor (HGF-R), in vitro with the recombinant proteins and in vivo using the N2 and E7 cells. The GST-fused HGF-R kinase domain, produced in the baculovirus system, and the GST-fused wild-type ezrin and NH2-terminal domain, produced in bacteria, were purified on glutathione–Sepharose and tested using an in vitro kinase assay, followed by a Western blot with anti-phosphotyrosine antibodies. Fig. 4 A shows that the kinase domain of HGF-R can phosphorylate itself and wild-type ezrin. We found the NH2-terminal domain of ezrin to be poorly phosphorylated by HGF-R in vitro (Fig. 4 A) and not at all in vivo in N2 cells (Fig. 4 C, left). On the contrary, when E7 cells were stimulated in vivo with 120 ng/ml HGF, ezrin and the β chain of HGF-R were phosphorylated (Fig. 4 B, left). Although ezrin did not coimmunoprecipitate with the HGF-R in LLC-PK1 cells, it did in MDCK cells, a cell line derived from kidney distal tubules (data not shown).
Figure 4.
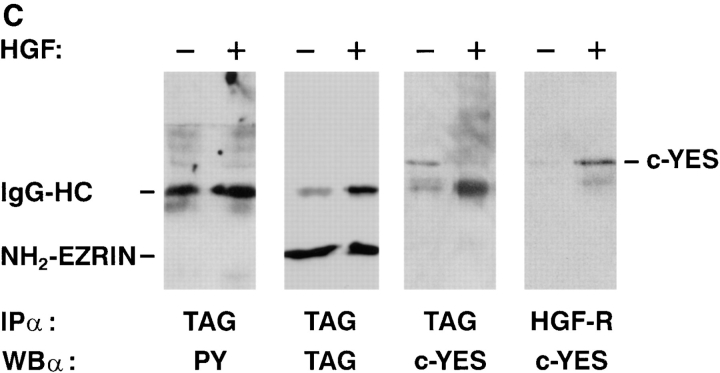
Wild-type ezrin is a substrate of the HGF-tyrosine kinase receptor. (A, left) In vitro kinase assay. GST-fused kinase domain of HGF-receptor (apparent molecular mass, 45 kD) was immobilized on glutathione–sepharose and incubated in the presence of 0.1 mM ATP alone (lane 1), with ezrin NH2-terminal domain (lane 2; apparent molecular mass, 38 kD), and with wild-type ezrin (lane 3; apparent molecular mass, 80 kD). Beads were washed, eluted in Laemmli buffer, and electrophoresed on 10% SDS-PAGE. Western blots were probed with rabbit anti–phosphotyrosine polyclonal antibodies. (Right) Western blot with anti–glutathione-S-transferase (GST) antibodies. 2 μg of GST proteins were loaded and detected with anti-GST polyclonal antibodies. (B) Tyrosine phosphorylation of ezrin and HGF-receptor and association of p62c-yes with ezrin and HGF receptor. E7 cells were unstimulated (−) and stimulated (+) in vivo with 120 ng/ml HGF, lysed, and immunocomplexes containing epitope-tagged ezrin and HGF-R were probed in Western blot with anti-phosphotyrosine antibodies (anti-PY; left two panels), and anti-p62c-yes polyclonal antibodies (right two panels). (C) Tyrosine phosphorylation of the NH2-terminal domain of ezrin, and association of p62c-yes with the ezrin NH2-terminal domain and HGF-R. N2 cells were unstimulated (−) and stimulated (+) in vivo with 120 ng/ml HGF, lysed, and immunocomplexes containing epitope-tagged NH2-terminal domain of ezrin were probed in Western blot with anti-phosphotyrosine, anti-tag, and anti-p62c-yes antibodies (left three panels). Immunocomplexes obtained with anti-HGF-R antibody were probed in Western blot with anti-p62c-yes antibodies (right).
A phosphoprotein of 62 kD, which we identified as p62c-yes, coprecipitated with ezrin in unstimulated E7 (Fig. 4 B, right). In unstimulated N2 cells, p62c-yes was also found associated with the NH2-terminal domain of ezrin (Fig. 4 C). On HGF stimulation of E7 and N2 cells, p62c-yes did not coimmunoprecipitate with ezrin or its NH2-terminal domain, but did associate with HGF-R (Fig. 4, B and C, right). We previously showed that the SH2 domain of the nonreceptor tyrosine kinase pp60c-src binds the HGF-R in vitro and in vivo (Ponzetto et al., 1994). p62c-yes in particular, another member of the src family, was found enriched in purified preparations of adherens junctions (Tsukita et al., 1991), indicating that it has a subcellular distribution similar to that of HGF-R.
HGF-induced Cell Migration Is Enhanced by Overproduction of Wild-Type Ezrin but Impaired by Overproduction of its NH2-terminal Domain
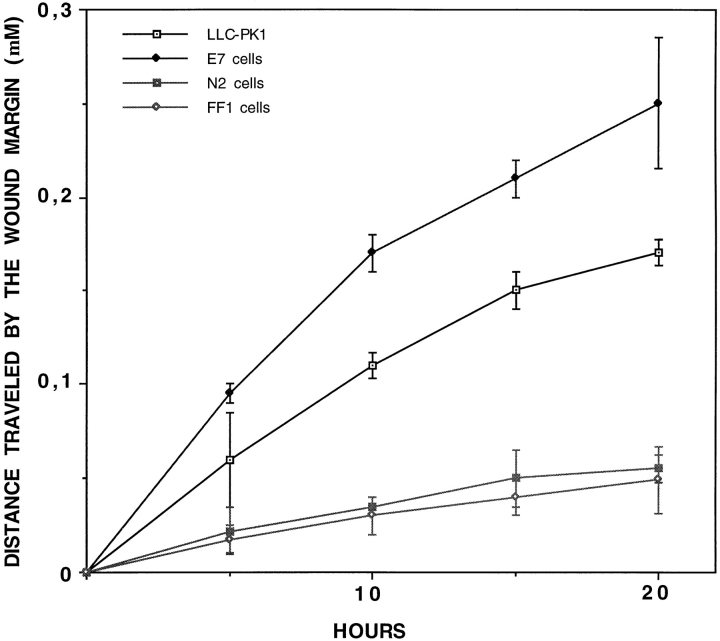
HGF treatment induces cell spreading and cell shape changes in LLC-PK1 cells. It also stimulates membrane ruffling on the apical surface of these cells, with a concomitant disassembly of the microvilli. However, LLC-PK1 cells do not totally disrupt their cell–cell contacts and do not move apart from each other in the conventional scatter assay used for MDCK cells (data not shown). This finding likely reflects either an elevated strength in cell–cell adhesion in LLC-PK1 cells (which is indicated by their higher resistance to low calcium treatment, compared to MDCK cells [data not shown]) or a lower intrinsic motility. Thus, a standard cell motility assay appeared unsuitable for LLC-PK1 cells. Instead, we determined the relative motility of untransfected and transfected LLC-PK1 cells by measuring how far they had traveled into a wound site after a 15-h incubation in the presence or absence of HGF. To quantify the migration rates, cells were inspected by videomicroscopy during the wound healing, and the mean distance traveled by the margins of the wound was plotted at definite time intervals (Fig. 5). E7 cells migrated faster than untransfected cells, in response to 15 ng/ml of HGF. On the contrary, N2 cells were almost unresponsive to HGF. Since cell proliferation could also contribute to wound healing in vitro, we estimated the degree of proliferation in untransfected and transfected LLC-PK1 clones by measuring the incorporation of BrdU into nascent DNA, by serum-starved monolayers grown in the presence of HGF for 9 h (Table I). A significant and comparable mitogenic activity was measured in transfected and untransfected cells.
Figure 5.
Wound healing abilities by LLC-PK1 cells transfectants. A wound was scored in a confluent monolayer. The average distance traveled by the cell margin during HGF-stimulated wound closure was measured with a micrometer for control cells (LLC-PK1), E7 cells, N2 cells, and FF1 cells. SEM bars are shown.
Table I.
Mitogenic Effect of HGF on LLC-PK1 Cells
| LLC-PK1 | E7 Clone | N2 Clone | ||||
|---|---|---|---|---|---|---|
| 1. −HGF | 88.2 ± 16.0 | 88.8 ± 5.6 | 90.0 ± 7.9 | |||
| 2. +HGF | 149.8 ± 18.0 | 150.0 ± 22.0 | 138.0 ± 12.4 |
4 × 104 cells were seeded in 96-well microtiter plate to reach confluence, starved in DME 0.2% FCS for 24 h, and then stimulated with 15 ng/ml of HGF. After 6 h, BrdU was added and left to incorporate for an additional 3 h. Incorporated BrdU was detected by a specific mAb and peroxidase-conjugated secondary antibody. After incubation with the peroxydase substrate, absorbance was read at 410 nm. Values are given as OD arbitrary units. Numbers are the mean (± SD) calculated from six duplicates of one representative experiment. The t test was applied for specific comparisons of the means of two pairs of samples in each column and in each row. Comparisons between HGF-treated and untreated cells samples (1 vs 2) gave P ⩽ 0.01, while comparisons between samples of each row did not give significant P.
To determine whether changes in the level of insoluble ezrin pool in the LLC-PK1 cells and their mutants could account for their different motility, we extracted the cells with the nonionic detergent Triton X-100, and fractionated the extracts between soluble and insoluble pool. In LLC-PK1 cells, 30–40% of ezrin was associated with the insoluble fraction. A similar ratio was observed with the E7 cells overproducing wild-type ezrin. On the contrary, in N2 transfectants, 70% of endogenous ezrin and the NH2-terminal domain was found in the insoluble pellet. After 10 min of HGF treatment (120 ng/ml), we observed a significant increase (10 to 20%) of the ezrin-insoluble pool in control and E7 cells. On the contrary, the treatment of N2 transfectants with HGF led to a decrease (30%) of ezrin and NH2-terminal domain in the insoluble fraction.
Taken together, these data suggest that ezrin is an important element in epithelial cell locomotion induced by HGF.
Ezrin Plays a Crucial Role in HGF-induced Morphogenesis
Kidney-derived epithelial cell lines retaining apical-basolateral polarity form cysts when grown in three-dimensional collagen gels and branch into tubular structures after HGF stimulation (Montesano et al., 1991; Barros et al., 1995). We thus assayed the ability of LLC-PK1 parental and transfected cells to undergo HGF-induced morphogenesis in vitro. When embedded in collagen gels, LLC-PK1 cells formed cysts in 2–3 d (Fig. 6 A). In the presence of 30 ng/ml HGF, the cysts were more abundant and some of them developed into tubules (Fig. 6 C). The E7 cells transfected with the full-length ezrin also formed cysts. In the presence of HGF, the cysts developed into very elongated tubules (three- to fivefold the control LLC-PK1 cells) with a lumen (Fig. 6 D, inset), as well as arborizations at the distal ends (Fig. 6 D). The N2 cells transfected with the NH2-terminal domain of ezrin formed small cysts, which scarcely developed into tubules in the presence of HGF (Fig. 6 E). After few days in culture, they frequently show cellular disorganization at the border of the cysts. These results suggest that ezrin functions as an important regulator of morphogenesis of kidney epithelial cells.
Figure 6.
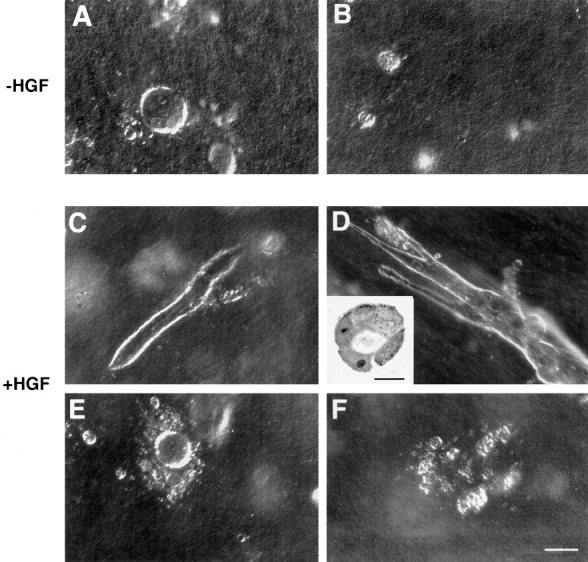
Tubulogenesis by LLC-PK1 cell transfectants within collagen gels. Spherical cysts were formed by control LLC-PK1 cells (A) and LLC-PK1 cells overproducing wild-type ezrin, grown under control conditions for 3 d. Small colonies were formed by LLC-PK1 cells overproducing the double phosphotyrosine-mutated ezrin-FF1 cells (B), and by cells overproducing the NH2-terminal domain of ezrin under the same growth conditions. In the presence of 30 ng/ml of HGF, some cysts developed into tubules in LLC-PK1 cells (C); the cysts developed in elongated tubules (three- to fivefold the control LLC-PK1 cells) in E7 cells (D). Inset represents a section of tubules formed and demonstrates lumen formation. N2 cells (E) or the tyrosine mutants (F) did not form tubules. Bars: (A–F) 100 μm; (inset) 25 μm.
Tyrosine Phosphorylation of Ezrin Is Involved in HGF-induced Motility and Morphogenesis
Since HGF stimulation leads to ezrin phosphorylation on tyrosine residues (Fig. 4), we wanted to assess the possible role of this phosphorylation in HGF-mediated responses. Ezrin contains several potential tyrosine phosphorylation sites fitting the consensus sequences defined for phosphotyrosine kinases (Songyang and Cantley, 1995). Two tyrosine residues Y145 and Y353 have been previously shown to be phosphorylated by EGF-R (Krieg and Hunter, 1992). Hence, we changed the tyrosines Y145 and Y353 to phenylalanine by site-directed mutagenesis. The mutated ezrin cDNA, again with the VSVG epitope, was stably transfected into LLC-PK1 cells. Two G-418–resistant clones (FF1; FF4) were isolated and characterized. These two clones produced a high level of mutated ezrin by Western blot (fivefold the endogenous protein; Fig. 1 C). Upon HGF treatment, mutated ezrin was still phosphorylated on tyrosine residues, suggesting that other tyrosine residues must be phosphorylated upon exposure of cells to HGF. Moreover, a two-dimensional gel analysis of the in vitro phosphorylated wild-type and mutated proteins showed a different pattern (data not shown).
No apparent phenotypic changes were observed in FF1 cells grown on plastic or filters. By immunofluorescence, the mutated ezrin was localized in microvilli and intracellularly as in the E7 cells (Fig. 2, C and C′). However, the HGF-induced motility of cells transfected with this double tyrosine mutant was markedly reduced (Fig. 5). Moreover, the tubulogenesis was completely abolished (Fig. 6, B and F). After 2 or 3 d in collagen, the small cysts showed cellular disorganization (Fig. 6 F) and never formed tubules. These results indicate that mutations of the two tyrosine residues in ezrin impair cell motility and tubulogenesis mediated by this protein.
Discussion
In this report we have shown that ezrin is an essential component of epithelial morphogenesis and that it controls processes induced by HGF signaling in a specific cell line. Overproduction of the wild-type protein and suppression of its function by dominant-negative mutants were pivotal in uncovering its relevance in HGF signaling and in novel cellular functions like cell motility and morphogenesis.
Ezrin Is an Essential Component of Brush Border Microvilli
As a model, we used a cell line derived from kidney proximal tubules. During terminal differentiation these cells develop a brush border composed of highly ordered microvilli; this cellular structure is specialized for absorption. The microvilli core is organized by villin and fimbrin, two actin bundling proteins, and is linked to the plasma membrane by the brush border myosin I (Arpin and Friederich, 1992). Although the microvilli also contain ezrin, its role in microvilli assembly is still poorly understood. Here we show that ezrin is an essential component of microvilli formation. A truncated variant of ezrin overproduced in LLC-PK1 cells interferes with microvilli formation through a dominant-negative mechanism. This is concomitant with lack of localization of both the transfected NH2-terminal domain and endogenous ezrin to the apical cell surface. Notably, both molecules accumulate at the lateral surfaces, as do the few developed microvilli detected by EM. It has been shown that ezrin suppression using an antisense approach does not affect microvilli formation in a thymoma cell line, unless performed in combination with antisense oligonucleotides complementary to the sequence coding for radixin and moesin (Takeuchi et al., 1994). Together these results indicate that microvilli from different tissue-derived cell lines can have diverse ERM protein composition.
We cannot exclude the possibility that the dominant-negative action of the NH2-terminal domain of ezrin also affects radixin and moesin functions, since heterooligomerization between ezrin and moesin has been observed (Gary and Bretscher, 1993). However, this hypothesis appears unlikely, since overproduction of radixin in LLC-PK1 cells does not lead to the same phenotype as overproduction of ezrin (unpublished observations).
How does the NH2-terminal domain of ezrin impede microvilli formation? There are several different hypotheses: first, the NH2-terminal domain could prevent the interaction of ezrin with membrane components essential for development of the brush border, since it is this domain that localizes to the plasma membrane (Algrain et al., 1993). However, no microvillar membrane components which associate with ezrin have yet been identified. In BHK cells, ERM proteins have been found associated with the integral membrane protein CD44 (Tsukita et al., 1994). The NH2-terminal domain of moesin binds to CD44 with higher affinity than full-length moesin (Hirao et al., 1996). However, CD44 is present in several cell types, including epithelial cells. In this latter case, CD44 is localized to the basolateral surface of the polarized cells.
Although ezrin is mainly localized to microvilli in mature epithelial cells, it can be detected on the basolateral surface of immature cells of intestinal crypts (Berryman et al., 1993). Furthermore, in cells which do not have a well-developed brush border, such as MDCK cells, a significant proportion of ezrin is found associated with the lateral membrane (unpublished results; Sato et al., 1992). We propose that, depending on the cellular context, ezrin can be recruited and can associate with membrane components present on both the apical and the basolateral surface. In addition, our results suggest that control of microvilli assembly could occur at sites associated with the lateral membranes, and this function could be suppressed by the NH2-terminal domain through a dominant-negative effect.
Second, the NH2-terminal domain of ezrin could also prevent microfilament organization. Although little is known about such a role, an F-actin binding site has been localized in the COOH-terminal domain of ezrin (Turunen et al., 1994). Furthermore, there is substantial evidence that the NH2-terminal domain of ezrin negatively regulates the morphogenic activity of the COOH-terminal domain (Martin et al., 1995) probably by an intra- or intermolecular association between these two domains (Berryman et al., 1995; Gary and Bretscher, 1995). Thus overproduction of the truncated ezrin could impair ezrin oligomerization. According to the model proposed by Berryman et al. (1995), this oligomerization step could be essential for microvillus assembly.
Third, it is conceivable that ezrin exists as an inactive molecule whose activation depends on signals regulating the cryptic sites in the COOH- and NH2-terminal domains. The ezrin NH2-terminal domain might interfere with the activation of endogenous ezrin by competing with signaling molecules that regulate the accessibility of cryptic binding sites. For example, it has been shown that the association of ERM proteins with CD44 is regulated by phosphatidylinositol turnover and activation of Rho (Hirao et al., 1996).
Ezrin Is a Downstream Target of HGF-R
The properties of ezrin and its ability to localize to different compartments of epithelial cells prompted us to analyze whether ezrin could be a downstream effector of HGF signaling. It has been shown that EGF stimulation of cultured cells induces tyrosine phosphorylation of ezrin and its concomitant oligomerization (Berryman et al., 1995). The assembly of cell surface projections could require both ezrin oligomerization and stabilization of oligomers by cytoskeleton interactions. Indeed, HGF stimulation causes enrichment of ezrin in the detergent-insoluble, cytoskeleton fraction. Here we show that ezrin is a substrate of HGF-R both in vivo and in vitro.
We have already shown that the HGF receptor forms complexes with Shc (Pelicci et al., 1995), PI3K (Graziani et al., 1991), PLC-γ, Grb-2-SOS, and pp60c-src (Ponzetto et al., 1994). In LLC-PK1 cells ezrin is phosphorylated on tyrosine residues upon activation of the cells with HGF. However, ezrin did not coprecipitate with the HGF-R in LLC-PK1 cells while such an association was found in MDCK cells. This might be due to the fact that, in LLC-PK1 cells, a higher proportion of ezrin is associated with actin cytoskeleton, in particular with microvilli microfilaments.
In LLC-PK1 cells the HGF-R associates in vivo with the p62c-yes intracellular tyrosine kinase in a ligand-dependent manner. We propose that p62c-yes, which is also found associated with ezrin, could mediate ezrin interaction with HGF-R in vivo. However, it is likely that other factors, stimulated by HGF, including the above-mentioned lipid kinases, mediate this interaction and possibly regulate ezrin function. In vitro association experiments will tell us whether the molecular association between ezrin and p62c-yes that we have uncovered is direct or indirect. Whereas p60c-src has a fundamental role in the cytoskeleton assembly of focal contacts (Thomas et al., 1995), a similar role on p62c-yes is less clear, although a report of its enrichment in adherens junctions is intriguing (Tsukita et al., 1991). However, p62c-yes is also located near the apical plasma membrane of epithelial cells in several tissues in vivo (Zhao et al., 1990) and is enriched in detergent-resistant membrane complexes containing glycosylphosphatidylinositol-anchored apical membrane proteins in MDCK cells (Arreaza et al., 1994). Altogether, these data suggest that ezrin and p62c-yes together play a role in the cross talk between the lateral and apical surfaces of epithelial cells.
Ezrin Triggers the Actin Cytoskeleton Dynamics Required for HGF-mediated Cell Migration and Tubulogenesis
Receptor tyrosine kinases play important roles in the differentiation of epithelial cells. It has been recently shown that among tyrosine kinase receptors present on epithelial cells only the HGF-receptor is able to induce branching morphogenesis of kidney epithelial cells in collagen matrix, whereas the other receptors induce only scattering of cells (Sachs et al., 1996). It has been proposed that growth factor–induced cell motility and morphogenesis imply specific signals. Here we have shown that overproduction of the ezrin protein and suppression of its function by two different dominant-negative mutants critically affect the HGF-mediated signaling. One of the important conclusions that can be drawn from this study, although limited to this specific cell line, is that ezrin is an effector of the HGF-induced cell migration and morphogenesis.
Rearrangements of actin cytoskeleton underlie the HGF-induced cell motility, as suggested by cytochalasin B prevention of MDCK cell scattering (Rosen et al., 1990). The small GTP-binding proteins, Ras and Rac, have been shown to act downstream of the HGF-receptor and to mediate cell spreading but not motility (Ridley et al., 1995). Thus, scattering requires additional signals which are likely to include regulation of specific actin-binding proteins. Overproduction of ezrin increased cell motility in response to HGF treatment as measured by the ability of LLC-PK1 cells to close a wound. Similar results were obtained with overproduction of gelsolin in fibroblast cells (Cunningham et al., 1991). Conversely, the HGF-mediated cell migration was blocked by the introduction of the NH2-terminal domain of ezrin and by the ezrin mutated on two tyrosine residues. We propose that changes in ezrin phosphorylation and/or conformation induced by HGF stimulation modify the state of actin organization in the cell cortex.
Our observation that overproduction of ezrin induces formation of very long tubules when LLC-PK1 cells are cultured in collagen gels in the presence of HGF and that ezrin mutants inhibit tubule formation confirms our view that ezrin plays an important role in HGF-induced morphogenesis. At present, the mechanisms are unclear. However, the results obtained in motility assays suggest that cytoskeletal rearrangements may affect tubule formation. Of course, ezrin could be involved in other significant aspects of tubulogenesis. Tubulogenesis requires extensive remodeling of the extracellular matrix and modulation of adhesion. HGF participates in this remodeling by increasing the synthesis of matrix-degrading enzymes (Pepper et al., 1992), and by down-regulating the expression of specific collagen adhesion integrins (Berdichevsky et al., 1994). Ezrin might transmit and integrate signals elicited by HGF-R and cell adhesion molecules, since it has been shown to interact with CD44, the hyaluronan adhesion receptor (Tsukita et al., 1994).
It is known that control of cell shape influences gene expression and cell differentiation (Boudreau et al., 1995). Increased ezrin content might also potentiate signaling pathways important for morphogenesis. It has been shown that in NIH3T3 cells transformed with v-Fos, overproduction of c-Fos leads to an increase of ezrin level and to morphological changes (Jooss and Muller, 1995).
Lastly, ezrin might have a role in regulating cell growth. This role has been shown for a member of the band 4.1 family, merlin/schwannomin, whose gene has been characterized as the tumor suppressor gene responsible for neurofibromatosis type 2 (Rouleau et al., 1993; Trofatter et al., 1993). Moreover, the level of ezrin has been found to be increased in immortalized mouse fibroblasts (Kaul et al., 1996).
We propose that ezrin is able to convey the signals elicited by growth factor receptors to the actin cytoskeleton machinery. Different signals can be integrated by ezrin to engage actin cytoskeleton in different, but possibly related functions such as apical domain morphogenesis and cell locomotion. Our findings may unveil new roles for ezrin in epithelial differentiation and carcinogenesis.
Acknowledgments
We thank M. Algrain for generating stable LLC-PK1 cell lines, R. Hellio and P. Gounon for their help with the confocal and scanning EM, and G. Raposo for her help with cryosections. We thank Dr. A. Hubbard for critically reading the manuscript. The excellent technical assistance of R. Callipo and L. Del Maestro, and secretarial help of E. Wright are gratefully acknowledged.
Abbreviations used in this paper
- BrdU
5-bromo-2′-deoxyuridine
- CSLM
confocal laser scanning microscopy
- ERM
ezrin-radixin-moesin
- GST
glutathione-S-transferase
- HGF
hepatocyte growth factor
- HGF-R
HGF-receptor
- VSVG
vesicular stomatitis virus glycoprotein G
Footnotes
T. Crepaldi was supported by a fellowship from the European Community (BMH1-CT-94-7309). This work was supported by grants from the Association pour la Recherche sur le Cancer (1825), the Ligue Nationale Française contre le Cancer, the Human Capital Mobility (ERB CHR-XCT 940 430), the Biomed Program (BMH4-CT95-0090), and the Associazione Italiana delle Ricerche sul Cancro.
Please address all correspondence to Monique Arpin, Institut Curie-UMR 144 CNRS, Laboratoire de Morphogenèse et Signalisation Cellulaires, 26 rue d'Ulm, 75231 Paris Cedex 05, France. Tel.: (33) 1-42-34-63-72. Fax: (33) 1-42-34-63-77. e-mail: marpin@curie.fr
References
- Algrain M, Turunen O, Vaheri A, Louvard D, Arpin M. Ezrin contains cytoskeleton and membrane binding domains accounting for its proposed role as a membrane–cytoskeletal linker. J Cell Biol. 1993;120:129–139. doi: 10.1083/jcb.120.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amicone L, Spagnoli FM, Spath G, Giordano S, Tommasini C, Bernardini S, De Luca V, Della C, Rocca, Weiss MC, Comoglio PM, Tripodi M. Transgenic expression in the liver of truncated Met blocks apoptosis and permits immortalization of hepatocytes. EMBO (Eur Mol Biol Organ) J. 1997;16:495–503. doi: 10.1093/emboj/16.3.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreoli C, Martin M, Le Borgne R, Reggio H, Mangeat P. Ezrin has properties to self-associate at the plasma membrane. J Cell Sci. 1994;107:2509–2521. doi: 10.1242/jcs.107.9.2509. [DOI] [PubMed] [Google Scholar]
- Arpin, M., and E. Friederich. 1992. Cytoskeletal components in intestinal brush border morphogenesis: an evaluation of their function. In Epithelial Organization and Development. T. P. Fleming, editor. Chapman & Hall, London. 245–271.
- Arpin M, Algrain M, Louvard D. Membrane-actin microfilament connections: an increasing diversity of players related to band 4.1. Curr Opin Cell Biol. 1994;6:136–141. doi: 10.1016/0955-0674(94)90127-9. [DOI] [PubMed] [Google Scholar]
- Arreaza G, Melkonian KA, LaFevre-Bernt M, Brown D A. Triton X-100-resistant membrane complexes from cultured kidney epithelial cells contain the Src family protein tyrosine kinase p62yes. J Biol Chem. 1994;269:19123–19127. [PubMed] [Google Scholar]
- Bardelli A, Maina F, Gout I, Fry MJ, Waterfield MD, Comoglio PM, Ponzetto C. Autophosphorylation promotes complex formation of recombinant hepatocyte growth factor receptor with cytoplasmic effectors containing SH2 domains. Oncogene. 1992;7:1973–1978. [PubMed] [Google Scholar]
- Bardelli, A., P. Longati, D. Albero, C. Ponzetto, and P.M. Comoglio. 1996. HGF receptor associates the anti-apoptotic protein BAG–1 and prevents cell death. EMBO (Eur. Mol. Biol. Organ.) J. 6205–6212. [PMC free article] [PubMed]
- Barros EJ, Santos OF, Matsumoto K, Nakamura T, Nigam SK. Differential tubulogenic and branching morphogenetic activities of growth factors: implications for epithelial tissue development. Proc Natl Acad Sci USA. 1995;92:4412–4416. doi: 10.1073/pnas.92.10.4412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berdichevsky F, Alford D, D'Souza B, Taylor-Papadimitriou J. Branching morphogenesis of human mammary epithelial cells in collagen gels. J Cell Sci. 1994;107:3557–3568. doi: 10.1242/jcs.107.12.3557. [DOI] [PubMed] [Google Scholar]
- Berryman M, Franck Z, Bretscher A. Ezrin is concentrated in the apical microvilli of a wide variety of epithelial cells whereas moesin is found primarily in endothelial cells. J Cell Sci. 1993;105:1025–1043. doi: 10.1242/jcs.105.4.1025. [DOI] [PubMed] [Google Scholar]
- Berryman M, Gary R, Bretscher A. Ezrin oligomers are major cytoskeletal components of placental microvilli: a proposal for their involvement in cortical morphogenesis. J Cell Biol. 1995;131:1231–1242. doi: 10.1083/jcb.131.5.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bladt F, Riethmacher D, Isenmann S, Aguzzi A, Birchmeier C. Essential role for the c-met receptor in the migration of myogenic precursor cells into the limb bud [see comments] Nature (Lond) 1995;376:768–771. doi: 10.1038/376768a0. [DOI] [PubMed] [Google Scholar]
- Bottaro DP, Rubin JS, Faletto DL, Chan AM, Kmiecik TE, Vande GF, Woude, Aaronson SA. Identification of the hepatocyte growth factor receptor as the c-met proto-oncogene product. Science (Wash DC) 1991;251:802–804. doi: 10.1126/science.1846706. [DOI] [PubMed] [Google Scholar]
- Boudreau N, Myers C, Bissell MJ. From laminin to lamin: regulation of tissue-specific gene expression by the ECM. Trends Cell Biol. 1995;5:1–4. doi: 10.1016/s0962-8924(00)88924-2. [DOI] [PubMed] [Google Scholar]
- Bretscher A. Purification of an 80,000-dalton protein that is a component of the isolated microvillus cytoskeleton, and its localization in nonmuscle cells. J Cell Biol. 1983;97:425–432. doi: 10.1083/jcb.97.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher A. Rapid phosphorylation and reorganization of ezrin and spectrin accompany morphological changes induced in A-431 cells by epidermal growth factor. J Cell Biol. 1989;108:921–930. doi: 10.1083/jcb.108.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher A, Gary R, Berryman M. Soluble ezrin purified from placenta exists as stable monomers and elongated dimers with masked C-terminal ezrin-radixin-moesin association domains. Biochemistry. 1995;34:16830–16837. doi: 10.1021/bi00051a034. [DOI] [PubMed] [Google Scholar]
- Brinkmann V, Foroutan H, Sachs M, Weidner KM, Birchmeier W. Hepatocyte growth factor/scatter factor induces a variety of tissue– specific morphogenic programs in epithelial cells. J Cell Biol. 1995;131:1573–1586. doi: 10.1083/jcb.131.6.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronner-Fraser M. Hepatocyte growth factor/scatter factor (HGF/SF) in early development: evidence for a role in neural induction. Trends Genet. 1995;11:423–425. doi: 10.1016/s0168-9525(00)89136-2. [DOI] [PubMed] [Google Scholar]
- Bussolino F, Di Renzo MF, Ziche M, Bocchietto E, Olivero M, Naldini L, Gaudino G, Tamagnone L, Coffer A, Comoglio P M. Hepatocyte growth factor is a potent angiogenic factor which stimulates endothelial cell motility and growth. J Cell Biol. 1992;119:629–641. doi: 10.1083/jcb.119.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987;7:2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comoglio, P.M., and E. Vigna. 1995. Structure and functions of the HGF receptor (c-Met). In Liver Regeneration and Carcinogenesis. R.L. Jirtle, editor. Academic Press Inc., San Diego. 51–70.
- Crepaldi T, Pollack AL, Prat M, Zborek A, Mostov K, Comoglio PM. Targeting of the SF/HGF receptor to the basolateral domain of polarized epithelial cells. J Cell Biol. 1994;125:313–320. doi: 10.1083/jcb.125.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham CC, Stossel TP, Kwiatkowski DJ. Enhanced motility in NIH 3T3 fibroblasts that overexpress gelsolin. Science (Wash DC) 1991;251:1233–1236. doi: 10.1126/science.1848726. [DOI] [PubMed] [Google Scholar]
- Derman MP, Chen JY, Spokes KC, Songyang Z, Cantley LG. An 11-amino acid sequence from c-metinitiates epithelial chemotaxis via phosphatidylinositol 3-kinase and phospholipase C. J Biol Chem. 1996;271:4251–4255. doi: 10.1074/jbc.271.8.4251. [DOI] [PubMed] [Google Scholar]
- Dransfield DT, Bradford AJ, Smith J, Martin M, Roy C, Mangeat PH, Goldenring JR. Ezrin is a cyclic AMP–dependent protein kinase anchoring protein. EMBO (Eur Mol Biol Organ) J. 1997;16:35–43. doi: 10.1093/emboj/16.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazioli F, Wong WT, Ullrich SJ, Sakaguchi K, Appella E, Di Fiore PP. The ezrin-like family of tyrosine kinase substrates: receptor-specific pattern of tyrosine phosphorylation and relationship to malignant transformation. Oncogene. 1993;8:1335–1345. [PubMed] [Google Scholar]
- Frisch SM, Francis H. Disruption of epithelial cell–matrix interactions induces apoptosis. J Cell Biol. 1994;124:619–626. doi: 10.1083/jcb.124.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gary R, Bretscher A. Heterotypic and homotypic associations between ezrin and moesin, two putative membrane-cytoskeletal linking proteins. Proc Natl Acad Sci USA. 1993;90:10846–10850. doi: 10.1073/pnas.90.22.10846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gary R, Bretscher A. Ezrin self-association involves binding of an N-terminal domain to a normally masked C-terminal domain that includes the F-actin binding site. Mol Biol Cell. 1995;6:1061–1075. doi: 10.1091/mbc.6.8.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant DS, Kleinman HK, Goldberg ID, Bhargava MM, Nickoloff BJ, Kinsella JL, Polverini P, Rosen EM. Scatter factor induces blood vessel formation in vivo. Proc Natl Acad Sci USA. 1993;90:1937–1941. doi: 10.1073/pnas.90.5.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziani A, Gramaglia D, Cantley LC, Comoglio PM. The tyrosine-phosphorylated hepatocyte growth factor/scatter factor receptor associates with phosphatidylinositol 3-kinase. J Biol Chem. 1991;266:22087–22090. [PubMed] [Google Scholar]
- Hartmann G, Weidner KM, Schwarz H, Birchmeier W. The motility signal of scatter factor/hepatocyte growth factor mediated through the receptor tyrosine kinase met requires intracellular action of Ras. J Biol Chem. 1994;269:21936–21939. [PubMed] [Google Scholar]
- Helander TS, Carpén O, Turunen O, Kovanen PE, Vaheri A, Timonen T. ICAM-2 redistributed by ezrin as a target for killer cells. Nature (Lond) 1996;382:265–268. doi: 10.1038/382265a0. [DOI] [PubMed] [Google Scholar]
- Hirao M, Sato N, Kondo T, Yonemura S, Monden M, Sasaki T, Takai Y, Tsukita S, Tsukita S. Regulation mechanism of ERM (ezrin/radixin/ moesin) protein/plasma membrane association: possible involvement of phosphatidylinositol turnover and rho-dependent signaling pathway. J Cell Biol. 1996;135:37–51. doi: 10.1083/jcb.135.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jooss KU, Muller R. Deregulation of genes encoding microfilament-associated proteins during Fos-induced morphological transformation. Oncogene. 1995;10:603–608. [PubMed] [Google Scholar]
- Kaul SC, Mitsui Y, Komatsu Y, Reddel RR, Wadhwa R. A highly expressed 81 kD protein in immortalized mouse fibroblasts: its proliferative function and identity with ezrin. Oncogene. 1996;13:1231–1237. [PubMed] [Google Scholar]
- Kreis TE. Microinjected antibodies against the cytoplasmic domain of vesicular stomatitis virus glycoprotein block its transport to the cell surface. EMBO (Eur Mol Biol Organ) J. 1986;5:931–941. doi: 10.1002/j.1460-2075.1986.tb04306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg J, Hunter T. Identification of the two major epidermal growth factor-induced tyrosine phosphorylation sites in the microvillar core protein ezrin. J Biol Chem. 1992;267:19258–19265. [PubMed] [Google Scholar]
- Maina F, Casagranda F, Audero E, Simeone A, Comoglio PM, Klein R, Ponzetto C. Uncoupling of Grb2 from the met receptor in vivo reveals complex roles in muscle development. Cell. 1996;87:531–542. doi: 10.1016/s0092-8674(00)81372-0. [DOI] [PubMed] [Google Scholar]
- Martin M, Andreoli C, Sahuquet A, Montcourrier P, Algrain M, Mangeat P. Ezrin NH2-terminal domain inhibits the cell extension activity of the COOH-terminal domain. J Cell Biol. 1995;128:1081–1093. doi: 10.1083/jcb.128.6.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto K, Matsumoto K, Nakamura T, Kramer RH. Hepatocyte growth factor/scatter factor induces tyrosine phosphorylation of focal adhesion kinase (p125FAK) and promotes migration and invasion by oral squamous cell carcinoma cells. J Biol Chem. 1994;269:31807–31813. [PubMed] [Google Scholar]
- Medico E, Mongiovì AM, Huff J, Jelinek MA, Follenzi A, Gaudino G, Parsons JT, Comoglio PM. The tyrosine kinase receptors Ron and Sea control “scattering” and morphogenesis of liver progenitor cells in vitro. Mol Biol Cell. 1996;7:495–504. doi: 10.1091/mbc.7.4.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montesano R, Matsumoto K, Nakamura T, Orci L. Identification of a fibroblast-derived epithelial morphogen as hepatocyte growth factor. Cell. 1991;67:901–908. doi: 10.1016/0092-8674(91)90363-4. [DOI] [PubMed] [Google Scholar]
- Naldini L, Weidner KM, Vigna E, Gaudino G, Bardelli A, Ponzetto C, Narsimhan RP, Hartmann G, Zarnegar R, Michalopoulos GK, et al. Scatter factor and hepatocyte growth factor are indistinguishable ligands for the MET receptor. EMBO (Eur Mol Biol Organ) J. 1991;10:2867–2878. doi: 10.1002/j.1460-2075.1991.tb07836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naldini L, Vigna E, Bardelli A, Follenzi A, Galimi F, Comoglio PM. Biological activation of pro–HGF (hepatocyte growth factor) by urokinase is controlled by a stoichiometric reaction. J Biol Chem. 1995;270:603–611. doi: 10.1074/jbc.270.2.603. [DOI] [PubMed] [Google Scholar]
- Nishiyama T, Sasaki T, Takaishi K, Kato M, Yaku H, Araki K, Matsuura Y, Takai Y. rac p21 is involved in insulin-induced membrane ruffling and rho p21 is involved in hepatocyte growth factor- and 12-O- tetradecanoylphorbol-13-acetate (Tpa)-induced membrane ruffling in Kb cells. Mol Cell Biol. 1994;14:2447–2456. doi: 10.1128/mcb.14.4.2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelicci G, Giordano S, Zhen Z, Salcini AE, Lanfrancone L, Bardelli A, Panayotou G, Waterfield MD, Ponzetto C, Pelicci PG, et al. The motogenic and mitogenic responses to HGF are amplified by the Shc adaptor protein. Oncogene. 1995;10:1631–1638. [PubMed] [Google Scholar]
- Pepper MS, Matsumoto K, Nakamura T, Orci L, Montesano R. Hepatocyte growth factor increases urokinase-type plasminogen activator (u-PA) and u-PA receptor expression in Madin-Darby canine kidney epithelial cells. J Biol Chem. 1992;267:20493–20496. [PubMed] [Google Scholar]
- Pestonjamasp K, Amieva MR, Strassel CP, Nauseef WM, Furthmayr H, Luna EJ. Moesin, ezrin, and p205 are actin-binding proteins associated with neutrophil plasma membranes. Mol Biol Cell. 1995;6:247–259. doi: 10.1091/mbc.6.3.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaller W, Gstraunthaler G, Loidl P. Morphology of the differentiation and maturation of LLC-PK1 epithelia. J Cell Physiol. 1990;142:247–254. doi: 10.1002/jcp.1041420205. [DOI] [PubMed] [Google Scholar]
- Ponzetto C, Bardelli A, Zhen Z, Maina F, dalla Zonca P, Giordano S, Graziani A, Panayotou G, Comoglio PM. A multifunctional docking site mediates signaling and transformation by the hepatocyte growth factor/scatter factor receptor family. Cell. 1994;77:261–271. doi: 10.1016/0092-8674(94)90318-2. [DOI] [PubMed] [Google Scholar]
- Prat M, Narsimhan RP, Crepaldi T, Nicotra MR, Natali PG, Comoglio PM. The receptor encoded by the human c-MET oncogene is expressed in hepatocytes, epithelial cells and solid tumors. Int J Cancer. 1991a;49:323–328. doi: 10.1002/ijc.2910490302. [DOI] [PubMed] [Google Scholar]
- Prat M, Crepaldi T, Gandino L, Giordano S, Longati P, Comoglio P. C-terminal truncated forms of Met, the hepatocyte growth factor receptor. Mol Cell Biol. 1991b;11:5954–5962. doi: 10.1128/mcb.11.12.5954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley AJ, Comoglio PM, Hall A. Regulation of scatter factor/ hepatocyte growth factor responses by Ras, Rac, and Rho in MDCK cells. Mol Cell Biol. 1995;15:1110–1122. doi: 10.1128/mcb.15.2.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen EM, Meromsky L, Goldberg I, Bhargava M, Setter E. Studies on the mechanism of scatter factor. Effects of agents that modulate intracellular signal transduction, macromolecule synthesis and cytoskeleton assembly. J Cell Sci. 1990;96:639–649. doi: 10.1242/jcs.96.4.639. [DOI] [PubMed] [Google Scholar]
- Rouleau GA, Merel P, Lutchman M, Sanson M, Zucman J, Marineau C, Hoang K, Xuan, Demczuk S, Desmaze C, et al. Alteration in a new gene encoding a putative membrane-organizing protein causes neuro-fibromatosis type 2. Nature (Lond) 1993;363:515–521. doi: 10.1038/363515a0. [DOI] [PubMed] [Google Scholar]
- Royal I, Park M. Hepatocyte growth factor-induced scatter of Madine-Darby canine kidney cells requires phosphatidylinositol 3-kinase. J Biol Chem. 1995;270:27780–27787. doi: 10.1074/jbc.270.46.27780. [DOI] [PubMed] [Google Scholar]
- Sachs M, Weidner KM, Brinkmann V, Walther I, Obermeier A, Ullrich A, Birchmeier W. Motogenic and morphogenic activity of epithelial receptor tyrosine kinases. J Cell Biol. 1996;133:1095–1107. doi: 10.1083/jcb.133.5.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato N, Funayama N, Nagafuchi A, Yonemura S, Tsukita S, Tsukita S. A gene family consisting of ezrin, radixin and moesin. Its specific localization at actin filament/plasma membrane association sites. J Cell Sci. 1992;103:131–143. doi: 10.1242/jcs.103.1.131. [DOI] [PubMed] [Google Scholar]
- Schmidt C, Bladt F, Goedecke S, Brinkmann V, Zschiesche W, Sharpe M, Gherardi E, Birchmeier C. Scatter factor/hepatocyte growth factor is essential for liver development. Nature (Lond) 1995;373:699–702. doi: 10.1038/373699a0. [DOI] [PubMed] [Google Scholar]
- Shibamoto S, Hayakawa M, Takeuchi K, Hori T, Oku N, Miyazawa K, Kitamura N, Takeichi M, Ito F. Tyrosine phosphorylation of beta-catenin and plakoglobin enhanced by hepatocyte growth factor and epidermal growth factor in human carcinoma cells. Cell Adhes Commun. 1994;1:295–305. doi: 10.3109/15419069409097261. [DOI] [PubMed] [Google Scholar]
- Songyang Z, Cantley LC. Recognition and specificity in protein tyrosine kinase-mediated signalling. Trends Biochem Sci. 1995;20:470–475. doi: 10.1016/s0968-0004(00)89103-3. [DOI] [PubMed] [Google Scholar]
- Soriano JV, Pepper MS, Nakamura T, Orci L, Montesano R. Hepatocyte growth factor stimulates extensive development of branching duct-like structures by cloned mammary gland epithelial cells. J Cell Sci. 1995;108:413–430. doi: 10.1242/jcs.108.2.413. [DOI] [PubMed] [Google Scholar]
- Takeuchi K, Sato N, Kasahara H, Funayama N, Nagafuchi A, Yonemura S, Tsukita S, Tsukita S. Perturbation of cell adhesion and microvilli formation by antisense oligonucleotides to ERM family members. J Cell Biol. 1994;125:1371–1384. doi: 10.1083/jcb.125.6.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas SM, Soriano P, Imamoto A. Specific and redundant roles of Src and Fyn in organizing the cytoskeleton. Nature (Lond) 1995;376:267–271. doi: 10.1038/376267a0. [DOI] [PubMed] [Google Scholar]
- Trofatter JA, MacCollin MM, Rutter JL, Murrell JR, Duyao MP, Parry DM, Eldridge R, Kley N, Menon AG, Pulaski K, et al. A novel moesin-, ezrin-, radixin-like gene is a candidate for the neurofibromatosis 2 tumor suppressor. Cell. 1993;72:791–800. doi: 10.1016/0092-8674(93)90406-g. [DOI] [PubMed] [Google Scholar]
- Tsukita S, Oishi K, Akiyama T, Yamanashi Y, Yamamoto T, Tsukita S. Specific proto-oncogenic tyrosine kinases of src family are enriched in cell-to-cell adherens junctions where the level of tyrosine phosphorylation is elevated. J Cell Biol. 1991;113:867–879. doi: 10.1083/jcb.113.4.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukita S, Oishi K, Sato N, Sagara J, Kawai A, Tsukita S. ERM family members as molecular linkers between the cell surface glycoprotein CD44 and actin-based cytoskeletons. J Cell Biol. 1994;126:391–401. doi: 10.1083/jcb.126.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukita S, Yonemura S, Tsukita S. ERM proteins: head-to-tail regulation of actin-plasma membrane interaction. Trends Biochem Sci. 1997;22:53–58. doi: 10.1016/s0968-0004(96)10071-2. [DOI] [PubMed] [Google Scholar]
- Turunen O, Wahlstrom T, Vaheri A. Ezrin has a COOH-terminal actin-binding site that is conserved in the ezrin protein family. J Cell Biol. 1994;126:1445–1453. doi: 10.1083/jcb.126.6.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehara Y, Minowa O, Mori C, Shiota K, Kuno J, Noda T, Kitamura N. Placental defect and embryonic lethality in mice lacking hepatocyte growth factor/scatter factor. Nature (Lond) 1995;373:702–705. doi: 10.1038/373702a0. [DOI] [PubMed] [Google Scholar]
- Weidner KM, Di Cesare S, Sachs M, Brinkmann V, Behrens J, Birchmeier W. Interaction between gab1 and the c-met receptor tyrosine kinase is responsible for epithelial morphogenesis. Nature. 1996;384:173–176. doi: 10.1038/384173a0. [DOI] [PubMed] [Google Scholar]
- Woolf AS, Kolatsi-Joannou M, Hardman P, Andermarcher E, Moorby C, Fine LG, Jat PS, Noble MD, Gherardi E. Roles of hepatocyte growth factor/scatter factor and the met receptor in the early development of the metanephros. J Cell Biol. 1995;128:171–184. doi: 10.1083/jcb.128.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Spitzer E, Meyer D, Sachs M, Niemann C, Hartmann G, Weidner KM, Birchmeier C, Birchmeier W. Sequential requirement of hepatocyte growth factor and neuregulin in the morphogenesis and differentiation of the mammary gland. J Cell Biol. 1995;131:215–226. doi: 10.1083/jcb.131.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao YH, Krueger JG, Sudol M. Expression of cellular-yes protein in mammalian tissues. Oncogene. 1990;5:1629–1635. [PubMed] [Google Scholar]