Abstract
Through association with CDK1, cyclin B accumulation and destruction govern the G2/M/G1 transitions in eukaryotic cells. To identify CDK1 inactivation-dependent events during late mitosis, we expressed a nondestructible form of cyclin B (cyclin BΔ90) by microinjecting its mRNA into prometaphase normal rat kidney cells. The injection inhibited chromosome decondensation and nuclear envelope formation. Chromosome disjunction occurred normally, but anaphase-like movement persisted until the chromosomes reached the cell periphery, whereupon they often somersaulted and returned to the cell center. Injection of rhodamine-tubulin showed that this movement occurred in the absence of a central anaphase spindle. In 82% of cells cytokinesis was inhibited; the remainder split themselves into two parts in a process reminiscent of Dictyostelium cytofission. In all cells injected, F-actin and myosin II were diffusely localized with no detectable organization at the equator. Our results suggest that a primary effect of CDK1 inactivation is on spindle dynamics that regulate chromosome movement and cytokinesis. Prolonged CDK1 activity may prevent cytokinesis through inhibiting midzone microtubule formation, the behavior of proteins such as TD60, or through the phosphorylation of myosin II regulatory light chain.
The protein kinase CDK1 functions as a universal mitotic regulator (Nurse, 1990; see Pines, 1994, for terminology). As CDK1 expression is constant throughout the cell cycle, its cyclical activation is achieved through phosphoregulation and association with cyclins (Hunt, 1991). Levels of mitotic cyclins (B-type cyclins) rise and fall during the cell cycle, peaking at the G2/M transition (Evans et al., 1983; Hunt, 1991). Its destruction, which causes CDK1 inactivation, occurs at the metaphase to anaphase transition and is required for the exit from mitosis (Murray and Kirschner, 1989; Murray et al., 1989; Murray, 1995; Yamano et al., 1996).
Cyclin B is degraded via the ubiquitin pathway (Glotzer et al., 1991). Truncation of 90 amino acids from its NH2-terminal domain renders it nondegradable but still able to interact with and activate CDK1 (Murray et al., 1989; Glotzer et al., 1991). Thus this construct, referred to as cyclin BΔ90, can be used as a valuable tool for identifying events that require CDK1 inactivation. Introduction of this truncated cyclin into mitotic extracts of frog eggs allows sister chromosome disjunction but prevents chromosome decondensation and spindle disassembly (Holloway et al., 1993). Subsequent studies with other systems confirmed that chromosome separation can occur independent of CDK1 inactivation (Surana et al., 1993; Rimmington et al., 1994; Sigrist et al., 1995; Yamano et al., 1996).
Besides chromosome separation and spindle disassembly, many other events occur at or subsequent to anaphase onset, including the movement of chromosomes and spindle poles, the elongation of polar microtubules, the formation of interzonal microtubule bundles, the relocation of motor and nonmotor spindle proteins, and the reorganization of cortical actin and myosin filaments for cytokinesis. It is important to determine whether all of these events require the inactivation of CDK1, or if some of them could be triggered by proteolysis alone, as for sister chromosome disjunction. Although CDK1 has been shown to affect microtubule dynamics in cell extracts (Verde et al., 1990) and myosin activity in vitro (Satterwhite et al., 1992), there is no direct evidence that it regulates these proteins in the living cell.
A number of studies have been performed to address the role of CDK1 inactivation in late mitosis. In frog embryos (Murray, 1989), yeast (Surana et al., 1993, Yamano et al., 1996), and flies (Rimmington et al., 1994; Sigrist et al., 1995), expression of nondestructible cyclin B leads to arrest between late anaphase and telophase. However, little is known about the effects of sustained CDK1 activity on specific events as listed above. In addition, experiments with mammalian cells have yielded a disparate picture: HeLa cells transfected with nondegradable avian cyclin B2 appear to be arrested in a “pseudomitotic” state, with unsegregated chromosomes attached to multipolar spindles (Gallant and Nigg, 1992). As CDK1 interacts with multiple cyclins during interphase and mitosis (Sigrist et al., 1995), it is possible that some phenotypes of transfected cells may reflect a cumulative effect of disrupting a number of CDK1-dependent events.
Due to the transience of mitosis, the most pertinent way to assess the functional role of CDK1 inactivation after anaphase onset is to maintain its activity specifically in mitotic cells. To this end, we have injected cultured mammalian cells during prometaphase with mRNA of cyclin BΔ90 and monitored subsequent cell behavior. We report here that sustained activity of CDK1 has profound effects on spindle pole separation, microtubule organization, and the relocation of proteins implicated in regulating spindle assembly and cytokinesis.
Materials and Methods
Cell Culture
A subclone of normal rat kidney epithelial cells (NRK-52E;1 American Type Culture Collection, Rockville, MD) was cultured in Kaighn's modified F12 medium (Sigma Chemical Co., St. Louis, MO) supplemented with 10% FCS (JRH Biosciences, Lenexa, KS), 50 U/ml penicillin, and 50 μg/ml streptomycin, on glass chamber dishes as previously described (McKenna and Wang, 1989).
In Vitro Transcription of mRNA
Messenger RNA, containing a 5′ 7-methyl guanosine cap was synthesized from a pET3b vector (for T7Δ90) and from FpΔ90TF1 and FpΔ13TF1 vectors (for T7Δ90 and T7Δ13; see Glotzer et al., 1991 for cloning details) using an mMESSAGE mMACHINE™ in vitro Transcription Kit (Ambion Inc., Austin, TX). The yield of mRNA from each transcription reaction was determined as per kit instructions, using percent incorporation of a trace nucleotide ([α-32P]GTP) added to the reaction mixture. Transcribed mRNA was separated from unincorporated nucleotides by a G50 spin column, subjected to phenol/chloroform extraction, followed by chloroform extraction, then twice precipitated with ethanol, and stored at −20°C. Before use, the mRNA pellet was suspended in RNAse-free dH2O to appropriate concentrations and heat treated for 1 min at 60°C.
Fluorescent Staining
Localization of tubulin, F-actin, and TD-60 in fixed cells were performed as previously described (Wheatley and Wang, 1996). Myosin II was localized in cells fixed by the glutaraldehyde method (Wheatley and Wang, 1996) using a polyclonal antibody directed against the rod portion of human platelet myosin (diluted 1/40 with PBS/BSA; Fujiwara and Pollard, 1976), and FITC-labeled anti–rabbit antibody (TAGO Inc., Burlingame, CA; diluted 1/50 with PBS/BSA). To assess nuclear protein-associated mitotic apparatus (NuMA) distribution, cells were fixed using 4% formaldehyde for 10 min and then extracted with 0.5% triton (Wheatley and Wang, 1996), probed with anti-NuMA antibody (Matritech Inc., Cambridge, MA), and detected using a secondary FITC-anti–mouse antibody (Sigma Chemical Co.; 1:50). To locate the chromosomes, fixed cells were incubated for 15 min in 10 μg/ml Hoechst 33258, diluted from a 10 mg/ml DMSO stock with PBS, and then washed in PBS.
Preparation of Fluorescent Tubulin, Microinjection, and Microscopy
Tubulin was prepared according to Williams and Lee (1982) and labeled by the procedure of Sammak and Borisy (1988) as detailed in Wheatley and Wang (1996). Cells were microinjected and images collected with a microscope (Axiovert; Zeiss, Inc., Thornwood, NY) coupled to a cooled CCD camera as previously described (Wheatley and Wang, 1996).
Chromosomes were tracked by monitoring changes in pixel coordinates of a consistent region of the chromosomal mass in sequential images using phase optics. Poles were tracked in the same manner using fluorescent optics after injection with rhodamine tubulin.
Digital optical sectioning and three-dimensional reconstruction were performed as described in Fishkind and Wang (1993).
Drug Treatments
All drugs (Sigma Chemical Co.) used were stored at −20°C as 103× stocks in DMSO and diluted directly into prewarmed medium before application to cells. To inhibit protein translation, cells were exposed to a cocktail of 10 μM anisomycin and 100 μM emetine (Sluder et al., 1990). Taxol was used at a final concentration of 10 μM and cytochalasin B at 5 μM.
Results
Expression of Cyclin BΔ90 Causes Exaggerated Chromosome Separation and Inhibits Cytokinesis
Prometaphase NRK cells were microinjected with 1 mg/ml mRNA (3.1 μM) coding for cyclin BΔ90 and monitored using phase contrast time-lapse microscopy (Fig. 1). In all cases (n = 56), chromosomes aligned at the metaphase plate and underwent disjunction normally. The initial separation of chromosomes during anaphase A appeared indistinguishable from that in control cells. However, subsequent movements were abnormal, as spindle poles and chromosomes continued to travel towards opposite sides of the cell (Fig. 1, B and C, and F–H) while in control cells, this movement halted before cytokinesis. In injected cells the chromosomes frequently reached the cell periphery and then turned and traveled back towards the cell center (Fig. 1 D; 17 out of 48 cells). Similar phenomena were observed after microinjection of 0.05–0.6 mg/ml (0.12–1.45 μM) mRNA carrying cyclin BΔ90 sequence connected to a flanking untranslated region (cyclin BΔ90UTR), although in this case 33.3% (n = 84) of cells went through division without a clear phenotype (not shown).
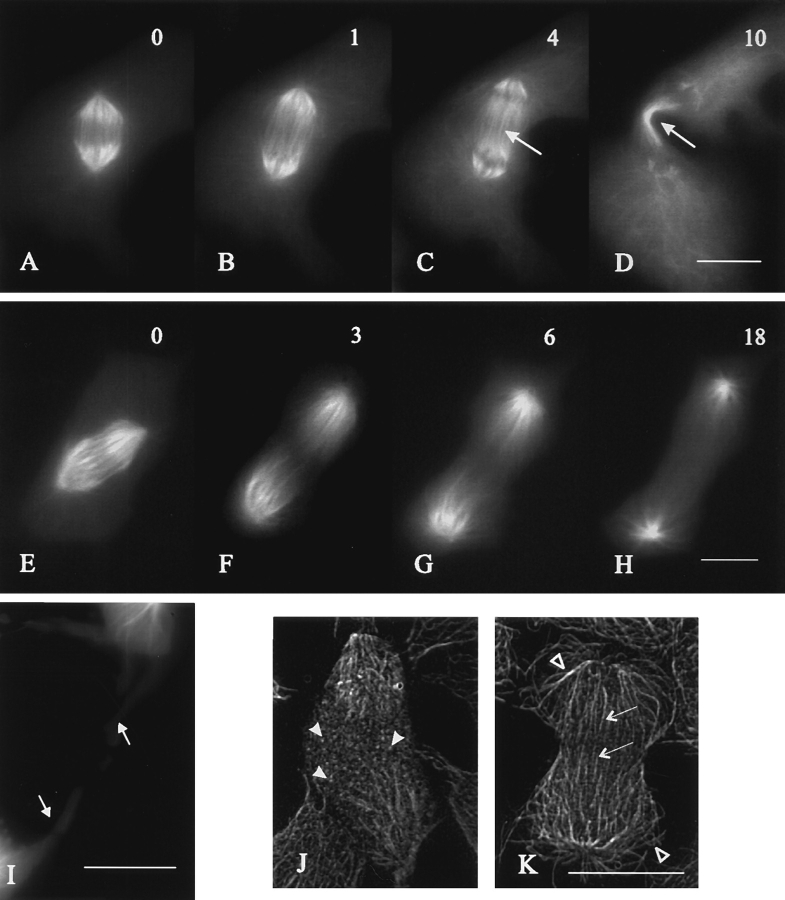
Figure 1.
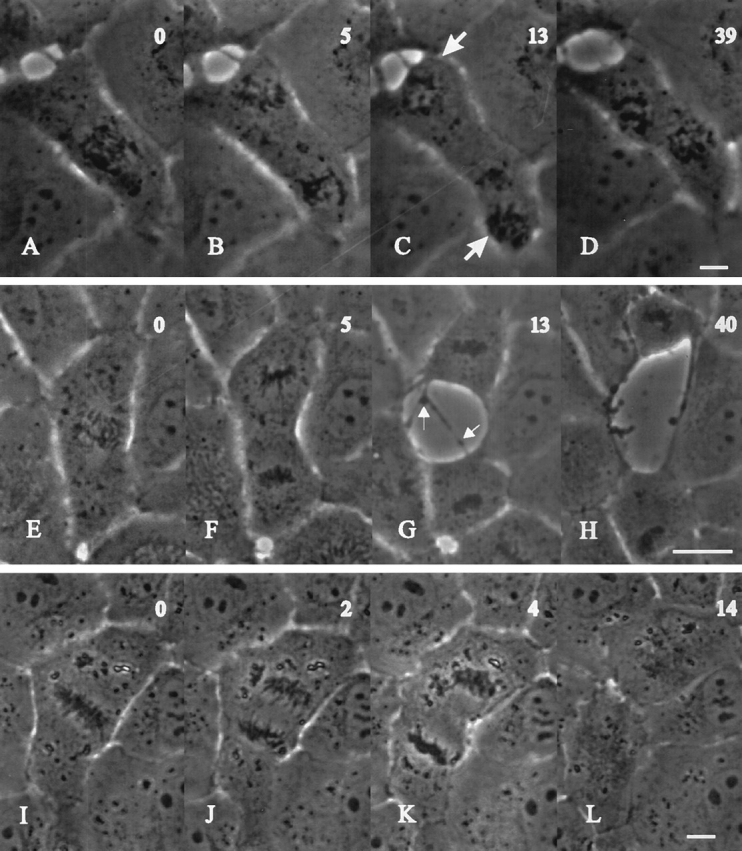
Effects of cyclin BΔ90 mRNA injection on mitosis and cytokinesis in NRK cells. Cells were injected at prometaphase with cyclin BΔ90 mRNA (A–H) and monitored by time lapse phase microscopy. Time elapsed since anaphase onset is given in minutes in the top right corner. The response shown in A–D represents 82% of injected cells: anaphase initiates normally (A), but chromosomes continue separating (B and C) until they reach the periphery where they cause the cell to bulge (C, arrows). Often the chromosomes pirouette and then travel back towards the cell center (D). There is no sign of cytokinesis throughout the period of observation. The remaining 18% of cells (E–H) separate their chromosomes similarly. However, a wide and ill defined ingression develops (F and G), which rips the cell into two, often unequal, parts (G and H). Long, thin strands of cytoplasm with finger-like projections remain associated with neighboring cells in the region of fission (G, arrows). No midbody forms (see also Fig. 2 I) and no post-mitotic respreading occurs in these cells, which accounts for the lingering gaps in the monolayer (D and H). When treated with emetine and anisomycin within 30 s of injection with cyclin BΔ90 mRNA (I–L), cells undergo anaphase and cytokinesis normally. Bars, 20 μm.
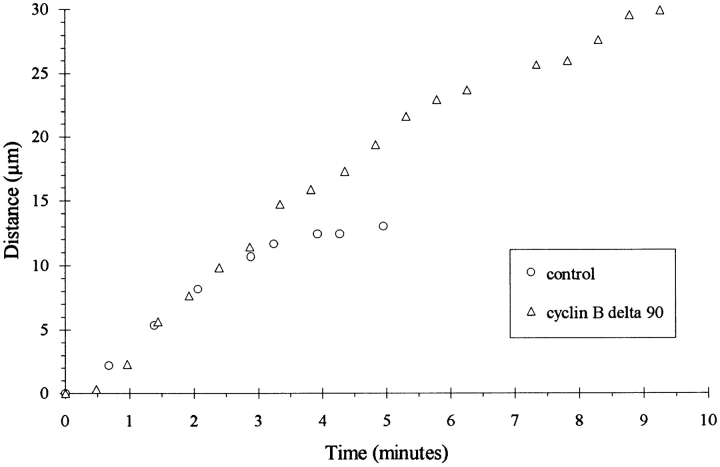
The movement of chromosomes and spindle poles was tracked with phase optics and with fluorescence after microinjection with rhodamine-tubulin. As shown in Fig. 2 and Table I, the average rate of movement for both chromosomes and spindle poles was remarkably similar between control and experimental cells. In addition, both controls and cells injected with mRNA for cyclin BΔ90 reached a similar final distance between chromosomes and spindle poles, as defined by the length of kinetochore microtubules (3.5 ± 1.3 μm, n = 10 for controls and 4.1 ± 1.0 μm, n = 6 for experimental cells). However, while chromosomal movement in control cells stopped ∼4 min after anaphase onset, in cells expressing cyclin BΔ90 the movement continued for 10–20 min. The prolonged anaphase B accounts for the exaggerated separation of chromosomes and spindle poles.
Figure 2.
Movement of individual chromosomes during anaphase in representative control (circles) and cyclin BΔ90 mRNA- injected (triangles) cells. The positions of a consistent region of the chromosomal mass were plotted as a function of time after anaphase onset. Chromosomes move at comparable speeds (∼2.5 μm/min) in both cells during the initial 3.5 min after anaphase onset. In the control cell this motility ceases after 4.5 min, and the cell enters telophase. By contrast, in the injected cell, chromosomes continue traveling at the same velocity until they reach the cell periphery, whereupon they rotate and travel back towards the cell center. The final time point (10 min) marks the commencement of this rotation.
Table I.
Effect of Cyclin BΔ90 mRNA Injection on Anaphase Motilities
| Type of movement | Sample | Average velocity | Sample number | |||
|---|---|---|---|---|---|---|
| (μm/min) ±SD | ||||||
| Anaphase A | Control | 2.6 ± 1.1 | 10 | |||
| chromosomes | cyclin BΔ90 | 2.4 ± 1.0 | 12 | |||
| Anaphase B* | Control | 2.8 ± 1.0 | 16 | |||
| chromosomes | cyclin BΔ90 | 2.7 ± 0.9 | 15 | |||
| Anaphase B | Control | 1.8 ± 0.6 | 8 | |||
| poles | cyclin BΔ90 | 1.7 ± 0.5 | 7 |
Anaphase B is determined based on the movement of spindle poles. Note that the poles move at a slower speed than the chromosomes during anaphase B, implying that anaphase A and B do not occur entirely sequentially in these cells.
Cytokinesis was inhibited in 82% of cells injected with mRNA for cyclin BΔ90 (Fig. 1 D; control cells are 96% successful in cleaving, n = 25). The remaining 18% tore themselves into two daughter cells despite the lack of a well defined cleavage furrow (Fig. 1, F–H). The daughter cells were often of unequal size, with numerous irregular projections but no microtubule-containing midbody present in the region of fission (Fig. 3 I). In addition, no post-mitotic spreading occurred after cell–cell separation, which often created a gap in the monolayer (Fig. 1, G and H). Interestingly, this fission process occurred in the presence of 5 μM cytochalasin B, further indicating that it was not a typical cleavage.
Figure 3.
Microtubule organization in NRK cells expressing cyclin BΔ90. Cells were injected at prometaphase first with cyclin BΔ90 mRNA and then with rhodamine-tubulin. Time in minutes after anaphase onset is shown in the top right corner of each image (A–H). Unlike control cells (A–D), where spindle poles separate to a limited extent and prominent microtubule bundles form in the spindle midzone (C, arrow), cells injected with cyclin BΔ90 mRNA (E–H) show exaggerated pole–pole separation and no interzonal bundles (F and G). Cells that rip apart (18% population, Fig. 1, E–H) are connected by thin cytoplasmic bridges (I, arrows) but lack a midbody which represents a prominent structure in control cells at the end of division (D, arrow). Microtubule organization in fixed cells is shown after deconvolving optical sections of immunofluorescence images and reconstructing a 90° view. In injected cells, punctate staining of tubulin is found throughout the cytoplasm (J, arrowheads). By contrast, in uninjected anaphase cells (K), prominent bundles of microtubules form in the midzone (arrows), and polar microtubules reach extensively into the cytoplasm (open arrowheads). Bars, 20 μm.
Several observations indicate that these phenomena were caused by the expression of cyclin BΔ90. First, chromosomes in injected cells remained condensed and nuclear envelopes never reformed (Fig. 1, D and H, and see Fig. 5 F), as expected with the sustained activity of CDK1. Second, treatment of cells with a cocktail of protein translation inhibitors, emetine (100 μM) and anisomycin (10 μM), immediately after the injection of cyclin BΔ90 mRNA allowed cells to proceed through mitosis and cytokinesis normally (Fig. 1, I–K). Third, injection of mRNA coding for Xenopus elongation factor or for a degradable cyclin B (cyclin BΔ13UTR) at the same molar concentrations (0.1–1.22 μM) as those for cyclin BΔ90UTR had no effect on mitosis or cytokinesis (n = 27/28; not shown).
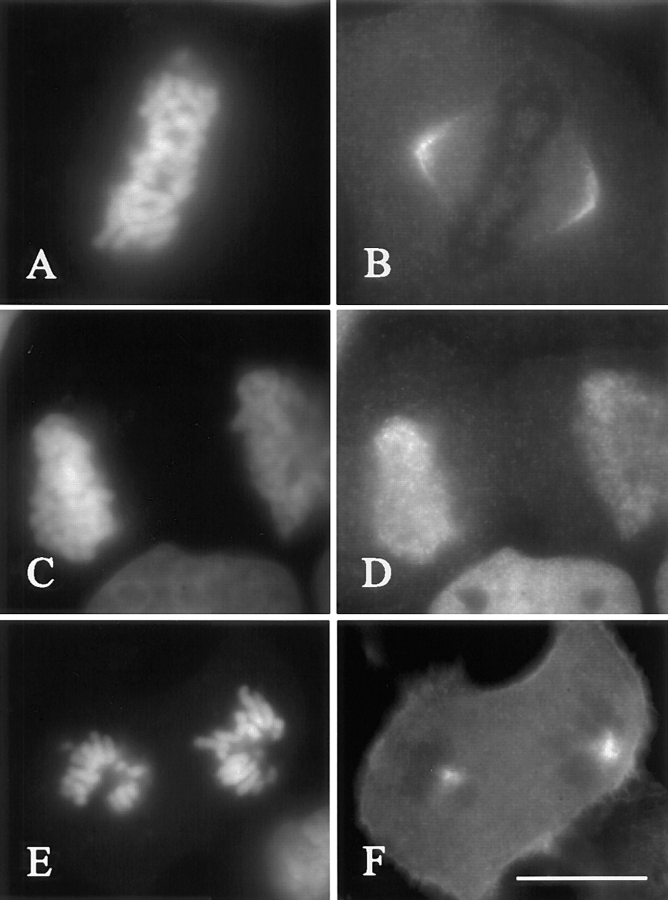
Figure 5.
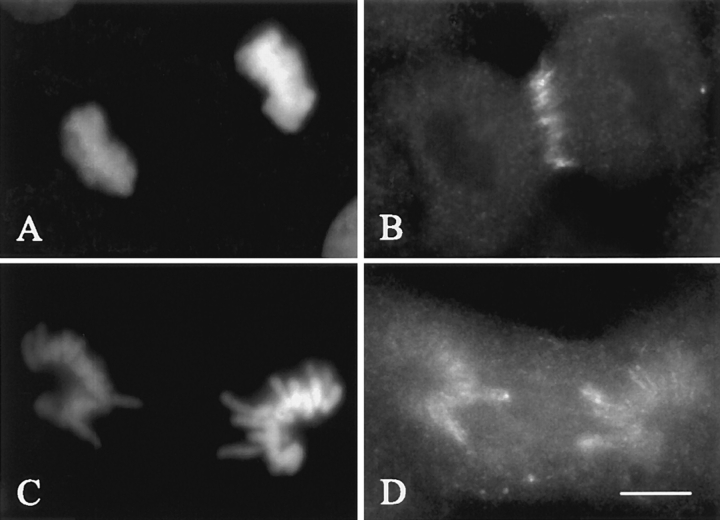
Effect of cyclin BΔ90 mRNA injection on the distribution of NuMA. Left panels show chromosomes stained with Hoechst 33258, and right panels show corresponding NuMA localization. In control cells, NuMA is apparent as a crescent at the poles and half spindles during anaphase A (B) and is found at the reformed nuclear membranes during cytokinesis (D). NuMA fails to redistribute from the centrosomes in cells injected with cyclin BΔ90 mRNA (F). Bar, 20 μm.
Expression of Cyclin BΔ90 Causes Aberrant Spindle Dynamics
We next examined the effect of prolonged CDK1 activity on microtubule organization in vivo. Double injection of prometaphase cells with cyclin BΔ90 mRNA and rhodamine-tubulin showed that, in contrast to control cells (Fig. 3, A–D), neither interzonal microtubule bundles nor a midbody formed in the central region of the anaphase/ telophase spindle (Fig. 3, E–H, and I). Immunofluorescence staining of fixed cells with anti-tubulin and three- dimensional reconstruction of optical sections confirmed that polar microtubules remained short (Fig. 3 J), while in control cells they extended throughout the cell after anaphase onset (Fig. 3 K). In addition, punctate staining of tubulin, which might represent short fragments of microtubules, was present throughout injected cells and was particularly abundant in the central region during cytokinesis (Fig. 3 J). Control cells contained no such punctate structures but instead developed prominent microtubule bundles in the midzone (Fig. 3 K).
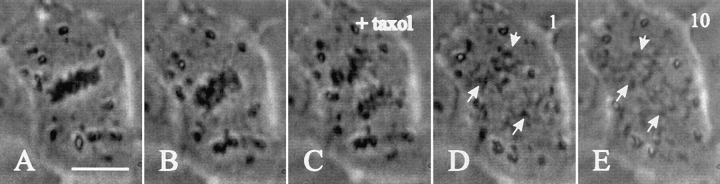
Despite the absence of microtubule bundles in the interzone, chromosome separation remained dependent upon microtubule dynamics, as treatment with 10 μM taxol caused abrupt cessation of chromosomal movement (Fig. 4) but 5 μM cytochalasin B had no effect (not shown). After taxol treatment, the chromosomes often appeared to disperse but remained condensed (Fig. 4 E).
Figure 4.
Dependence of chromosomal movement on microtubule dynamics. NRK cells injected with cyclin BΔ90 mRNA during prometaphase (A) were treated after chromatid disjunction (B) with 10 μM taxol (C). Time after treatment is shown in minutes in the top right hand corner (D and E). The treatment causes abrupt cessation of chromosome movement (D and E, arrows). Chromosomes become dispersed after the treatment but remain condensed. Bar, 20 μm.
We also examined the distribution of NuMA, a spindle component that contains four CDK1 substrate consensus motifs and is regulated by phosphorylation (Compton and Luo, 1995; Gaglio et al., 1995). In control cells NuMA is present as a crescent at the poles and half spindles during prometaphase through anaphase B (Fig. 5, A and B; n = 30/30). It then relocates to the reforming nuclear envelopes during telophase/cytokinesis (Compton et al., 1992; Fig. 5, C and D; n = 5/8). In injected cells NuMA remains highly concentrated at the centrosomes (Fig. 5, E and F; n = 4/4).
Expression of Cyclin BΔ90 Disrupts the Organization of Actin and Myosin
Probing with fluorescent phalloidin and anti-myosin II antibodies indicated that, unlike controls (Fig. 6, A and B) in which F-actin (n = 20/20) and myosin II (n = 18/20) became concentrated at the equatorial cortex (as defined by the spindle) after the separation of chromosomes, in injected cells these antigens remained diffuse throughout the cortex (Fig. 6, C and D; n = 10/10), even in cells that tore themselves into two.
Figure 6.
F-actin (A and C) and myosin II (B and D) localization in control (A and B) and cyclin BΔ90 mRNA injected NRK cells (C and D). Cells were probed with myosin II antibodies and counterstained with fluorescent phalloidin. Control cells display discrete equatorial bands of F-actin and myosin II, while in injected cells these proteins remain diffuse throughout the cortex. Bar, 20 μm.
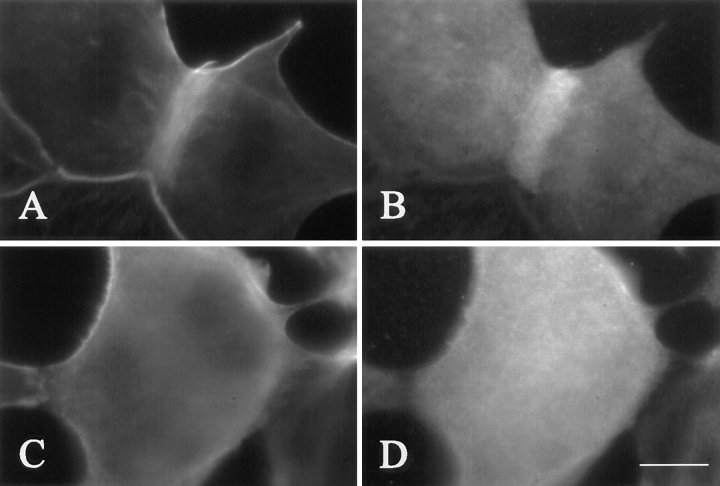
It has been suggested that the reorganization of actin and myosin in cultured cells may be directed by a number of spindle proteins, including TD60, a chromosomal passenger protein that relocates from kinetochores to the equatorial cortex during mid-anaphase (Andreassen et al., 1991; Martineau et al., 1995). In cells injected with mRNA for cyclin BΔ90, TD60 was found to remain associated with chromosomes (Fig. 7, C and D; n = 8/8), while in control cells it became localized at the furrow as short linear structures (Fig. 7, A and B; n = 23/23).
Figure 7.
Localization of TD60 in uninjected (A and B) and cyclin BΔ90 mRNA-injected NRK cells (C and D). Hoechst 33258 staining shows the position of chromosomes (A and C). Unlike control cells, where TD60 protein becomes concentrated at the equator as short linear segments (B), TD60 remains associated with the chromosomes in cells expressing cyclin BΔ90 (D). Bar, 20 μm.
Discussion
We prolonged CDK1 activity in mitotic NRK cells by microinjecting mRNA encoding a nondegradable form of cyclin B, cyclin BΔ90 (Glotzer et al., 1991). Maintenance of CDK1 activity is evident based on the inhibition of chromosome decondensation and nuclear envelope reformation, and on the sensitivity of the observed effects to protein synthesis inhibitors. In addition, as indicated by the negative results obtained with a degradable construct, cyclin BΔ13UTR, the effects are dependent upon the resistance of cyclin BΔ90 to proteolysis.
Regulation of Spindle Dynamics by CDK1
Our observations indicate that CDK1 inactivation is required for the regulation of spindle dynamics during anaphase. In normal cultured cells, microtubules undergo striking reorganizations after anaphase onset: kinetochore microtubules shorten as chromosomes migrate towards the poles, polar microtubules elongate extensively through the cytoplasm, and microtubule bundles form in the midzone. When CDK1 activity is maintained by cyclin BΔ90, there is neither detectable elongation of polar microtubules nor formation of midzone microtubule bundles, suggesting that CDK1 inactivation is required for these microtubule reorganizations. Since at least a fraction of mitotic cyclins has been found in the spindle in mammalian cells (Jackman et al., 1995) and the spindle pole bodies in yeast (Alfa et al., 1990), it is possible that the effects of CDK1 on microtubules are exerted through phosphorylation/dephosphorylation of microtubule-associated motors (Niclas et al., 1996; Nigg et al., 1996) or non-motor proteins such as katanin (McNally and Vale, 1993), MAP4 (Ookata et al., 1995), and oncoprotein 18 (Belmont and Mitchison, 1996). These proteins in turn may regulate the nucleation, elongation, and bundling of microtubules necessary to establish the appropriate geometric configuration(s) for completing anaphase and stimulating cytokinesis.
One protein that may assist in regulating microtubule reorganization is NuMA, a 236-kD protein that is localized at spindle poles during mitosis (Compton et al., 1992). NuMA has four consensus sequences for CDK1 phosphorylation; mutation of one or more of these sites results in the formation of a disorganized spindle and inability of cells to cleave (Compton and Luo, 1995). We found that NuMA fails to dissociate from the spindle pole region when CDK1 activity is maintained at a high level. Thus while CDK1 phosphorylation of NuMA is required for spindle assembly (Compton and Luo, 1995), its dephosphorylation appears necessary for the dissociation from the spindle poles, which may then allow the reorganization of associated microtubules.
Effects of CDK1 Inactivation on Anaphase Movement
A striking effect of maintained CDK1 activity is the exaggerated separation of spindle poles and chromosomes. However, the rate of neither polar nor chromosomal movement was affected to a significant extent. In addition, kinetochore microtubules reached a similar final length as in control cells. These results suggest that CDK1 activity has little or no effect on anaphase A or on the rate of anaphase B. Its effects on anaphase B could be explained solely by the deregulation of the duration and direction of polar movement.
That anaphase B movement does not require CDK1 inactivation suggests that the driving force for polar separation may be continuously active throughout mitosis. This suggestion was first made with newt lung epithelial cells, where half spindle motility was monitored in cells that failed to establish a bipolar spindle (Bajer, 1982; Waters et al., 1993). Monocentric spindles occur when pole–chromosome connections form before centrosome separation, while “anaphase-like prometaphase” results when pole– chromosome connection is established after the centrosomes have traveled too far apart (Bajer, 1982; Waters et al., 1993). Bajer (1982) observed that, regardless of whether half spindles with their associated chromosomes were undertaking normal prometaphase, anaphase-like prometaphase, true anaphase B, or monocentric mitosis, they moved in a similar manner, at the same speed, and had a tendency to migrate towards the cell periphery where they occasionally rotated (Waters et al., 1993). Bajer (1982) concluded that the mechanisms responsible for polar movement are intrinsic to each aster and may function in a similar manner from prometaphase through anaphase B. The present study provides experimental data to substantiate this notion that was previously based on insightful observations.
Our results further indicate that the extent and duration of pole–pole separation is controlled by CDK1 inactivation in normal cells. With sustained CDK1 activity, microtubules appear to be organized as two independent half spindles after chromosome disjunction, rather than forming one integral anaphase spindle. A similar conversion of a bipolar spindle into two half spindles was recently observed in cyclin BΔ90-treated frog egg extracts (Murray et al., 1996). The coordination between the two half spindles in the normal cell is most likely achieved by the congressing chromosomes during prometaphase and by the interzonal microtubule bundles during anaphase.
Our data also provide useful insight into the mechanism of anaphase B movement. The common view contends that anaphase B is achieved through antiparallel sliding of interzonal microtubules driven by kinesin-like motors (for reviews see Cande and Hogan, 1989; Hogan and Cande, 1990; Vernos and Karsenti, 1996). Support for this model comes from observations of diatom spindles (McIntosh et al., 1977), reactivation of spindles in vitro (Cande and McDonald, 1985), and analysis of kinesin-disrupted systems (Hagan and Yanagida, 1990; Nislow et al., 1992; Hogan et al., 1993). Our observation of normal rates but unrestricted extent of anaphase B in the absence of interzonal microtubule bundles, however, supports an alternative model that suggests that forces associated with the poles pull them apart and that interzonal microtubules act to limit the extent and direction of chromosome movement. Previous support for this “astral pulling model” comes from three-dimensional structural analysis of microtubule organization in PtK cells (Mastronarde et al., 1993); from the similar rates of polar movement during prometaphase and anaphase B (discussed above) and from several micromanipulation experiments. Specifically, severing the central spindle by laser (Aist et al., 1993) or by perforation (Kronebush and Borisy, 1982) causes an elevation in the rate of chromosome separation in PtK cells, while ablation of the astral region causes cessation of chromosome movement (Aist et al., 1993). It appears, therefore, that the half spindles are autonomous units capable of independent migration in the cell (Bajer, 1982; Waters et al., 1993).
How do the half spindles and associated chromosomes propel themselves? Most likely forces are generated through interactions between pole-associated microtubules and anchored motors (Bajer, 1982; Hyman, 1989; Waters et al., 1993), which could be associated directly with the cell cortex or indirectly through organelles and the actin–myosin system. In addition, to achieve net movement, forces must be asymmetric with respect to the spindle pole, possibly through the preferential involvement of a subset of microtubules on one side of the pole. Since free astral microtubules appeared very short in cells with prolonged CDK1 activity, we speculate that the kinetochore microtubules may participate in this process. However, to achieve pole–pole separation this would require anchored, plus end–directed microtubule motors, a notion at odds with the opinion that minus end–directed motors contribute to polar migration by pulling on the asters (Hyman and White, 1987; Saunders et al., 1995). Alternatively, as suggested by the sensitivity of the movement to taxol (Fig. 4; Hyman and White, 1987; Waters et al., 1996), it is possible that anaphase B is driven primarily by the assembly– disassembly of spindle pole-associated microtubules. This mechanism could involve either the dynamic instability of microtubules in combination with a “molecular ratchet” that constrains the direction of polar movement, or treadmilling of free polar microtubules.
Regulation of Cytokinesis by CDK1
The majority of cells injected with cyclin BΔ90 mRNA failed to divide. A similar effect was reported previously in frog embryos (Murray et al., 1989). There are several possible reasons why cytokinesis is inhibited. First, we recently demonstrated that in NRK cells, midzone microtubule bundles are continuously required for cytokinesis, possibly serving a role of guiding and maintaining the localization of cleavage signals (Wheatley and Wang, 1996). Cells expressing cyclin BΔ90 may fail to divide due to the absence of midzone microtubules. Second, a number of chromosomal passenger proteins have been found to relocate to the central anaphase spindle and then to the equatorial cortex during telophase. At least some of them may participate in signaling cytokinesis (Earnshaw and Mackay, 1994). We found one such protein, TD60 (Andreassen et al., 1991; Martineau et al., 1995), to remain associated with the chromosomes in cells with high CDK1 activity. Thus phosphorylation by CDK1 may directly or indirectly regulate the redistribution of molecules involved in triggering cytokinesis. Third, in vitro studies have shown that the regulatory light chain of myosin II is negatively regulated by CDK1 phosphorylation (Satterwhite et al., 1992). Prolonged phosphorylation of myosin light chain by CDK1 could inhibit division by preventing myosin II reorganization (Fig. 2) and actin–myosin II interactions.
A small percentage of cells broke into two daughter cells, in a manner distinctly different from cytokinesis as indicated by its insensitivity to cytochalasin B and the absence of a midbody. This result is intriguing, as it suggests that, as for Dictyostelium, which can undergo “traction-mediated cytoplasmic fission” in the absence of myosin II (DeLozanne and Spudich, 1987), there is an actin–myosin II independent mechanism that can affect division in cultured mammalian cells.
Conclusions
In a previous report, HeLa cells transfected with nondestructible avian cyclin B2 were found to arrest in a mitotic state, but in this case the condensed chromosomes appeared as a single mass attached to multiple poles through short microtubules (Gallant and Nigg, 1992). It is possible that the prolonged exposure to CDK1 activity (up to 36 h) may have caused the chromosomes to aggregate and the centrosomes to split and reorganize. By introducing continuously active CDK1 precisely when endogenous CDK1 is inactivated, we have avoided potential extraneous effects that might result from exposing other phases of the cell cycle to CDK1. This allows us to assess more specifically the impact of sustained CDK1 activity on the exit from M phase. Our results demonstrate that mammalian cells respond to prolonged CDK1 activity in a way consistent with what was hinted with yeast and flies: arresting with segregated chromosomes and failing to undergo cytokinesis.
We conclude that CDK1 inactivation is required for the reorganization of microtubules into an integrated anaphase spindle with interzonal microtubules and extended polar microtubules. Without the connection by interzonal microtubules of the central spindle during anaphase, the half spindles operate independently, a situation that leaves the transmission of the genome open to frequent errors. While extrapolation of in vitro studies on microtubule dynamics might have predicted some of the behavior of microtubules in this study (Verde et al., 1990), using living mammalian cells we were able to observe directly the consequences of altered microtubule organization. Our experiments have also demonstrated the requirement of CDK1 inactivation in the relocation of proteins implicated in mitosis and cytokinesis, thus providing new insights into the control of these events.
Acknowledgments
We would like to thank Drs. L. Hake and J. Richter for assistance with in vitro transcription; Dr. C. Wilkerson for providing tubulin; and Drs. K. Fujiwara (myosin), R. Margolis (TD60), and C. Sparks (NuMA) for antibodies.
Abbreviations used in this paper
- NRK
normal rat kidney
- NuMA
nuclear protein-associated mitotic apparatus
Footnotes
This project was supported by funds from the National Institutes of Health and Human Frontier Science Program.
S.P. Wheatley would like to dedicate this work to Professor Daniel Mazia, whose insights into cell division inspired her to follow the same path in cell biology. S.P. Wheatley met Professor Mazia during a United Nations Educational, Scientific, and Cultural Organization course entitled “Dynamics of intracellular structures during the cell cycle” held in L'Ile d'Yeu, France, in the summer of 1992.
Please address all correspondence to Yu-li Wang, Cell Biology Group, Worcester Foundation for Biomedical Research, Shrewsbury, MA 01545. Tel.: (508) 845-2651; Fax: (508) 842-3915.
References
- Aist JR, Berns MW. Mechanics of chromosome separation during mitosis in Fusarium(Fungi imperfecti): new evidence from ultrastructural and laser microbeam experiments. J Cell Biol. 1981;91:446–458. doi: 10.1083/jcb.91.2.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aist JR, Bayles CJ, Tao W, Berns MW. Direct experimental evidence for the existence, structural basis and function of astral forces during anaphase B in vivo. . J Cell Sci. 1991;100:279–288. doi: 10.1242/jcs.100.2.279. [DOI] [PubMed] [Google Scholar]
- Aist JR, Liang H, Berns MW. Astral and spindle forces in PtK2cells during anaphase B: a laser microbeam study. J Cell Sci. 1993;104:1207–1216. doi: 10.1242/jcs.104.4.1207. [DOI] [PubMed] [Google Scholar]
- Alfa CE, Ducommun B, Beach D, Hyams JS. Distinct nuclear and spindle pole body populations of cyclin-cdc2 in fission yeast. Nature (Lond) 1990;347:680–682. doi: 10.1038/347680a0. [DOI] [PubMed] [Google Scholar]
- Andreassen PR, Palmer DK, Wener MH, Margolis R. Telophase disc: a new mammalian mitotic organelle that bisects telophase cells with a possible function in cytokinesis. J Cell Sci. 1991;99:523–534. doi: 10.1242/jcs.99.3.523. [DOI] [PubMed] [Google Scholar]
- Bajer AS. Functional autonomy of monopolar spindle and evidence for oscillatory movement in mitosis. J Cell Biol. 1982;93:33–48. doi: 10.1083/jcb.93.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmont LD, Mitchison TJ. Identification of a protein that interacts with tubulin dimers and increases the catastrophe rate of microtubules. Cell. 1996;84:623–631. doi: 10.1016/s0092-8674(00)81037-5. [DOI] [PubMed] [Google Scholar]
- Cande WZ, McDonald KL. In vitro reactivation of anaphase spindle elongation using isolated diatom spindles. Nature (Lond) 1985;316:168–170. doi: 10.1038/316168a0. [DOI] [PubMed] [Google Scholar]
- Cande WZ, Hogan CJ. The mechanism of anaphase spindle elongation. Bioessays. 1989;11:5–9. doi: 10.1002/bies.950110103. [DOI] [PubMed] [Google Scholar]
- Compton DA, Luo C. Mutation of the predicted p34cdc2phosphorylation sites in NuMA impair the assembly of the mitotic spindle and block mitosis. J Cell Sci. 1995;108:621–633. doi: 10.1242/jcs.108.2.621. [DOI] [PubMed] [Google Scholar]
- Compton DA, Szilak I, Cleveland DW. Primary structure of NuMA, an intranuclear protein that defines a novel pathway for segregation of proteins at mitosis. J Cell Biol. 1992;116:1395–1408. doi: 10.1083/jcb.116.6.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lozanne A, Spudich JA. Disruption of the Dictyosteliummyosin heavy chain gene by homologous recombination. Science (Wash DC) 1987;236:1086–1091. doi: 10.1126/science.3576222. [DOI] [PubMed] [Google Scholar]
- Earnshaw WC, Mackay AM. Role of nonhistone proteins in the chromosomal events of mitosis. FASEB (Fed Am Soc Exp Biol) J. 1994;8:947–956. doi: 10.1096/fasebj.8.12.8088460. [DOI] [PubMed] [Google Scholar]
- Evans T, Rosenthal ET, Youngbloom J, Distel D, Hunt T. Cyclin: a protein specified by maternal mRNA in sea urchin eggs that is destroyed at each cleavage division. Cell. 1983;33:389–396. doi: 10.1016/0092-8674(83)90420-8. [DOI] [PubMed] [Google Scholar]
- Fishkind DJ, Wang Y-l. Orientation and three-dimensional organization of actin filaments in dividing cultured cells. J Cell Biol. 1993;123:837–848. doi: 10.1083/jcb.123.4.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara K, Pollard TD. Fluorescent antibody localization of myosin in the cytoplasm, cleavage furrow and mitotic spindle of human cells. J Cell Biol. 1976;71:847–875. doi: 10.1083/jcb.71.3.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaglio T, Saredi A, Compton DA. NuMA is required for the organization of microtubules into aster-like arrays. J Cell Biol. 1995;131:693–708. doi: 10.1083/jcb.131.3.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallant P, Nigg EA. Cyclin B2 undergoes cell cycle–dependent nuclear translocation and, when expressed as a non-destructible mutant, causes mitotic arrest in HeLa cells. J Cell Biol. 1992;117:213–224. doi: 10.1083/jcb.117.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glotzer M, Murray AM, Kirschner MW. Cyclin is degraded by the ubiquitin pathway. Nature (Lond) 1991;349:132–138. doi: 10.1038/349132a0. [DOI] [PubMed] [Google Scholar]
- Hagan IM, Yanagida M. Novel potential mitotic motor protein encoded by the fission yeast cut7+ gene. Nature (Lond) 1990;347:563–566. doi: 10.1038/347563a0. [DOI] [PubMed] [Google Scholar]
- Hogan CJ, Cande WZ. Antiparallel microtubule interactions: spindle formation and anaphase B. Cell Motil Cytosk. 1990;16:99–103. doi: 10.1002/cm.970160203. [DOI] [PubMed] [Google Scholar]
- Hogan CJ, Wein H, Wordeman L, Scholey JM, Sawin KE, Cande WZ. Inhibition of anaphase spindle elongation in vitro by a peptide antibody that recognizes kinesin motor domain. PNAS. 1993;90:6611–6615. doi: 10.1073/pnas.90.14.6611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway SL, Glotzer M, King RW, Murray AW. Anaphase is initiated by proteolysis rather than by the inactivation of maturation-promoting factor. Cell. 1993;73:1393–1402. doi: 10.1016/0092-8674(93)90364-v. [DOI] [PubMed] [Google Scholar]
- Hunt T. Destruction's our delight. Nature (Lond) 1991;349:100–101. doi: 10.1038/349100a0. [DOI] [PubMed] [Google Scholar]
- Hyman AA. Centrosome movement in the early divisions of Caenorhabditis elegans: a cortical site determining centrosome position. J Cell Biol. 1989;109:1185–1193. doi: 10.1083/jcb.109.3.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman AA, White JG. Determination of cell division axes in the early embryogenesis of Caenorhabditis elegans. . J Cell Biol. 1987;105:2123–2135. doi: 10.1083/jcb.105.5.2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackman M, Firth M, Pines J. Human cyclins B1 and B2 are localized to strikingly different structures: B1 to microtubules, B2 primarily to the Golgi apparatus. EMBO (Eur Mol Biol Organ) J. 1995;14:1646–1654. doi: 10.1002/j.1460-2075.1995.tb07153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronebusch, P.J., and G.G. Borisy. 1982. Mechanisms of anaphase B movement. In Biological Function of Mts and Related Structures. H. Sakai, H. Mohari, and G.G. Borisy, editors. Academic Press, New York. 233–245.
- Martineau SN, Andreassen PR, Margolis RL. Delay in HeLa cell cleavage into interphase using dihydrocytochalasin B: retention of a postmitotic spindle and telophase disc correlates with synchronous cleavage recovery. J Cell Biol. 1995;131:191–205. doi: 10.1083/jcb.131.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastronarde DN, McDonald KL, Ding R, McIntosh JR. Interpolar spindle microtubules in PTK cells. J Cell Biol. 1993;123:1475–1489. doi: 10.1083/jcb.123.6.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh KL, Pickett-Heaps JD, McIntosh JR, Tippit DH. On the mechanism of anaphase spindle elongation in Diatoma vulgare. . J Cell Biol. 1977;74:377–388. doi: 10.1083/jcb.74.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna NM, Wang Y-l. Culturing cells on the microscope stage. Methods Cell Biol. 1989;29:195–205. doi: 10.1016/s0091-679x(08)60195-8. [DOI] [PubMed] [Google Scholar]
- McNally FJ, Vale RD. Identification of katanin, an ATPase that severs and disassembles stable microtubules. Cell. 1993;75:419–429. doi: 10.1016/0092-8674(93)90377-3. [DOI] [PubMed] [Google Scholar]
- Murray AW. Cyclin ubiquitination: the destructive end of mitosis. Cell. 1995;81:149–152. doi: 10.1016/0092-8674(95)90322-4. [DOI] [PubMed] [Google Scholar]
- Murray AW, Solomon MJ, Kirschner MW. The role of cyclin synthesis and degradation in the control of maturation promoting factor activity. Nature (Lond) 1989;339:280–286. doi: 10.1038/339280a0. [DOI] [PubMed] [Google Scholar]
- Murray AW, Kirschner MW. Cyclin synthesis drives the early embryonic cell cycle. Nature (Lond) 1989;339:275–280. doi: 10.1038/339275a0. [DOI] [PubMed] [Google Scholar]
- Murray AW, Desai AB, Salmon ED. Real time observation of anaphase in vitro. . Proc Natl Acad Sci USA. 1996;93:12327–12332. doi: 10.1073/pnas.93.22.12327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niclas J, Allan VJ, Vale RD. Cell cycle regulation of dynein associated with membranes modulates microtubule-based organelle transport. J Cell Biol. 1996;133:585–593. doi: 10.1083/jcb.133.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg EA, Blangy A, Lane HA. Dynamic changes in nuclear architecture during mitosis: on the role of protein phosphorylation in spindle assembly and chromosome segregation. Exp Cell Res. 1996;229:174–180. doi: 10.1006/excr.1996.0356. [DOI] [PubMed] [Google Scholar]
- Nislow C, Lombillo V, Kuriyama R, McIntosh JR. A plus-end directed motor enzyme that moves antiparallel microtubules in vitrolocalizes to the interzone of mitotic spindles. Nature (Lond) 1992;359:543–547. doi: 10.1038/359543a0. [DOI] [PubMed] [Google Scholar]
- Nurse P. Universal control mechanism regulating onset of M-phase. Nature (Lond) 1990;344:503–508. doi: 10.1038/344503a0. [DOI] [PubMed] [Google Scholar]
- Ookata K, Hisanaga S, Bulinski JC, Murofushi H, Aizawa H, Itoh TJ, Hotani H, Okumura E, Tachibana E, Kishimoto T. Cyclin B interaction with microtubule-associated protein 4 (MAP4) targets p34cdc2kinase to microtubules and is a potential regulator of M-phase microtubule dynamics. J Cell Biol. 1995;128:849–862. doi: 10.1083/jcb.128.5.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pines J. The cell cycle kinases. Semin Cancer Biol. 1994;5:305–313. [PubMed] [Google Scholar]
- Rimmington G, Dalby B, Glover DM. Expression of N-terminally truncated cyclin B in the Drosophilalarval brain leads to mitotic delay at late anaphase. J Cell Sci. 1994;107:2729–2738. doi: 10.1242/jcs.107.10.2729. [DOI] [PubMed] [Google Scholar]
- Sammak PJ, Borisy GG. Detection of single fluorescent microtubules and methods for determining their dynamics in living cells. Cell Motil Cytoskeleton. 1988;10:237–245. doi: 10.1002/cm.970100128. [DOI] [PubMed] [Google Scholar]
- Satterwhite LL, Lohka MJ, Wilson KL, Cisek TY, Corden LJ, Pollard TD. Phosphorylation of myosin-II regulatory light chain by cyclin-p34cdc2: a mechanism for the timing of cytokinesis. J Cell Biol. 1992;118:595–605. doi: 10.1083/jcb.118.3.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders WS, Koshland D, Eshel D, Gibbons IR, Hoyt MA. Saccharomyces cerevisiaekinesin- and dynein-related proteins required for anaphase chromosome segregation. J Cell Biol. 1995;128:617–624. doi: 10.1083/jcb.128.4.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigrist S, Jacobs H, Stratmann R, Lehner CF. Exit from mitosis is regulated by Drosophila fizzyand the sequential destruction of cyclins A, B, and B3. EMBO (Eur Mol Biol Organ) J. 1995;14:4827–4838. doi: 10.1002/j.1460-2075.1995.tb00164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluder G, Miller FJ, Cole R, Rieder CL. Protein synthesis and the cell cycle: centrosome reproduction in sea urchin eggs is not under translational control. J Cell Biol. 1990;110:2025–2032. doi: 10.1083/jcb.110.6.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surana U, Amon A, Dowzer C, McGrew J, Byers B, Nasmyth K. Destruction of the CDC28/CLB mitotic kinase is not required for the metaphase to anaphase transition in budding yeast. EMBO (Eur Mol Biol Organ) J. 1993;12:1969–1978. doi: 10.1002/j.1460-2075.1993.tb05846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verde F, Labbe J-C, Doree M, Karsenti E. Regulation of microtubule dynamics by cdc2 protein kinase in cell-free extracts of Xenopuseggs. J Cell Biol. 1990;118:1097–1108. doi: 10.1038/343233a0. [DOI] [PubMed] [Google Scholar]
- Vernos I, Karsenti E. Motors involved in spindle assembly and chromosome segregation. Curr Opin Cell Biol. 1996;8:4–9. doi: 10.1016/s0955-0674(96)80041-x. [DOI] [PubMed] [Google Scholar]
- Waters JC, Cole RW, Rieder CL. The force-producing mechanism for centrosome separation during spindle formation in vertebrates is intrinsic to each aster. J Cell Biol. 1993;122:361–372. doi: 10.1083/jcb.122.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters JC, Mitchison TJ, Rieder CL, Salmon ED. The kinetochore microtubule minus-end disassembly associated with poleward flux produces a force that can do work. Mol Biol Cell. 1996;7:1547–1558. doi: 10.1091/mbc.7.10.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheatley SP, Wang Y-l. Midzone microtubule bundles are continuously required for cytokinesis in cultured epithelial cells. J Cell Biol. 1996;135:981–989. doi: 10.1083/jcb.135.4.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RC, Lee JC. Preparation of tubulin from brain. Methods Enzymol. 1982;85:376–381. doi: 10.1016/0076-6879(82)85038-6. [DOI] [PubMed] [Google Scholar]
- Yamano H, Gannon J, Hunt T. The role of proteolysis in cell cycle progression in Schizosaccharomyces pombe. . EMBO (Eur Mol Biol Organ) J. 1996;15:5268–5279. [PMC free article] [PubMed] [Google Scholar]