Abstract
Rab9 GTPase is required for the transport of mannose 6-phosphate receptors from endosomes to the trans-Golgi network in living cells, and in an in vitro system that reconstitutes this process. We have used the yeast two-hybrid system to identify proteins that interact preferentially with the active form of Rab9. We report here the discovery of a 40-kD protein (p40) that binds Rab9–GTP with roughly fourfold preference to Rab9–GDP. p40 does not interact with Rab7 or K-Ras; it also fails to bind Rab9 when it is bound to GDI. The protein is found in cytosol, yet a significant fraction (∼30%) is associated with cellular membranes. Upon sucrose density gradient flotation, membrane- associated p40 cofractionates with endosomes containing mannose 6-phosphate receptors and the Rab9 GTPase. p40 is a very potent transport factor in that the pure, recombinant protein can stimulate, significantly, an in vitro transport assay that measures transport of mannose 6-phosphate receptors from endosomes to the trans-Golgi network. The functional importance of p40 is confirmed by the finding that anti-p40 antibodies inhibit in vitro transport. Finally, p40 shows synergy with Rab9 in terms of its ability to stimulate mannose 6-phosphate receptor transport. These data are consistent with a model in which p40 and Rab9 act together to drive the process of transport vesicle docking.
Rab GTPases participate in the processes by which transport vesicles identify and fuse with their cognate target membranes (for reviews see Novick and Brennwald, 1993; Zerial and Stenmark, 1993; Nuoffer and Balch, 1994; Pfeffer, 1994). The strongest evidence suggesting that Rab GTPases function in transport vesicle docking is the finding that temperature-sensitive mutations in the Sec4p GTPase lead to the accumulation of secretory vesicles. Rabs are also rate-limiting factors in homotypic membrane fusion events: a GTPase-deficient, activated mutant of Rab5, for example, leads to the generation of enlarged endosomes (Bucci et al., 1992), presumably due to hyperactivation of endosome fusion. Over 30 different Rab proteins have been identified; distinct sets of Rab proteins are found on the ER, Golgi, intermediate compartment between the ER and Golgi, on early and late endosomes, and on the plasma membrane. Some are redundant isoforms that carry out a common function (Singer-Krüger et al., 1994); most others are unique and essential for a particular step of intracellular transport.
Rabs are doubly geranylgeranylated at their COOH termini, a modification that renders them hydrophobic and is essential for their activity. While the majority of Rabs are membrane associated, prenylated Rabs are also found in the cytosol bound to a protein named GDI (Sasaki et al., 1990). We have shown that Rab/GDI complexes represent active transport factors (Dirac-Svejstrup et al., 1994) that possess adequate information to deliver Rab GTPases to their correct membrane-bound compartments (Soldati et al., 1994, 1995; Ullrich et al., 1994). After membrane delivery, Rabs are converted to their active, GTP-bound conformations (Soldati et al., 1994; Ullrich et al., 1994), presumably by the action of a membrane-associated, GDI-displacement factor (Dirac-Svejstrup et al., 1997) and a distinct, subsequently acting nucleotide-exchange factor (Walch-Solimena et al., 1997).
Rabs, in their active, GTP-bound conformations, are believed to recruit cytosolic factors onto membranes to facilitate subsequent membrane–membrane recognition before fusion (Pfeffer, 1996). The best characterized example of this is the recruitment of Rabaptin5 onto Rab5–GTP-bearing endosomes (Stenmark et al., 1995). Rabaptin5 binds Rab5 preferentially in its GTP-bound conformation, a process that stimulates subsequent endosome fusion (Stenmark et al., 1995).
We study the Rab9 GTPase and its role in stimulating the transport of mannose 6-phosphate receptors from endosomes to the trans-Golgi network. We have shown that Rab9 is required for this process in living cells (Riederer et al., 1994) and in an in vitro system that reconstitutes this transport process (Lombardi et al., 1993; Dirac-Svejstrup et al., 1994). Given the importance of Rab-interacting proteins in driving vesicle-docking events, we sought to identify proteins with which Rab9 interacts. We report here the identification of a novel 40-kD protein, p40, that interacts specifically with the Rab9 GTPase in its GTP-bound conformation and can stimulate the transport of mannose 6-phosphate receptors (MPRs)1 from endosomes to the TGN in vitro.
Materials and Methods
Rab9 cDNA clones (Shapiro et al., 1993; Riederer et al., 1994), anti-Rab9 antibodies (Shapiro et al., 1993), monoclonal anti-mannose 6-phosphate receptor antibodies (Lombardi et al., 1993), prenyl Rab9, bovine brain GDI, and Rab9/GDI complexes were as described (Dirac-Svejstrup et al., 1994; Soldati et al., 1994). Anti-p40 antiserum was prepared in rabbits using recombinant, His-tagged p40. Endosome-to-TGN transport assays were carried out by a modification (Itin, C., C. Rancaño, Y. Nakajima, and S.R. Pfeffer, manuscipt submitted for publication) of the standard procedure (Goda and Pfeffer, 1988) using K562 cytosol. p40 and Rab9/ GDI were diluted in cytosol before addition to the assay. Cytosol-dependent transport was determined by subtracting the cpm obtained in the absence of cytosol (∼200 cpm). Transport in the presence of 1 mg/ml cytosol was defined as 100%. Rabbit IgG was purified using protein A Sepharose (Sigma Chemical Co., St. Louis, MO). Protein assays were carried out using reagent (Bio Rad; Hercules, CA) and bovine serum albumin as standard.
Yeast Two Hybrid Screen
Yeast strain Y190 (MATa gal4 gal80 his3 trp1-901 ade2-1-1 ura3-52 leu2-3,-112+URA3::GAL→ lacZ, LYS2::GAL→ HIS3) was transformed with the pASI–CYH-Rab9Δcc plasmid (Schiestl and Giest, 1989) and colonies selected by growth on plates lacking tryptophan. Rab9 fusion protein expression was verified by immunoblotting. These cells were then transformed with library DNA (human mature B cell cDNA in pACT) and plated on synthetic medium lacking tryptophan, leucine, and histidine and containing 25 mM 3′-aminotriazole (Sigma Chemical Co.). Colonies were picked after 5 d at 30°C and β-galactosidase activity assessed by colony filter assay (Vojtek et al., 1993) or quantitative liquid culture assay (Rose and Botstein, 1983). Positive clones (His+, LacZ+) were forced to lose the library plasmid by growth on YPAD containing 10 μg/ml cyclohexamide and were mated to yeast strain Y187 (MATα gal4 gal80 his3 trp1-901 ade2-101 ura3-52 leu2-3,-112 GAL→ lacZ) expressing pASI-CYH–Rab9S21NΔcc or -Rab7Δcc and tested for β-galactosidase activity. Several clones displayed Rab9–GTP-dependent β-galactosidase activity. Library plasmids were recovered in Escherichia coli XL1-blue cells and purified on a column (Qiagen, Chatsworth, CA) for subsequent sequencing.
Cloning and Expression
A human Jurkat lymphoma cDNA library (106 plaque-forming units) in λZAPII (Stratagene, La Jolla, CA) was screened with a 32P-labeled probe made by random-primed labeling (Boehringer Mannheim, Indianapolis, IN) of a 400-bp BglII–PvuII fragment representing the most 5′ region of the two hybrid clone. Plasmid DNA was recovered by in vivo excision of the pBluescript plasmid from the λZAPII vector in E. coli XL1-blue cells and purified on a column (Qiagen) for subsequent sequencing. An AvrII– SalI fragment containing the entire open reading frame of p40 was subcloned into pQE-31 and transformed into E. coli XL1-blue cells. Cells (OD = 0.9) were induced with 0.5 mM IPTG for 10 h at 22°C. Protein purification was carried out according to the manufacturer (Qiagen), except that cells were broken by two passes through a French press (medium power, 1400 U pressure) and subjected to Sephacryl-S100 gel filtration.
In Vitro Binding Assay
Reactions were in 20 mM Hepes, pH 7.4, 50 mM NaCl, 20 mM imidazole, 5 mM MgCl2, 100 μg/ml BSA. Each prenylated GTPase (1 μg = 571 nM in complex with BSA or GDI) was incubated with 1 μg (357 nM) recombinant p40 and 100 μM GDP or GTP at 37°C for 1.5 h. p40-bound GTPase was recovered by copurification on Ni-NTA resin (Qiagen; 20 μl of a 50% slurry) and elution in 20 μl, 100 mM EDTA; eluted amounts were quantitated by immunoblot analysis. GTPase standards (1–40 ng) were analyzed in parallel; the amount of GTPase bound to the resin in the absence of p40 (⩽2 ng) was subtracted.
GTPase Assays
Rab9 (50 nM) GTPase activity was measured as described (Shapiro et al., 1993); reactions were analyzed by thin layer chromatography.
Sucrose Gradient Flotation
K562 cell postnuclear supernatant (PNS) was fractionated by sucrose gradient flotation according to Balch et al. (1984). The PNS (6 ml) in 1.4 M sucrose was overlaid with 3 ml, 1.2 M sucrose and 3 ml, 0.8 M sucrose in an SW41 tube. Gradients were centrifuged for 3 h at 36,000 rpm. Fractions (0.5 ml) were collected from the top. Marker protein distributions were determined by immunoblot after trichloroacetic acid precipitation of 200 μl samples and 12% SDS-PAGE separation.
p40-depleted Cytosol
IgG from preimmune or anti-p40 serum (0.25 ml) was precipitated with 50% ammonium sulfate and pelleted at 95,000 rpm for 10 min in a centrifuge (TLA100; Beckman Instr., Fullerton, CA). Pellets were dissolved in 1 ml K562 cytosol (5 mg/ml) and incubated 5 h at 4°C; protein-A Sepharose (0.4 ml) was then added for 30 min at 4°C. The slurry was poured into a column, and the flow through was collected as “depleted cytosol.”
Results
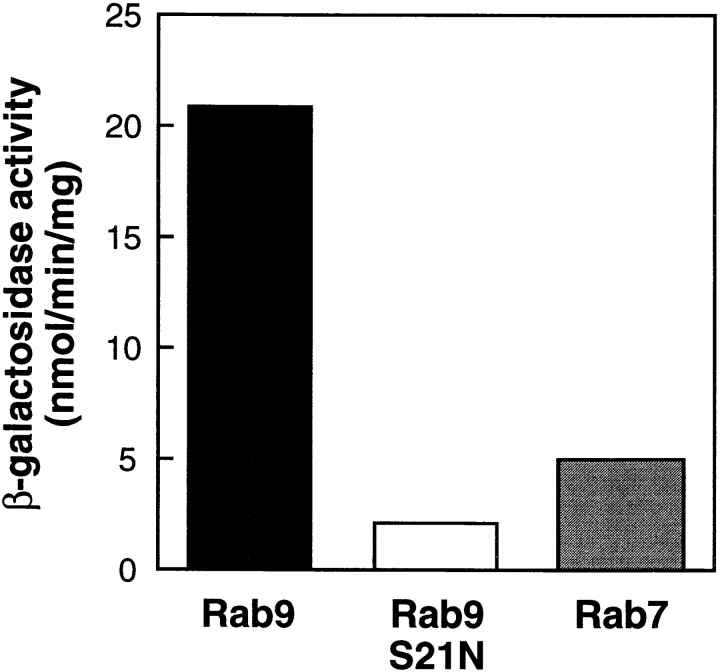
We used the yeast two hybrid system (Fields and Song, 1989) to identify proteins that interact with Rab9 in its active, GTP-bound form. A GAL4 DNA-binding domain hybrid was constructed using wild-type Rab9 lacking the two COOH-terminal cysteine residues (Rab9Δcc) to avoid interference due to protein prenylation. To enrich for proteins that interacted specifically with active Rab9–GTP, we discarded clones that interacted with a mutant of Rab9 (Rab9S21NΔcc) that binds GDP with >50-fold preference to GTP (Riederer et al., 1994) or a related Rab family member (Rab7Δcc). Two hybrid screening of 1.4 × 106 GAL4 activation domain hybrid transformants led to the identification of clone 361, which interacted preferentially with Rab9Δcc but not Rab9S21NΔcc or Rab7Δcc in a quantitative, β-galactosidase liquid culture assay (Fig. 1). Clone 361 showed at least fourfold higher β-galactosidase activity with Rab9Δcc than with Rab7Δcc (Fig. 1), even though these proteins are 54% identical (Chavrier et al., 1990).
Figure 1.
Discovery of a yeast two hybrid cDNA clone encoding a peptide that preferentially binds Rab9– GTP. β-galactosidase activity of yeast strains co-expressing the clone 361-GAL4 activation domain hybrid and GAL4 DNA binding domain hybrids of either Rab9 (black), Rab9S21N (white), or Rab7 (gray).
A Jurkat cDNA library was screened to obtain a full length 361 cDNA. Of 6 independent clones identified, clone 361.6 extended the 5′ region of the two hybrid clone 361 by 589 bp (Fig. 2) and contained a potential ATG start codon (Kozak, 1987). The 5′ region of this clone was virtually identical to a human EST (these sequence data are available from GenBank/EMBL/DDBT under accession number N36641). Furthermore, two in-frame stop codons were found upstream of the potential initiator codon (Fig. 2, underlined). Thus, clone 361.6 represents a full length cDNA.
Figure 2.
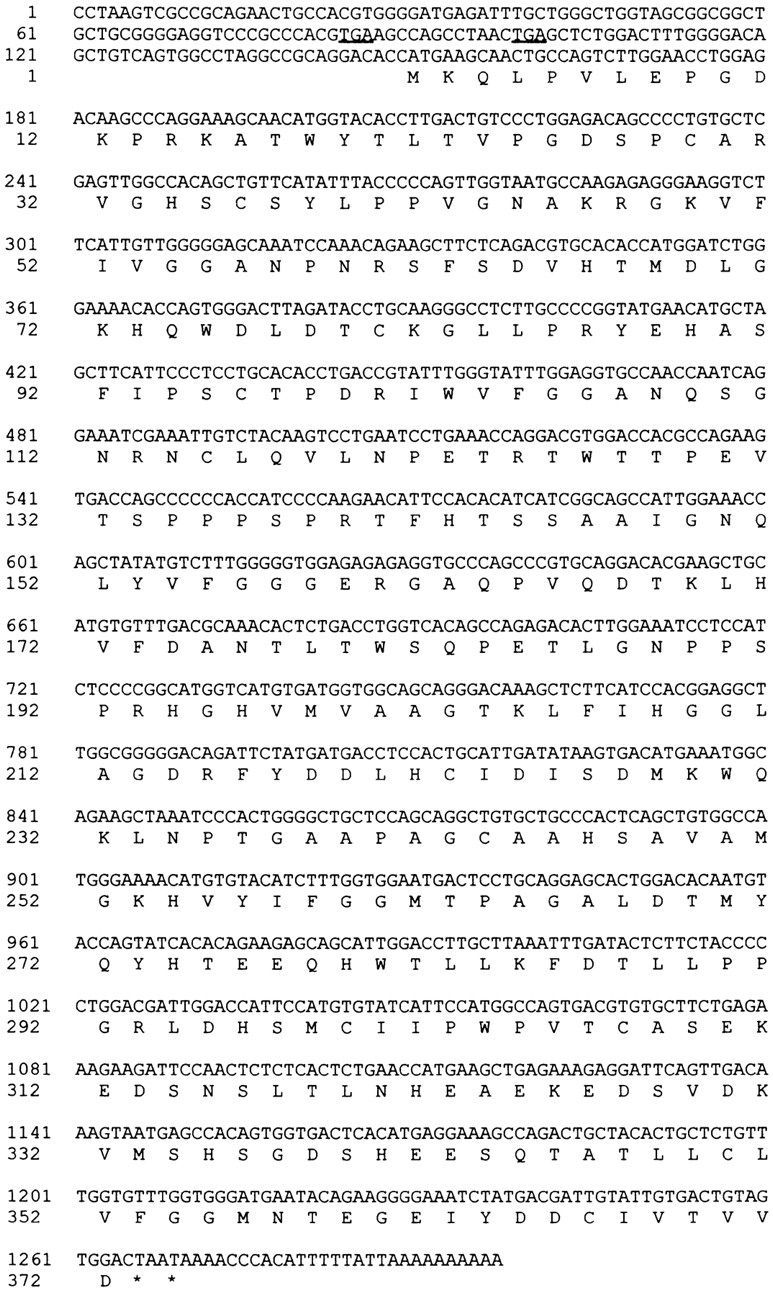
cDNA and predicted amino acid sequence of p40. The 1,298-bp cDNA and predicted 372 amino acid protein are shown. The two in frame stop codons are underlined. The portion of the cDNA identified in the two hybrid screen began at base pair 564.
Clone 361 encodes a hydrophilic protein of 372 amino acids with a predicted molecular weight of 40,566. We have termed this protein p40. p40 shares a 50 amino acid stretch (44% identity) with the Saccharomyces pombe protein, Ral2p (these sequence data are available from GenBank/EMBL/DDBJ index accession number M30827). RAL2 shows genetic interaction with S. pombe RAS1 and is thought to be involved in the activation of Ras1p (Fukui et al., 1989); thus, p40 contains a domain in common with another small GTPase activator.
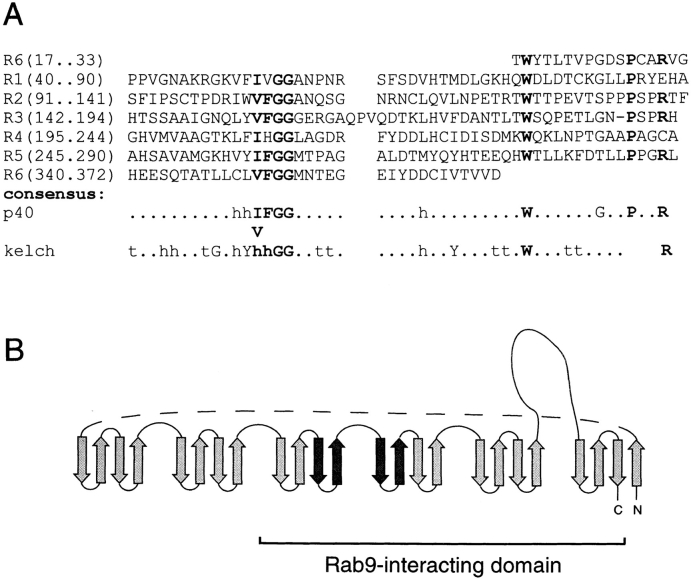
The p40 sequence is comprised almost entirely of six internally repeated sequences of ∼50 amino acids in length (Fig. 3 A). These represent so-called kelch repeats, which were first detected in the Drosophila kelch protein (Xue and Cooley, 1993) and are found in a wide variety of proteins of completely unrelated function (Bork and Doolittle, 1994). Kelch repeats are predicted to form four-stranded, anti-parallel β sheets that assemble into propeller-like barrel structures. The repeat is characterized by a pair of glycine residues at positions 15 and 16, immediately preceded by two hydrophobic amino acids, a tyrosine, and a fourth hydrophobic residue (Bork and Doolittle, 1994). In p40, phenylalanine is found at position minus one relative to the glycine pair in four of six of the repeats, and valine or isoleucine is always present at position minus two. However, only two of the p40 kelch repeats contain the upstream tyrosine residue. A tryptophan residue (found in other kelch motif-containing proteins) and a proline (unique in p40) are found in all of the repeats at position +20 and +30, respectively, downstream of the glycine pair. Due to the kelch repeats, the entire p40 structure can be predicted as drawn in Fig. 3 B. In this representation, the sequences homologous to S. pombe Ral2p are shown in bold; they fit readily into a connected pair of β strands that may comprise a portion of the Rab9-interacting region. Circular dichroism analysis of p40 failed to reveal the presence of any predominant α helices, consistent with the structure shown (data not shown). In kelch proteins of known tertiary structure, enzyme active sites are created by the loops located at the top of the barrel structure (Bork and Doolittle, 1994). Thus, p40 is predicted to fold into a compact β barrel that may interact with Rab9 via the β sheet connecting loops.
Figure 3.
(A) Alignment of kelch repeats in p40. h, Hydrophobic residues; t, turns. Bold residues indicate conservation with the original kelch motif. Also highlighted in bold in p40 are proline residues, whose positions are conserved among p40 repeats but are not present in other kelch-motif proteins. (B) Schematic diagram of the postulated β barrel structure of p40 showing the region of Ral2p similarity (bold) and a large loop domain. The dashed line does not represent additional amino acid sequences. The portion of the protein shown to interact with Rab9 by two hybrid analysis is indicated at the bottom.
Purified p40 Binds Rab9–GTP
p40 was expressed in E. coli as an NH2-terminally His-tagged protein and purified to homogeneity (Fig. 4 A, lane 1). Antibodies raised against the recombinant protein recognized a 44-kD band on immunoblots of human cell extracts (Fig. 4 A, lanes 2–4). Fractionation of HeLa cells revealed that p40 is predominantly cytosolic, but a significant fraction (∼30%) was found associated with membranes (Fig. 4 A, lanes 3 and 4).
Figure 4.
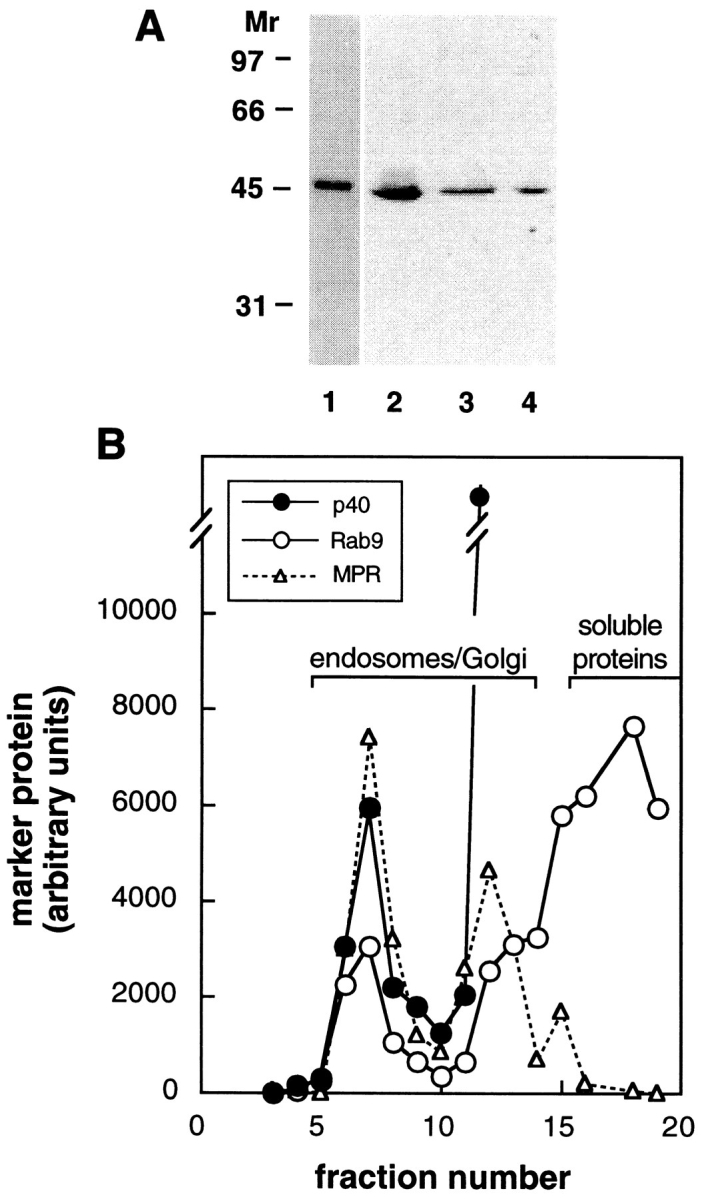
Subcellular distribution of p40. (A) Coomassie blue-stained SDS-PAGE of purified, recombinant, His-tagged p40 and immunoblot of cell extracts. Lane 1, recombinant p40 (1 μg); lane 2, K562 cytosol (100 μg); lanes 3 and 4, HeLa cytosol or membranes (each ∼100 μg, or 10% of a 10-cm dish). (B) A portion of p40 cofractionates with Rab9 and mannose 6-phosphate receptors upon sucrose gradient flotation of K562 cell postnuclear supernatant. The top of the gradient is at the left.
Fig. 4 B shows that a portion of p40 cofractionates with Rab9 and MPRs upon sucrose gradient flotation. Endosomes, detected by their content of MPRs (Fig. 4 B, triangles) and the Rab9 GTPase (open circles), fractionated in two discrete peaks corresponding to the 0.8/1.2 M sucrose interface and the 1.2/1.4 M sucrose interfaces of this gradient. Soluble proteins remained at the bottom, along with other dense membrane-bound compartments. Since 70% of p40 is cytosolic (Fig. 4 A), it was not unexpected that the majority of the protein was found in the lower half of the density gradient (fractions 11–19).
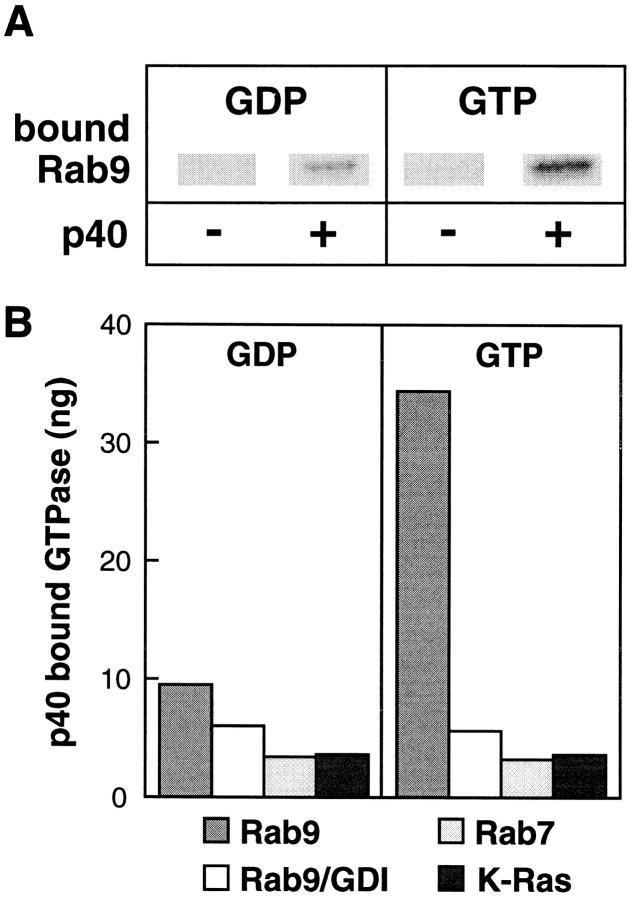
To test whether the full length p40 protein interacted directly with Rab9, the proteins were mixed in the presence of GTP or GDP, and after a period of incubation, p40 and bound proteins were collected via p40's histidine tag using Ni-agarose; bound proteins were then identified by immunoblot. As shown in Fig. 5, purified, recombinant p40 bound Rab9–GTP in preference to Rab9–GDP. No Rab9 was detected bound to the resin if p40 was omitted from the reactions (Fig. 5 A).
Figure 5.
Purified p40 binds Rab9–GTP in strong preference to Rab9–GDP. (A) An example of immunoblot binding data obtained is shown. Values shown in B were determined by PhosphorImager quantitation; Rab9 standards were included on the gels to permit determination of the nanogram amounts of Rab9 bound.
Rab9 in complex with GDI bound much less p40 than Rab9–GDP (Fig. 5 B, white bars). Since GDI retains Rab proteins in their GDP-bound conformations, this result confirms the lack of interaction of p40 with Rab9–GDP; GDI could equally well mask a p40 binding site. Consistent with the original two hybrid screen results, p40 bound very little Rab7 (Fig. 5 B, light gray bars); it also failed to bind K-Ras (Fig. 5 B, dark gray bars). These experiments demonstrate that the Rab9-interaction domain detected by the two-hybrid screen is competent for Rab9–GTP association, when present within the structure of the full length p40 protein. Moreover, the nucleotide-state preference of the interaction was also recapitulated with the full length protein.
Binding of p40 did not influence the intrinsic nucleotide exchange rate of Rab9 (Fig. 6 A). In contrast, at micromolar concentrations, p40 inhibited the intrinsic rate of GTP hydrolysis by Rab9 (Fig. 6 B). While the physiological significance of this observation is not known, this result provides independent confirmation of a direct interaction between p40 and Rab9. Indeed, GTPase inhibition may not reflect the true role of p40, since Rab9 has a very low intrinsic rate of GTP hydrolysis (Shapiro et al., 1993). Together, these data demonstrate that p40 interacts directly with Rab9–GTP.
Figure 6.

p40 inhibits Rab9's GTPase activity but not its rate of nucleotide exchange. (A) Nucleotide exchange was assayed with 10 nM Rab9 and 100 nM p40 according to Soldati et al. (1994). (B) Effect of p40 on the Rab9 GTPase. Reactions contained 50 nM Rab9. Control reactions displayed a rate of 0.0122 min−1, in agreement with previously reported values (Shapiro et al., 1993).
p40 Is a Potent Transport Factor
To explore the function of p40, we tested its influence on an in vitro transport assay that reconstitutes MPR transport from endosomes to the TGN (Goda and Pfeffer, 1988). Transport requires Rab9 (Lombardi et al., 1993; Dirac-Svejstrup et al., 1994), NSF, and α SNAP (Itin, C., C. Racaño, Y. Nakajima, and S.R. Pfeffer, manuscript submitted for publication) and shows all of the biochemical characteristics unique to this transport process (Goda and Pfeffer, 1988).
Our most satisfying result was obtained when we tested the activity of purified, recombinant p40. As shown in Fig. 7 A, p40 greatly stimulated the overall extent of endosome-to-TGN transport. Under conditions of limiting cytosol to provide other essential transport factors, nanomolar concentrations of p40 enhanced transport to 150% of the level seen with saturating concentrations of crude cytosolic proteins (Fig. 7 A). Thus, p40 represents a novel and potent transport factor that stimulates MPR transport.
Figure 7.
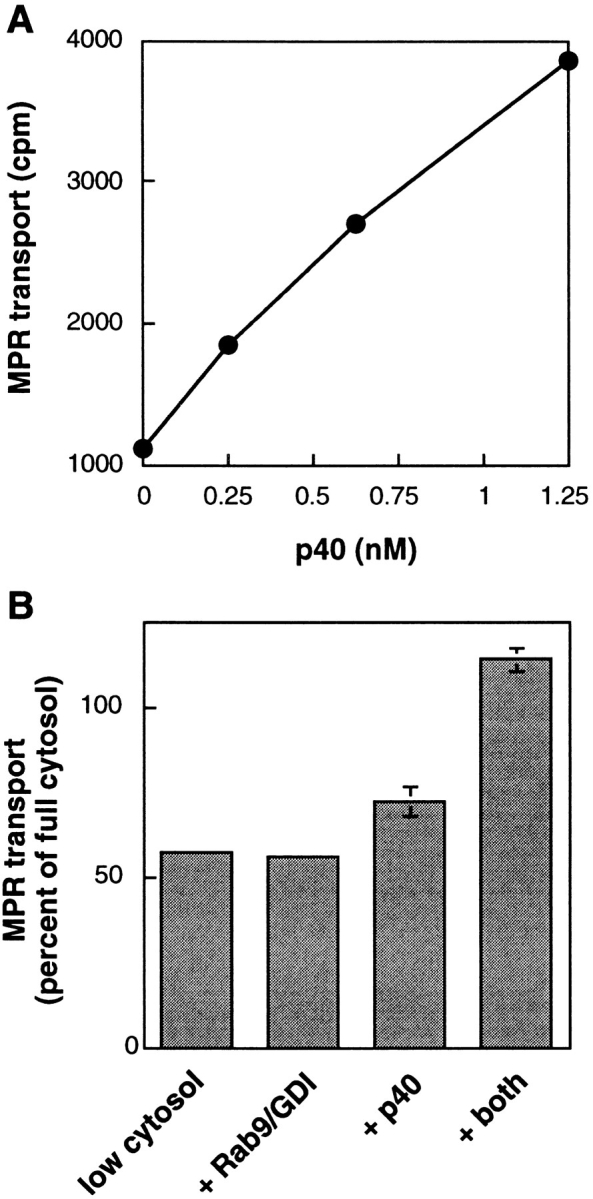
p40 functions in endosome-to-TGN transport. (A) Recombinant p40 was added to reactions containing 0.6 mg/ml cytosol. No additional stimulation was observed at higher p40 concentrations. (B) p40 and Rab9/GDI act synergistically in MPR transport. Recombinant p40 (10 ng/ml), Rab9 in an equimolar complex with GDI (100 ng/ml), or both components were added to transport reactions containing 0.65 mg/ml cytosol. Transport is presented in relation to that seen with 1 mg/ml cytosol (2,345 cpm). Error bars represent standard error of the mean (n = 2).
If p40 functions by virtue of direct interaction with Rab9, the two proteins might be expected to act synergistically in transport. In reactions containing limiting cytosol, low concentrations of either p40 or Rab9 (added as an active complex with GDI) alone showed very little stimulation. However, addition of both of these components stimulated transport to maximum levels (Fig. 7 B). These data strongly suggest that p40 functions in concert with Rab9 to facilitate MPR transport.
The experiments described above showed that p40 can stimulate MPR transport from endosomes to the TGN. Evidence that the molecule normally functions in this process came from antibody inhibition experiments. Anti-p40 IgG inhibited transport by almost 50% compared with preimmune IgG (Fig. 8). Furthermore, the inhibition was neutralized by addition of recombinant p40 (Fig. 8). These data demonstrate that p40 is normally required for efficient transport. The fact that only partial inhibition was observed may reflect the fact that our antibodies were raised against a human antigen while the assay utilizes CHO cell components.
Figure 8.

Anti-p40 antibodies inhibit MPR transport. Immune or preimmune IgG (75 μg/ml) was added to MPR transport reactions containing 1 mg/ml cytosol and where indicated, p40 (100 ng/ml). Error bars represent standard error of the mean (n = 2). Control reactions yielded 724 cpm.
Membrane Association Accompanies p40 Function
A number of independent lines of evidence support the conclusion that membrane-associated p40 represents the active form of the protein. As discussed earlier, ∼30% of p40 is present on membranes. In addition, p40 acts in a synergistic fashion with presumably membrane-associated Rab9–GTP. Moreover, recombinant p40 can also bind directly to endosome-enriched membranes (E. Díaz, unpublished observation). Is this the active form of the protein? While anti-p40 IgG inhibited the overall transport reaction (Fig. 8), cytosol lacking p40 as a consequence of immunodepletion was still fully active in supporting in vitro transport (Fig. 9), consistent with this possibility. Moreover, preincubation of cytosol alone with anti-p40 IgG did not inhibit transport in reactions containing untreated membranes (not shown). Together, these data imply that anti-p40 IgG blocks transport by inactivating an active, membrane-associated p40 pool. At minimum, these data show that membrane-associated p40 is sufficient for MPR transport.
Figure 9.

Depletion of cytosolic p40 does not inhibit MPR transport. (A) Anti-p40 immunoblot analysis of cytosols (100 μg each) preadsorbed with either preimmune IgG (control) or anti-p40 immune IgG (depleted). (B) Transport activity of the cytosols shown in A. Cytosols were assayed at 0.4 mg/ml; 100% transport of control cytosol represents 556 cpm. Values shown represent the average of duplicate determinations (standard error ⩽ 5%) in a representative experiment.
How, then, does soluble, recombinant p40 stimulate transport? It is important to note that p40 stimulated transport under conditions of limiting cytosolic proteins. We have shown elsewhere that α SNAP is among the most limiting cytosolic factors under these conditions (Itin C., C. Racaño, Y. Nakajima, and S.R. Pfeffer, manuscript submitted for publication). Presumably, by driving more p40 onto membranes, the reaction can bypass limitations of other factors. Further experiments will be needed to identify directly the proteins to which p40 binds on membranes.
Discussion
We have reported here the discovery of a novel transport factor, p40, that acts together with the active Rab9 GTPase to drive MPR trafficking from endosomes to the TGN. p40 is present in cytosol and on membranes, a feature common to a variety of important transport factors including NSF (Block et al., 1988), α SNAP (Clary and Rothman, 1990), p115 (Waters et al., 1992), and Rabaptin 5 (Stenmark et al., 1995). An important characteristic of p40 is its specific interaction with the active conformation of the Rab9 GTPase. Since Rabs likely function in the process of vesicle docking, we infer that p40 also functions at this stage of vesicular transport.
How might p40 act? The newly discovered Rab5 effector, Rabaptin 5, has been shown to interact specifically with the active Rab5 GTPase to drive endosome fusion (Stenmark et al., 1995). Rabaptin 5 is part of a much larger multiprotein complex. This feature led us to propose that GTP-bearing Rabs function by recruiting large, coiled-coil, macromolecular docking factors to the surface of transport vesicles to facilitate the docking reaction (Pfeffer, 1996). However, unlike Rabaptin 5, p40 occurs as a monomer in cytosol (as determined by gel filtration of K562 cytosol), and thus it might represent a different class of Rab-interacting transport factor. Alternatively, p40 may be part of a larger complex when present on membranes. Indeed, p40 could act to catalyze the assembly of other docking factors on the surface of transport vesicles carrying MPRs from late endosomes to the TGN. It will be of interest to characterize further the interaction of p40 with membrane-associated components.
In addition to stimulating endosome-to-TGN transport at nanomolar concentrations, p40 blocked the intrinsic GTPase of Rab9, at least when added at micromolar concentrations. We estimate that K562 cytosol contains ⩽0.05 μg/mg cytosol protein, or ∼50 nM p40. Unless there are significant, local concentration effects on the surface of membranes, it does not seem likely that p40 acts to block Rab9 GTPase in vivo, since it is present in insufficient levels to do so. It is quite possible, however, that local membrane concentration effects are significant. In any event, it is essential to note that the ability of a protein to block GTPase activity may simply be an indirect consequence of tight binding to the GTP-bound form. A secondary outcome of this interaction would be to retain the Rab in its active conformation. Whether or not p40 blocks Rab9 GTPase, it is satisfying to note that a minimal estimate for membrane-associated Rab9 is also ∼50 nM, a value quite close to the level of p40.
Other Rab-interacting proteins have been identified that may also slow GTP hydrolysis. For example, Rabphilin binds Rab3A–GTP and blocks its GAP-stimulated GTPase (Kishida et al., 1993). At the nerve terminal where vesicles may wait days before exocytosis, such a factor could be important to retain synaptic vesicles in an exocytosis-competent state. Whether this feature is also critical for a constitutive process such as endosome-to-TGN transport is not yet at all clear.
We and others have shown that Rabs are delivered to distinct membrane-bound compartments in their GDP-bound conformations and only subsequently are these GTPases converted to their active, GTP-bound forms (Soldati et al., 1994; Ullrich et al., 1994). Walch-Solimena et al. (1997) have now reported that Sec2p has the capacity to stimulate nucleotide exchange on Sec4p, converting it to the GTP-bound, active form. Satisfyingly, Sec2p interacts directly with the vesicle-associated, Sec4 GTPase and is necessary for subsequent secretory vesicle localization to the bud tips in yeast (Walch-Solimena et al., 1997). If this paradigm holds true for other transport steps, it would seem that Sec2p-like nucleotide exchange factors would act before p40 or Rabaptin, to convert the respective GTPase to its active form. It has been postulated that proteins related in sequence to Sec2p, of which there are several, including the Rab3A-interacting, Rabin 3 protein (Brondyk et al., 1995), activate Rabs once delivered to cellular membranes (Walch-Solimena et al., 1997). The action of such proteins would then lead to the recruitment of docking factors onto the vesicle surface. Not yet known is whether Sec2p-like proteins remain bound to the GTPase after converting the proteins to their active conformations.
Rab GTPase-catalyzed docking interactions will eventually link to SNARE complex formation, before membrane fusion (Söllner et al., 1993; Lian et al., 1994; Rothman, 1994; Søgaard et al., 1994; Pfeffer, 1996). In this regard it is interesting to consider the need for 30 distinct Rab GTPases. While v- and t-SNAREs are likely to encode specific, cognate, vesicle-targeting information, genetic studies in yeast suggest that pairing of v- and t-SNAREs requires additional and distinct sets of both docking factors and Rab GTPases. Some of the functions of these distinct components may be to activate distinct t-SNAREs, perhaps by releasing SNARE-protecting proteins (Pfeffer, 1996). Thus, some of the specificity of vesicle docking is indirectly provided by Rab GTPases.
Vesicles bearing activated Rabs with their recruited docking factors must then find their targets and undergo SNARE pairing. In yeast secretion, this would involve the multisubunit, “Exocyst” complex that contains Sec3p, Sec5p, Sec6p, Sec8p, and Sec15p (Terbush et al., 1996), which appears to be localized to the bud tip (Terbush et al., 1995). For homotypic endosome fusion, Rabaptin5 may be sufficient to facilitate v- and t-SNARE pairing. With molecules in hand, the molecular gymnastics accomplished by these enzymes are now amenable to study.
In summary, we have discovered a highly active transport factor that stimulates the transport of MPRs from endosomes to the TGN. A future challenge will be to determine the subsequent molecular events that are catalyzed by this novel, Rab-interacting transport factor.
Acknowledgments
We are grateful to R. Lesley, B. Oh, and W. Choi for their assistance, C. Itin for help with the transport assay, D. Laurents for CD analysis, S. Elledge for the yeast strains and plasmids, W. Wang and G. Crabtree for the Jurkat cDNA library, and M. Garrett for purified K-Ras and anti–K-Ras antibodies.
Footnotes
This research was funded by a research grant to S.R. Pfeffer from the National Institutes of Health (DK37332), a predoctoral fellowship from the National Science Foundation to E. Díaz, and by a postdoctoral fellowship from the Human Frontier Science Program Organization and CIBA-Geigy Jubiläumsstiftung to F. Schimmöller.
Please address all correspondence to Suzanne R. Pfeffer, Department of Biochemistry, Stanford University School of Medicine, Stanford, CA 94305-5307. Tel.: (650) 723-6169; Fax: (650) 725-6776; E-Mail: pfeffer@cmgm.stanford.edu
1. Abbreviation used in this paper: MPR, mannose 6-phosphate receptor.
References
- Block MR, Glick BS, Wilcox CA, Wieland FT, Rothman JE. Purification of an N-ethylmaleimide-sensitive protein catalyzing vesicular transport. Proc Natl Acad Sci USA. 1988;85:7852–7856. doi: 10.1073/pnas.85.21.7852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bork P, Doolittle RF. Drosophila kelchmotif is derived from a common enzyme fold. J Mol Biol. 1994;236:1277–1282. doi: 10.1016/0022-2836(94)90056-6. [DOI] [PubMed] [Google Scholar]
- Brondyk WH, McKiernan CJ, Fortner KA, Stabila P, Holz RW, Macara IG. Interaction cloning of Rabin3, a novel protein that associates with the Ras-like GTPase Rab3A. Mol Cell Biol. 1995;15:1137–1143. doi: 10.1128/mcb.15.3.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucci C, Parton RG, Mather IH, Stunnenberg H, Simons K, Hoflack B, Zerial M. The small GTPase Rab5 functions as a regulatory factor in the early endocytic pathway. Cell. 1992;70:715–728. doi: 10.1016/0092-8674(92)90306-w. [DOI] [PubMed] [Google Scholar]
- Chavrier P, Vingron M, Sander C, Simons K, Zerial M. Molecular cloning of YPT1/SEC4-related cDNAs from an epithelial cell line. Mol Cell Biol. 1990;10:6578–6585. doi: 10.1128/mcb.10.12.6578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clary DO, Rothman JE. Purification of three related peripheral membrane proteins needed for vesicular transport. J Biol Chem. 1990;265:10109–10117. [PubMed] [Google Scholar]
- Dirac-Svejstrup, A.B., T. Soldati, A.D. Shapiro, and S.R. Pfeffer. 1994. Rab-GDI presents functional rab9 to the intracellular transport machinery and contributes selectivity to rab9 membrane recruitment. J. Biol. Chem., 269: 15427–15430. [PubMed]
- Dirac-Svejstrup, A.B., D. Sumizawa, and S.R. Pfeffer. 1997. Identification of an endosomal GDI displacement factor that displaces prenylated Rab GTPases from Rab-GDI. EMBO (Eur. Mol. Biol. Organ.) J., 16:465–472. [DOI] [PMC free article] [PubMed]
- Fields S, Song O. A novel genetic system to detect protein-protein interactions. Nature (Lond) 1989;340:245–246. doi: 10.1038/340245a0. [DOI] [PubMed] [Google Scholar]
- Fukui Y, Miyake S, Satoh M, Yamamoto M. Characterization of the Schizosaccharomyces pomberal 2 gene implicated in the activation of the ras1 gene product. Mol Cell Biol. 1989;9:5617–5622. doi: 10.1128/mcb.9.12.5617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goda Y, Pfeffer SR. Selective recycling of the mannose 6 phosphate/ICIF-11 receptor to one trans-Golgi network in vitro. . Cell. 1988;55:309–320. doi: 10.1016/0092-8674(88)90054-2. [DOI] [PubMed] [Google Scholar]
- Kishida S, Shirataki H, Sasaki T, Kato M, Kaibuchi K, Takai Y. Rab3A GTPase-activating protein-inhibiting activity of Rabphilin-3A, a putative Rab3A target protein. J Biol Chem. 1993;268:22529–22261. [PubMed] [Google Scholar]
- Kozak M. At least six nucleotides preceding the AUG initiator codon enhance translation in mammalian cells. J Mol Biol. 1987;196:947–950. doi: 10.1016/0022-2836(87)90418-9. [DOI] [PubMed] [Google Scholar]
- Lian JP, Stone S, Jiang Y, Lyons P, Ferro-Novick S. Ypt1p implicated in v-SNARE activation. Nature (Lond) 1994;372:698–701. doi: 10.1038/372698a0. [DOI] [PubMed] [Google Scholar]
- Lombardi D, Soldati T, Riederer MA, Goda Y, Zerial M, Pfeffer SR. Rab9 functions in transport between late endosomes and the trans-Golgi network in vitro. . EMBO (Eur Mol Biol Organ) J. 1993;12:677–682. doi: 10.1002/j.1460-2075.1993.tb05701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick P, Brennwald P. Friends and family: the role of Rab GTPases in vesicular traffic. Cell. 1993;75:597–601. doi: 10.1016/0092-8674(93)90478-9. [DOI] [PubMed] [Google Scholar]
- Nuoffer C, Balch WE. GTPases: multifunctional molecular switches regulating vesicular traffic. Annu Rev Biochem. 1994;63:949–990. doi: 10.1146/annurev.bi.63.070194.004505. [DOI] [PubMed] [Google Scholar]
- Pfeffer SR. Rab GTPases: master regulators of membrane trafficking. Curr Opin Cell Biol. 1994;6:522–526. doi: 10.1016/0955-0674(94)90071-x. [DOI] [PubMed] [Google Scholar]
- Pfeffer SR. Transport vesicle docking––SNAREs and Associates. Annu Rev Cell Dev Biol. 1996;12:441–461. doi: 10.1146/annurev.cellbio.12.1.441. [DOI] [PubMed] [Google Scholar]
- Riederer MA, Soldati T, Shapiro AD, Lin J, Pfeffer SR. Lysosome biogenesis requires Rab9 function and receptor recycling from endosomes to the trans-Golgi network . J Cell Biol. 1994;125:573–582. doi: 10.1083/jcb.125.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose M, Botstein D. Construction and use of gene fusions lacZ (β-galactosidase) which are expressed in yeast. Methods Enzymol. 1983;101:167–180. doi: 10.1016/0076-6879(83)01012-5. [DOI] [PubMed] [Google Scholar]
- Rothman JE. Mechanisms of intracellular protein transport. Nature (Lond) 1994;372:55–63. doi: 10.1038/372055a0. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Kikuchi A, Araki S, Hata Y, Isomura M, Kuroda S, Takai Y. Purification and characterization from bovine brain cytosol of a protein that inhibits the dissociation of GDP from and the subsequent binding of GTP to smg p25A, a ras p21-like GTP-binding protein. J Biol Chem. 1990;265:2333–2337. [PubMed] [Google Scholar]
- Schiestl RH, Gietz RD. High efficiency transformation of intact yeast cells using single stranded nucleic acids as a carrier. Curr Genet. 1989;16:339–346. doi: 10.1007/BF00340712. [DOI] [PubMed] [Google Scholar]
- Shapiro AD, Riederer MS, Pfeffer SR. Biochemical analysis of rab9, a ras-like ATPase involved in protein transport from late endosomes to the trans-Golgi network. J Biol Chem. 1993;268:6925–6931. [PubMed] [Google Scholar]
- Singer-Krüger B, Stenmark H, Düsterhöft A, Philippsen P, Yoo J-S, Gallwitz D, Zerial M. Role of three Rab5-like GTPases, Ypt51p, Ypt52p, and Ypt53p, in the endocytic and vacuolar protein sorting pathways of yeast. J Cell Biol. 1994;125:283–298. doi: 10.1083/jcb.125.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Søgaard M, Tani K, Ye RR, Geromanos S, Tempst P, Kirchhausen T, Rothman JE, Söllner T. A rab protein is required for the assembly of SNARE complexes in the docking of transport vesicles. Cell. 1994;78:937–948. doi: 10.1016/0092-8674(94)90270-4. [DOI] [PubMed] [Google Scholar]
- Soldati T, Shapiro AD, Svejstrup AB, Pfeffer SR. Membrane targeting of the small GTPase Rab9 is accompanied by nucleotide exchange. Nature (Lond) 1994;369:76–78. doi: 10.1038/369076a0. [DOI] [PubMed] [Google Scholar]
- Soldati T, Rancaño C, Geissler H, Pfeffer SR. Rab7 and Rab9 are recruited onto late endosomes by biochemically distinguishable processes. J Biol Chem. 1995;270:25541–25548. doi: 10.1074/jbc.270.43.25541. [DOI] [PubMed] [Google Scholar]
- Söllner T, Whiteheart SW, Brunner M, Erdjument–Bromage H, Geromanos S, Tempst P, Rothman JE. SNAP receptors implicated in vesicle targeting and fusion. Nature (Lond) 1993;362:318–324. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- Stenmark H, Vitale G, Ullrich O, Zerial M. Rabaptin5 is a direct effector of the small GTPase Rab5 in endocytic membrane fusion. Cell. 1995;83:423–432. doi: 10.1016/0092-8674(95)90120-5. [DOI] [PubMed] [Google Scholar]
- TerBush DR, Novick P. Sec6, Sec8, amd Sec15 are components of a multisubunit complex which localizes to small bud tips in Saccharomyces cerevisiae. . J Cell Biol. 1995;130:299–312. doi: 10.1083/jcb.130.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TerBush DR, Maurice T, Roth D, Novick P. The Exocyst is a multiprotein complex required for exocytosis in Saccharomyces cerevisiae. . EMBO (Eur Mol Biol Organ) J. 1996;15:6483–6494. [PMC free article] [PubMed] [Google Scholar]
- Ullrich O, Horiuchi H, Bucci C, Zerial M. Membrane association of Rab5 mediated by GDP-dissociation inhibitor and accompanied by GDP/ GTP exchange. Nature (Lond) 1994;368:157–160. doi: 10.1038/368157a0. [DOI] [PubMed] [Google Scholar]
- Vojtek AB, Hollenberg SM, Cooper JA. Mammalian Ras interacts directly with the serine/threonine kinase Raf. Cell. 1993;74:205–214. doi: 10.1016/0092-8674(93)90307-c. [DOI] [PubMed] [Google Scholar]
- Walch-Solimena C, Collins RN, Novick PJ. Sec2p mediates nucleotide exchange on Sec4p and is involved in polarized delivery of post-Golgi vesicles. J Cell Biol. 1997;137:1495–1510. doi: 10.1083/jcb.137.7.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters MG, Clary DO, Rothman JE. A novel 115-kD peripheral membrane protein is required for intercisternal transport in the Golgi stack. J Cell Biol. 1992;118:1015–1026. doi: 10.1083/jcb.118.5.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue F, Cooley L. Kelch encodes a component of intercellular bridges in Drosophilaegg chambers. Cell. 1993;72:681–693. doi: 10.1016/0092-8674(93)90397-9. [DOI] [PubMed] [Google Scholar]
- Zerial M, Stenmark H. Rab GTPases in vesicular transport. Curr Opin Cell Biol. 1993;5:613–620. doi: 10.1016/0955-0674(93)90130-i. [DOI] [PubMed] [Google Scholar]