Abstract
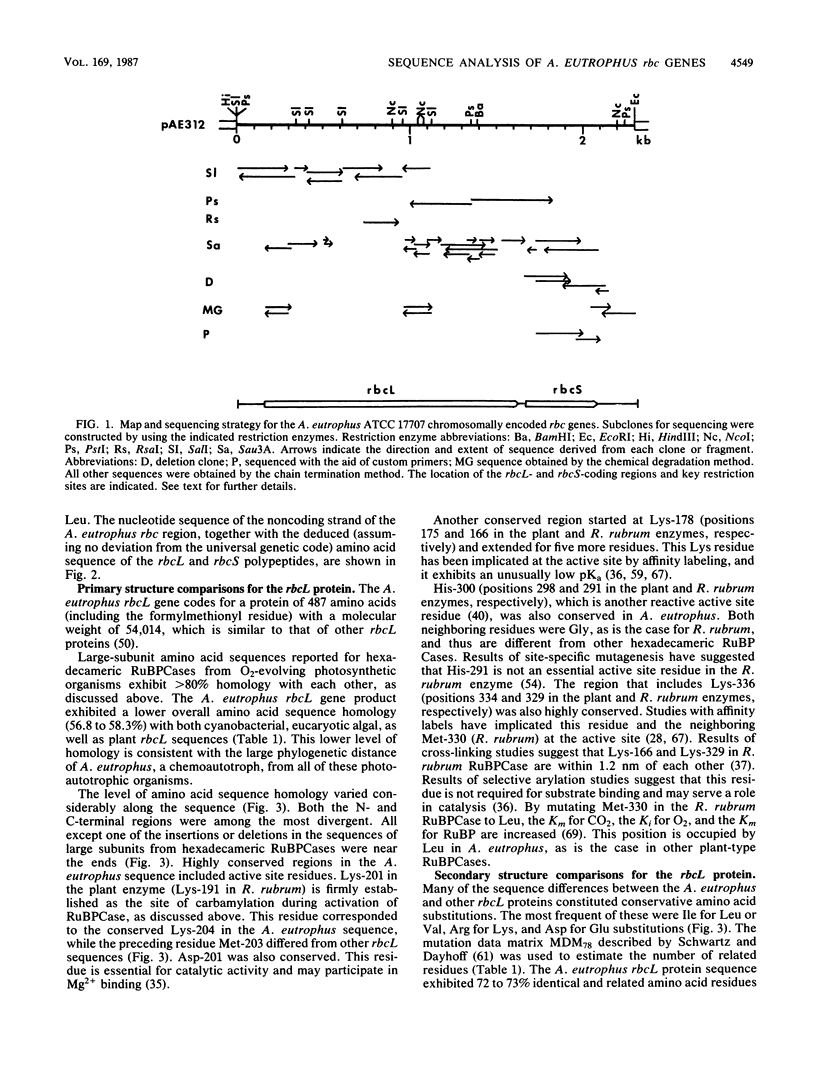
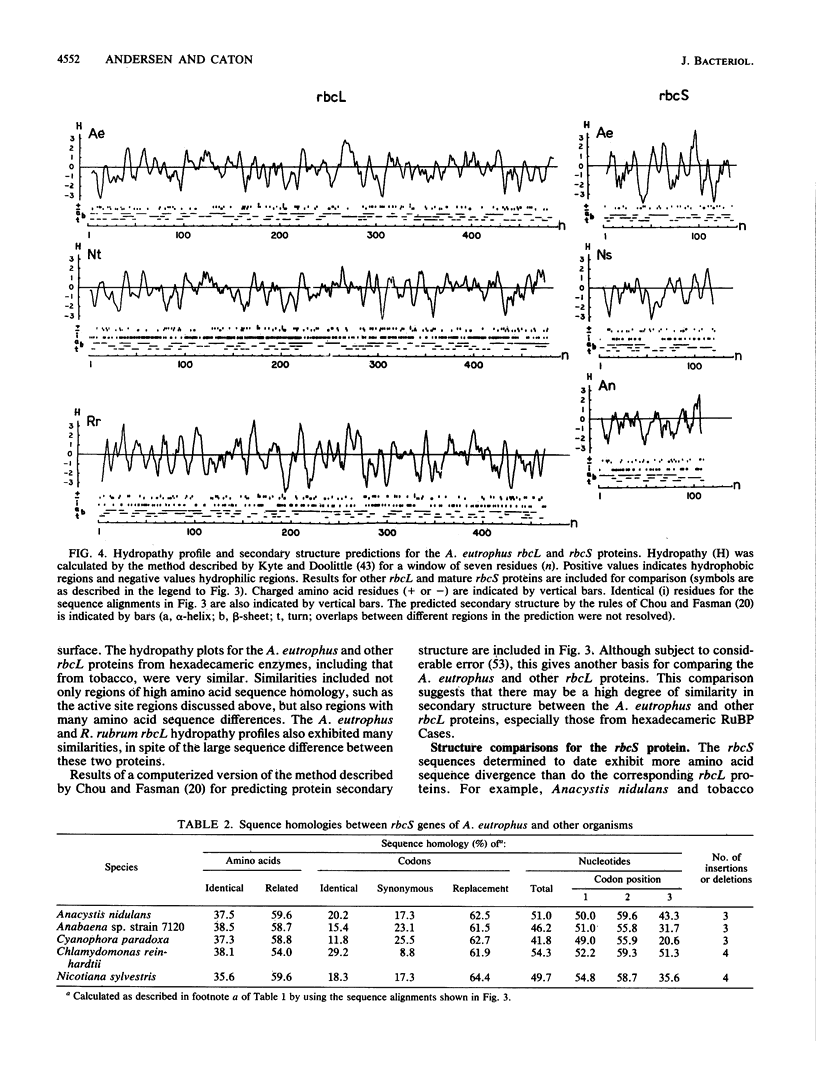
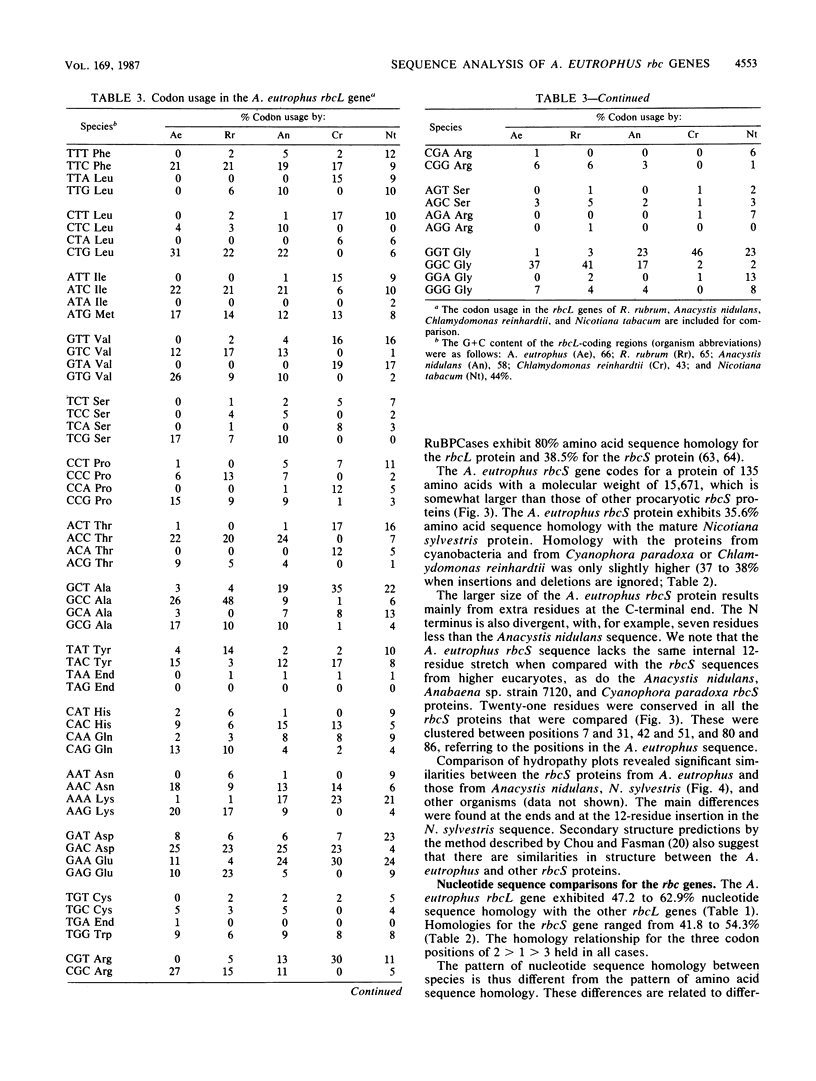
The nucleotide sequence of the chromosomally encoded ribulose bisphosphate carboxylase/oxygenase (RuBPCase) large (rbcL) and small (rbcS) subunit genes of the hydrogen bacterium Alcaligenes eutrophus ATCC 17707 was determined. We found that the two coding regions are separated by a 47-base-pair intergenic region, and both genes are preceded by plausible ribosome-binding sites. Cotranscription of the rbcL and rbcS genes has been demonstrated previously. The rbcL and rbcS genes encode polypeptides of 487 and 135 amino acids, respectively. Both genes exhibited similar codon usage which was highly biased and different from that of other organisms. The N-terminal amino acid sequence of both subunit proteins was determined by Edman degradation. No processing of the rbcS protein was detected, while the rbcL protein underwent a posttranslational loss of formylmethionyl. The A. eutrophus rbcL and rbcS proteins exhibited 56.8 to 58.3% and 35.6 to 38.5% amino acid sequence homology, respectively, with the corresponding proteins from cyanobacteria, eucaryotic algae, and plants. The A. eutrophus and Rhodospirillum rubrum rbcL proteins were only about 32% homologous. The N- and C-terminal sequences of both the rbcL and the rbcS proteins were among the most divergent regions. Known or proposed active site residues in other rbcL proteins, including Lys, His, Arg, and Asp residues, were conserved in the A. eutrophus enzyme. The A. eutrophus rbcS protein, like those of cyanobacteria, lacks a 12-residue internal sequence that is found in plant RuBPCase. Comparison of hydropathy profiles and secondary structure predictions by the method described by Chou and Fasman (P. Y. Chou and G. D. Fasman, Adv. Enzymol. 47:45-148, 1978) revealed striking similarities between A. eutrophus RuBPCase and other hexadecameric enzymes. This suggests that folding of the polypeptide chains is similar. The observed sequence homologies were consistent with the notion that both the rbcL and rbcS genes of the chemoautotroph A. eutrophus and the thus far characterized rbc genes of photosynthetic organisms have a common origin. This suggests that both subunit genes have a very ancient origin. The role of quaternary structure as a determinant of the rate of accepted amino acid substitution was examined. It is proposed that the sequence of the dimeric R. rubrum RuBPCase may be less conserved because there are fewer structural constraints for this RuBPCase than there are for hexadecameric enzymes.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen K. Mutations altering the catalytic activity of a plant-type ribulose biphosphate carboxylase/oxygenase in Alcaligenes eutrophus. Biochim Biophys Acta. 1979 Jun 1;585(1):1–11. doi: 10.1016/0304-4165(79)90319-2. [DOI] [PubMed] [Google Scholar]
- Andersen K., Wilke-Douglas M., Caton J. Ribulose-bisphosphate carboxylase manipulation in the hydrogen bacterium Alcaligenes eutrophus. Biochem Soc Trans. 1986 Feb;14(1):29–31. doi: 10.1042/bst0140029. [DOI] [PubMed] [Google Scholar]
- Andersen K., Wilke-Douglas M. Construction and use of a gene bank of Alcaligenes eutrophus in the analysis of ribulose bisphosphate carboxylase genes. J Bacteriol. 1984 Sep;159(3):973–978. doi: 10.1128/jb.159.3.973-978.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen K., Wilke-Douglas M. Genetic and physical mapping and expression in Pseudomonas aeruginosa of the chromosomally encoded ribulose bisphosphate carboxylase genes of Alcaligenes eutrophus. J Bacteriol. 1987 May;169(5):1997–2004. doi: 10.1128/jb.169.5.1997-2004.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews T. J., Ballment B. Active-site carbamate formation and reaction-intermediate-analog binding by ribulosebisphosphate carboxylase/oxygenase in the absence of its small subunits. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3660–3664. doi: 10.1073/pnas.81.12.3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews T. J., Lorimer G. H. Catalytic properties of a hybrid between cyanobacterial large subunits and higher plant small subunits of ribulose bisphosphate carboxylase-oxygenase. J Biol Chem. 1985 Apr 25;260(8):4632–4636. [PubMed] [Google Scholar]
- Bibb M. J., Findlay P. R., Johnson M. W. The relationship between base composition and codon usage in bacterial genes and its use for the simple and reliable identification of protein-coding sequences. Gene. 1984 Oct;30(1-3):157–166. doi: 10.1016/0378-1119(84)90116-1. [DOI] [PubMed] [Google Scholar]
- Bowien B., Gottschalk E. M. Influence of the activation state on the sedimentation properties of ribulose bisphosphate carboxylase from Alcaligenes eutrophus. J Biol Chem. 1982 Oct 25;257(20):11845–11847. [PubMed] [Google Scholar]
- Bowien B., Mayer F., Codd G. A., Schlegel H. G. Purification, some properties and quaternary structure of the D-ribulose 1,5-diphosphate carboxylase of Alcaligenes eutrophus. Arch Microbiol. 1976 Nov 2;110(23):157–166. doi: 10.1007/BF00690223. [DOI] [PubMed] [Google Scholar]
- Bowien B., Mayer F., Spiess E., Pähler A., Englisch U., Saenger W. On the structure of crystalline ribulosebisphosphate carboxylase from Alcaligenes eutrophus. Eur J Biochem. 1980 May;106(2):405–410. doi: 10.1111/j.1432-1033.1980.tb04586.x. [DOI] [PubMed] [Google Scholar]
- Chapman M. S., Smith W. W., Suh S. W., Cascio D., Howard A., Hamlin R., Xuong N. H., Eisenberg D. Structural studies of Rubisco from tobacco. Philos Trans R Soc Lond B Biol Sci. 1986 Oct 14;313(1162):367–378. doi: 10.1098/rstb.1986.0044. [DOI] [PubMed] [Google Scholar]
- Chothia C., Lesk A. M. The relation between the divergence of sequence and structure in proteins. EMBO J. 1986 Apr;5(4):823–826. doi: 10.1002/j.1460-2075.1986.tb04288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- Curtis S. E., Haselkorn R. Isolation and sequence of the gene for the large subunit of ribulose-1,5-bisphosphate carboxylase from the cyanobacterium Anabaena 7120. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1835–1839. doi: 10.1073/pnas.80.7.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale R. M., McClure B. A., Houchins J. P. A rapid single-stranded cloning strategy for producing a sequential series of overlapping clones for use in DNA sequencing: application to sequencing the corn mitochondrial 18 S rDNA. Plasmid. 1985 Jan;13(1):31–40. doi: 10.1016/0147-619x(85)90053-8. [DOI] [PubMed] [Google Scholar]
- Donnelly M. I., Hartman F. C., Ramakrishnan V. The shape of ribulose bisphosphate carboxylase/oxygenase in solution as inferred from small angle neutron scattering. J Biol Chem. 1984 Jan 10;259(1):406–411. [PubMed] [Google Scholar]
- Dron M., Rahire M., Rochaix J. D. Sequence of the chloroplast DNA region of Chlamydomonas reinhardii containing the gene of the large subunit of ribulose bisphosphate carboxylase and parts of its flanking genes. J Mol Biol. 1982 Dec 25;162(4):775–793. doi: 10.1016/0022-2836(82)90547-2. [DOI] [PubMed] [Google Scholar]
- Estelle M., Hanks J., McIntosh L., Somerville C. Site-specific mutagenesis of ribulose-1,5-bisphosphate carboxylase/oxygenase. Evidence that carbamate formation at Lys 191 is required for catalytic activity. J Biol Chem. 1985 Aug 15;260(17):9523–9526. [PubMed] [Google Scholar]
- Fitch W. M. The estimate of total nucleotide substitutions from pairwise differences is biased. Philos Trans R Soc Lond B Biol Sci. 1986 Jan 29;312(1154):317–324. doi: 10.1098/rstb.1986.0010. [DOI] [PubMed] [Google Scholar]
- Fraij B., Hartman F. C. Isolation and sequencing of an active-site peptide from Rhodospirillum rubrum ribulosebisphosphate carboxylase/oxygenase after affinity labeling with 2-[(bromoacetyl)amino]pentitol 1,5-bisphosphate. Biochemistry. 1983 Mar 15;22(6):1515–1520. doi: 10.1021/bi00275a028. [DOI] [PubMed] [Google Scholar]
- Gingrich J. C., Hallick R. B. The Euglena gracilis chloroplast ribulose-1,5-bisphosphate carboxylase gene. II. The spliced mRNA and its product. J Biol Chem. 1985 Dec 25;260(30):16162–16168. [PubMed] [Google Scholar]
- Go M., Miyazawa S. Relationship between mutability, polarity and exteriority of amino acid residues in protein evolution. Int J Pept Protein Res. 1980 Mar;15(3):211–224. doi: 10.1111/j.1399-3011.1980.tb02570.x. [DOI] [PubMed] [Google Scholar]
- Gold L., Pribnow D., Schneider T., Shinedling S., Singer B. S., Stormo G. Translational initiation in prokaryotes. Annu Rev Microbiol. 1981;35:365–403. doi: 10.1146/annurev.mi.35.100181.002053. [DOI] [PubMed] [Google Scholar]
- Goldschmidt-Clermont M., Rahire M. Sequence, evolution and differential expression of the two genes encoding variant small subunits of ribulose bisphosphate carboxylase/oxygenase in Chlamydomonas reinhardtii. J Mol Biol. 1986 Oct 5;191(3):421–432. doi: 10.1016/0022-2836(86)90137-3. [DOI] [PubMed] [Google Scholar]
- Gutteridge S., Sigal I., Thomas B., Arentzen R., Cordova A., Lorimer G. A site-specific mutation within the active site of ribulose-1,5-bisphosphate carboxylase of Rhodospirillum rubrum. EMBO J. 1984 Dec 1;3(12):2737–2743. doi: 10.1002/j.1460-2075.1984.tb02204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman F. C., Milanez S., Lee E. H. Ionization constants of two active-site lysyl epsilon-amino groups of ribulosebisphosphate carboxylase/oxygenase. J Biol Chem. 1985 Nov 15;260(26):13968–13975. [PubMed] [Google Scholar]
- Herndon C. S., Hartman F. C. 2-(4-Bromoacetamido)anilino-2-deoxypentitol 1,5-bisphosphate, a new affinity label for ribulose bisphosphate carboxylase/oxygenase from Rhodospirillum rubrum. Determination of reaction parameters and characterization of an active site peptide. J Biol Chem. 1984 Mar 10;259(5):3102–3110. [PubMed] [Google Scholar]
- Igarashi Y., McFadden B. A., el-Gul T. Active site histidine in spinach ribulosebisphosphate carboxylase/oxygenase modified by diethyl pyrocarbonate. Biochemistry. 1985 Jul 16;24(15):3957–3962. doi: 10.1021/bi00336a024. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Mazur B. J., Chui C. F. Sequence of a genomic DNA clone for the small subunit of ribulose bis-phosphate carboxylase-oxygenase from tobacco. Nucleic Acids Res. 1985 Apr 11;13(7):2373–2386. doi: 10.1093/nar/13.7.2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden B. A., Torres-Ruiz J., Daniell H., Sarojini G. Interaction, functional relations and evolution of large and small subunits in Rubisco from prokaryota and eukaryota. Philos Trans R Soc Lond B Biol Sci. 1986 Oct 14;313(1162):347–358. doi: 10.1098/rstb.1986.0042. [DOI] [PubMed] [Google Scholar]
- Meisenberger O., Pilz I., Bowien B., Pal G. P., Saenger W. Small angle x-ray study on the structure of active and inactive ribulose bisphosphate carboxylase from Alcaligenes eutrophus. Evidence for a configurational change. J Biol Chem. 1984 Apr 10;259(7):4463–4465. [PubMed] [Google Scholar]
- Meyer T. E., Cusanovich M. A., Kamen M. D. Evidence against use of bacterial amino acid sequence data for construction of all-inclusive phylogenetic trees. Proc Natl Acad Sci U S A. 1986 Jan;83(2):217–220. doi: 10.1073/pnas.83.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miziorko H. M., Lorimer G. H. Ribulose-1,5-bisphosphate carboxylase-oxygenase. Annu Rev Biochem. 1983;52:507–535. doi: 10.1146/annurev.bi.52.070183.002451. [DOI] [PubMed] [Google Scholar]
- Nierzwicki-Bauer S. A., Curtis S. E., Haselkorn R. Cotranscription of genes encoding the small and large subunits of ribulose-1,5-bisphosphate carboxylase in the cyanobacterium Anabaena 7120. Proc Natl Acad Sci U S A. 1984 Oct;81(19):5961–5965. doi: 10.1073/pnas.81.19.5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa K. Assessment of secondary-structure prediction of proteins. Comparison of computerized Chou-Fasman method with others. Biochim Biophys Acta. 1983 Oct 28;748(2):285–299. doi: 10.1016/0167-4838(83)90306-0. [DOI] [PubMed] [Google Scholar]
- Niyogi S. K., Foote R. S., Mural R. J., Larimer F. W., Mitra S., Soper T. S., Machanoff R., Hartman F. C. Nonessentiality of histidine 291 of Rhodospirillum rubrum ribulose-bisphosphate carboxylase/oxygenase as determined by site-directed mutagenesis. J Biol Chem. 1986 Aug 5;261(22):10087–10092. [PubMed] [Google Scholar]
- Pinck M., Guilley E., Durr A., Hoff M., Pinck L., Fleck J. Complete sequence of one of the mRNAs coding for the small subunit of ribulose bisphosphate carboxylase of Nicotiana sylvestris. Biochimie. 1984 Jul-Aug;66(7-8):539–545. doi: 10.1016/0300-9084(84)90148-2. [DOI] [PubMed] [Google Scholar]
- Reichelt B. Y., Delaney S. F. The nucleotide sequence for the large subunit of ribulose 1,5-bisphosphate carboxylase from a unicellular cyanobacterium, Synechococcus PCC6301. DNA. 1983;2(2):121–129. doi: 10.1089/dna.1983.2.121. [DOI] [PubMed] [Google Scholar]
- Robison P. D., Tabita F. R. Modification of ribulose bisphosphate carboxylase from Rhodospirillum rubrum with tetranitromethane. Biochem Biophys Res Commun. 1979 May 14;88(1):85–91. doi: 10.1016/0006-291x(79)91699-1. [DOI] [PubMed] [Google Scholar]
- Schloss J. V., Stringer C. D., Hartman F. C. Identification of essential lysyl and cysteinyl residues in spinach ribulosebisphosphate carboxylase/oxygenase modified by the affinity label N-bromoacetylethanolamine phosphate. J Biol Chem. 1978 Aug 25;253(16):5707–5711. [PubMed] [Google Scholar]
- Schneider G., Lindqvist Y., Brändén C. I., Lorimer G. Three-dimensional structure of ribulose-1,5-bisphosphate carboxylase/oxygenase from Rhodospirillum rubrum at 2.9 A resolution. EMBO J. 1986 Dec 20;5(13):3409–3415. doi: 10.1002/j.1460-2075.1986.tb04662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinozaki K., Sugiura M. The gene for the small subunit of ribulose-1,5-bisphosphate carboxylase/oxygenase is located close to the gene for the large subunit in the cyanobacterium Anacystis nidulans 6301. Nucleic Acids Res. 1983 Oct 25;11(20):6957–6964. doi: 10.1093/nar/11.20.6957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinozaki K., Sugiura M. The nucleotide sequence of the tobacco chloroplast gene for the large subunit of ribulose-1,5-bisphosphate carboxylase/oxygenase. Gene. 1982 Nov;20(1):91–102. doi: 10.1016/0378-1119(82)90090-7. [DOI] [PubMed] [Google Scholar]
- Shinozaki K., Yamada C., Takahata N., Sugiura M. Molecular cloning and sequence analysis of the cyanobacterial gene for the large subunit of ribulose-1,5-bisphosphate carboxylase/oxygenase. Proc Natl Acad Sci U S A. 1983 Jul;80(13):4050–4054. doi: 10.1073/pnas.80.13.4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalker D. M., Hiatt W. R., Comai L. A single amino acid substitution in the enzyme 5-enolpyruvylshikimate-3-phosphate synthase confers resistance to the herbicide glyphosate. J Biol Chem. 1985 Apr 25;260(8):4724–4728. [PubMed] [Google Scholar]
- Stringer C. D., Hartman F. C. Sequences of two active-site peptides from spinach ribulosebisphosphate carboxylase/oxygenase. Biochem Biophys Res Commun. 1978 Feb 28;80(4):1043–1048. doi: 10.1016/0006-291x(78)91351-7. [DOI] [PubMed] [Google Scholar]
- Tabita F. R., McFadden B. A. D-ribulose 1,5-diphosphate carboxylase from Rhodospirillum rubrum. II. Quaternary structure, composition, catalytic, and immunological properties. J Biol Chem. 1974 Jun 10;249(11):3459–3464. [PubMed] [Google Scholar]
- Terzaghi B. E., Laing W. A., Christeller J. T., Petersen G. B., Hill D. F. Ribulose 1,5-bisphosphate carboxylase. Effect on the catalytic properties of changing methionine-330 to leucine in the Rhodospirillum rubrum enzyme. Biochem J. 1986 May 1;235(3):839–846. doi: 10.1042/bj2350839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese C. R., Stackebrandt E., Macke T. J., Fox G. E. A phylogenetic definition of the major eubacterial taxa. Syst Appl Microbiol. 1985;6:143–151. doi: 10.1016/s0723-2020(85)80047-3. [DOI] [PubMed] [Google Scholar]
- Zurawski G., Perrot B., Bottomley W., Whitfeld P. R. The structure of the gene for the large subunit of ribulose 1,5-bisphosphate carboxylase from spinach chloroplast DNA. Nucleic Acids Res. 1981 Jul 24;9(14):3251–3270. doi: 10.1093/nar/9.14.3251. [DOI] [PMC free article] [PubMed] [Google Scholar]



