Abstract
Increased energy metabolism in the circulating blood platelet plays an essential role in platelet plug formation and clot retraction. This increased energy consumption is mainly due to enhanced anaerobic consumption of glucose via the glycolytic pathway. The aim of the present study was to determine the role of glucose transport as a potential rate-limiting step for human platelet glucose metabolism. We measured in isolated platelet preparations the effect of thrombin and ADP activation, on glucose transport (2-deoxyglucose uptake), and the cellular distribution of the platelet glucose transporter (GLUT), GLUT-3. Thrombin (0.5 U/ml) caused a pronounced shape change and secretion of most α-granules within 10 min. During that time glucose transport increased approximately threefold, concomitant with a similar increase in expression of GLUT-3 on the plasma membrane as observed by immunocytochemistry. A major shift in GLUT-3 labeling was observed from the α-granule membranes in resting platelets to the plasma membrane after thrombin treatment. ADP induced shape change but no significant α-granule secretion. Accordingly, ADP-treated platelets showed no increased glucose transport and no increased GLUT-3 labeling on the plasma membrane. These studies suggest that, in human blood platelets, increased energy metabolism may be precisely coupled to the platelet activation response by means of the translocation of GLUT-3 by regulated secretion of α-granules. Observations in megakaryocytes and platelets freshly fixed from blood confirmed the predominant GLUT-3 localization in α-granules in the isolated cells, except that even less GLUT-3 is present at the plasma membrane in the circulating cells (∼15%), indicating that glucose uptake may be upregulated five to six times during in vivo activation of platelets.
Platelets play a crucial role in hemostasis and thrombosis. They originate from megakaryocytes in the bone marrow and adhere to sites of injury. In their resting state, platelets are nonadhesive, have a discoid shape, and contain a large number of α-granules. The α-granules, which are the most abundant granule type in these cells, contain adhesive proteins such as fibrinogen, fibronectin, von Willebrand factor (Sixma et al., 1989; Sander et al., 1983), and growth factors such as TGF-β and PDGF (Linder et al., 1979). At sites of injury the platelets become activated, a process that promotes the formation of a platelet thrombus and wound healing. Platelet activation can be subdivided into several discrete steps. The first step is the adhesion to exposed components of the subendothelium, which is rapidly followed by a change in cell shape (Sixma et al., 1987a ,b), due to rearrangement of actin filaments and microtubules, both of which maintain the discoid nature of the resting platelet (Behnke, 1974; Hartwig, 1992). The next step involves the aggregation of platelets, which occurs simultaneously with the exocytosis of the α-granules. Finally, the actin filaments associate with myosin in a process that resembles skeletal muscle contraction (Cohen et al., 1982; Fox et al., 1992). This step facilitates platelet retraction, which is responsible for clot contraction.
Each of these steps in the functional activation sequence of the platelet must result in a significant increase in the energy requirements of this cell. However, there is little information available regarding the type of fuel used by the platelet under these conditions, which of these steps is the most energy costly, or the points of regulation within the intermediary metabolic pathways that facilitate these changes. The resting platelet relies predominantly on the anaerobic metabolism of blood-borne glucose as its major source of energy (Akkerman, 1978, 1987) despite the presence of mitochondria and glycogen particles within these cells. It is likely that platelet plug formation and clot retraction are also highly dependent on extracellular glucose as it has been shown that there is a direct relationship between platelet retraction efficiency and glycolytic flux (Bettex-Galland and Luscher, 1959). In fact, these and further studies showed that glucose improves platelet retraction efficiency in in vitro assays, suggesting that the consumption of glucose by platelets and the efficiency of clot retraction must be somehow linked.
Skeletal and heart myocytes are other cell types in which rapid and marked changes in carbohydrate metabolism meet the increased energy demands of actomyosin contraction, similarly to the platelet. In these cells, the very first step in the glycolytic breakdown of extracellular glucose, i.e., glucose entry into the cell, appears to be a major rate-determining step (Ziel et al., 1988). Muscle contraction and other stimuli such as insulin stimulate glucose transport by triggering the movement of a facilitative glucose transporter (GLUT)1 known as GLUT-4 from an intracellular compartment to the cell surface (James et al., 1994; Cushman and Wardzala, 1980; Suzuki and Kono, 1980). Upon cessation of muscle contraction or removal of the agonist, GLUT-4 is retrieved from the cell surface, and glucose transport and glucose use return to resting levels.
In view of the possible similarity between platelets and myocytes in terms of the contraction-induced changes in carbohydrate metabolism, we have investigated the potential role of glucose transport in platelet activation. We present evidence that activation of platelets with thrombin results in a significant increase in 2-deoxyglucose (2-DG) uptake. Stimulation with ADP, under conditions where platelet secretion was inhibited, did not cause a significant change in platelet 2-DG uptake. Recently it was reported that GLUT-3 is the main glucose transporter isoform expressed in platelets (Craik et al., 1995). Accordingly, we show immunocytochemically that in resting platelets GLUT-3 is localized to the α-granule membrane from where it moves to the cell surface in response to thrombin. This regulated translocation of GLUT-3 is responsible for the increased uptake of 2-DG.
Materials and Methods
Antibodies
The anti–human GLUT-3 antibody was raised against a synthetic peptide corresponding to the last 12 amino acids in the carboxy terminus of the protein. This antibody has been extensively characterized and is highly specific for human GLUT-3 (Harris et al., 1992). Gold particles, prepared as described previously (Slot and Geuze, 1985), were conjugated to protein A (Roth et al., 1978).
Platelet Isolation
Blood was taken from healthy donors and was anticoagulated with 0.1 vol of 0.13 M sodium citrate. Platelet-rich plasma was obtained by centrifugation of whole blood at 180 g for 10 min at 22°C, and then mixed in a 1:1 ratio with Krebs-Ringer buffer, pH 5.0 (4 mM KCl, 100 mM NaCl, 20 mM NaHCO3, 2 mM Na2SO4, 4.7 mM citric acid, 14.2 tri-sodium citrate). The platelet pellet was washed twice with the same buffer at pH 6.0. Alternatively, fresh whole blood was collected directly from the vein into fixative containing 2.4% paraformaldehyde and 0.24% glutaraldehyde in 0.1 M sodium phosphate buffer, pH 7.4 (1:5 volume ratio). For biochemical analysis, samples of the washed platelet suspension were resuspended in Krebs-Ringer buffer, pH 7.4, adjusted to 2 × 109 platelets per ml, and used within 1 h.
Bone Marrow Megakaryocytes
Normal bone marrow samples were obtained after informed consent from a patient undergoing heart surgery. The megakaryocyte-rich cell suspension was obtained by centrifugation on Ficoll-Paque (Research Grade; Pharmacia, Uppsala, Sweden). The cells were fixed with a mixture of 2% paraformaldehyde and 0.2% glutaraldehyde, and then processed for cryosectioning and immunolabeling as described below.
[14C]2-DG Uptake
Aliquots (200 μl) of the washed platelet suspension were diluted in 1.8 ml 0.1 M Hepes- NaCl (0.01 M Hepes, 0.15 M NaCl, pH 7.4) to obtain a final platelet concentration of 2 × 108/ml. The platelet samples were warmed to 37°C and incubated either in the absence or presence of thrombin. A dose-dependent stimulation was achieved using concentrations of 0.02, 0.2, and 0.5 U/ml thrombin, respectively. After stimulation for 7 min, [14C]2-DG was added to the platelet suspension at a final concentration of 10 μM, and the incubation was continued for 3 min. Alternatively, platelets were incubated for 7 min with ADP (10 μM), followed by the addition of 10 μM [14C]2-DG as described above. In this case the platelets were preincubated at 37°C for 10 min with 30 μM indomethacin, a specific inhibitor of cyclo-oxygenase, which prevents secretion during ADP stimulation (Kaplan et al., 1979). The [14C]2-DG uptake was arrested by the addition of 10 μM cytochalasin B. The platelet samples were washed three times with 0.1 M PBS, 50-μl samples were taken, and the total protein content was measured. The platelet pellet was dissolved in 500 μl 1 N NaOH and counted in a liquid scintillation counter.
Cryosectioning and Immunolabeling
Platelet samples were incubated as described for the measurements of 2-DG uptake except that nonradiolabeled 2-DG was used for the incubation. At the conclusion of the 10 min, incubation platelets were immediately fixed using 2% paraformaldehyde and 0.2% glutaraldehyde in 0.1 M sodium phosphate buffer. Samples were then rinsed three times in 0.1 M sodium phosphate buffer and embedded in 10% gelatin in PBS at 37°C. The gelatin was solidified at 4°C, and cut into small blocks. After overnight infiltration in 2.3 M sucrose at 4°C, the cells were frozen in liquid nitrogen. Ultrathin cryosectioning and immunogold labeling were performed as described before (Slot et al., 1991). Sections were picked up with a drop of a 1:1 mixture of 2.3 M sucrose and 1.8% methyl cellulose (25 centipoise; Fluka AG, Buchs, Switzerland), thawed, and transferred to Formvar carbon-coated copper grids (Liou et al., 1996). The sections were subsequently immunolabeled at room temperature by floating on drops containing the diluted antibody. After incubation, the sections were washed on distilled water, stained for 5 min with uranyl-oxalate, pH 7.0, washed again, and embedded in a mixture of 1.8% methyl cellulose and 0.3% uranyl acetate at 4°C. For quantitative analysis of the GLUT-3 labeling, random electron micrographs were taken at a nominal magnification of 12,000 from directly fixed platelets, nonstimulated washed platelets, and after stimulation with ADP and thrombin. Gold particles were counted over α-granules, plasma membrane, including the open canalicular system (OCS), and undefined structures. Gold particle numbers were expressed as a percentage of the total.
Results
[14C]2-DG Uptake in Platelets
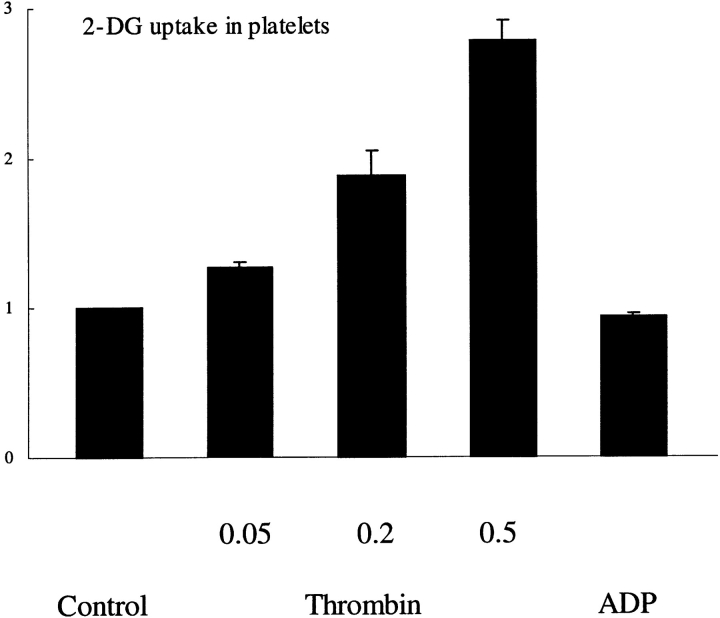
The measurement of 2-DG uptake provides an estimate of glucose transport, as this analogue is transported and phosphorylated like glucose but does not undergo subsequent metabolism. Stimulation with thrombin caused a rapid increase in 2-DG uptake in human platelets (Fig. 1). The amount of 2-DG uptake was 1.2-fold when a low dose of thrombin (0.05 U/ml) was used, while at a dose of 0.5 U/ml a 2.8-fold increase was observed. The specificity of the 2-DG uptake was confirmed by using cytochalasin B, a specific inhibitor of the facilitative glucose transporter (Jung and Rampal, 1977). 2-DG uptake was inhibited by >90% in the presence of 10 μM cytochalasin B, irrespective of the activation state of the platelets (data not shown).
Figure 1.
Glucose transport activity in human platelets. Isolated platelets were either nonstimulated or stimulated with 10 μM ADP or 0.05, 0.2, and 0.5 U/ml thrombin, respectively. The [14C]2-DG uptake was measured as described in Materials and Methods. The increase in 2- DG uptake upon stimulation is expressed relative to the basal 2-DG values of nonstimulated platelets. The values represent the mean ± SEM of three separate experiments.
In accordance with a previous study on Western blots of whole platelet lysates (Craik et al., 1995), we were unable to detect any significant expression of GLUT-1, GLUT-2, or GLUT-4 using isoform-specific antibodies. It is unlikely that platelets express GLUT-5 because this isoform has a selective preference for fructose (Burant et al., 1992), and previous studies have shown that platelets do not transport fructose (Akkerman, 1978). Significant labeling was, however, observed using an antibody specific for human GLUT-3, confirming that this is probably the major glucose transporter isoform expressed in these cells (Craik et al., 1995). We therefore next investigated the intracellular localization of GLUT-3 in human platelets and their precursor, the megakaryocyte, to further explain the activation-dependent increase in 2-DG uptake.
Localization of GLUT-3 in Resting Platelets
Ultrathin cryosections of fixed human platelets were immunolabeled with antibodies specific for GLUT-3. In resting platelets, from blood that was collected in fixative, significant immunolabeling of the α-granules was observed (Fig. 2). Most of the immunogold labeling was situated at the cytoplasmic face of the granules, consistent with the fact that the antibody was raised against the cytoplasmic carboxy terminus of GLUT-3 (Harris et al., 1992). Low labeling of the platelet plasma membrane, including the connected OCS, was also encountered under these conditions. The relative distribution of GLUT-3 was quantified by counting the gold particles associated with α-granules, surface membrane, OCS, and undefined structures (Table I). In resting platelets, 75% of the total gold labeling was associated with the limiting membrane of the α-granules and 15% was detected at the platelet outer surface and the OCS. The remaining 10% was attributed to structures that were not easily identified. This may represent in part labeling of grazing sections over α-granule membranes and nonspecific background staining. When megakaryocytes isolated from bone marrow were immunolabeled with antibodies specific for GLUT-3, we found labeling patterns similar to that in the resting platelets: predominant labeling of the α-granules, and additionally a low expression in the Golgi complex and demarcating membranes, the precursor of the platelet cell surface (data not shown).
Figure 2.
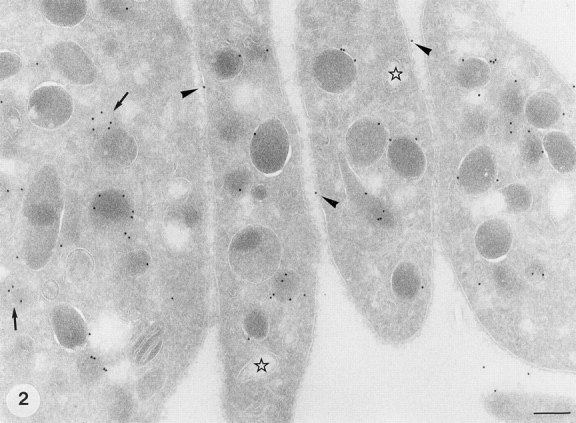
Ultrastructural localization of GLUT-3 in human platelets. Frozen thin-sections of resting platelets immediately fixed in whole blood were immunolabeled with an antibody specific for GLUT-3, followed by protein A conjugated to 10 nm gold. The platelets still have their discoid shape. The majority of the label is associated with the limiting membrane of the α-granules. Note that the gold particles are often situated at the cytoplasmic face of the membrane, and occasionally in a grazing section with the tip of an α-granule membrane (arrow). Some label is also associated with the plasma membrane (arrowheads). (Stars) OCS. Bar, 200 nm.
Table I.
Intracellular Distribution of GLUT-3 in Blood Platelets
| Isolated platelets | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fresh fixed platelets | Thrombin | ADP | ||||||||||
| Resting | Control | 0.05 | 0.2 | 0.5 | 10 | |||||||
| U/ml | μM | |||||||||||
| Plasma membrane | 14.8 (1.1) | 23.5 (1.6) | 32.0 | 66.9 (3.9) | 78.8 | 25.6 | ||||||
| Cell surface | 9.9 (1.3) | 14.3 (1.0) | 22.0 | 45.4 (2.0) | 57.0 | 17.3 | ||||||
| OCS | 4.9 (1.3) | 9.2 (1.0) | 10.0 | 21.5 (2.4) | 21.8 | 8.3 | ||||||
| α-Granules | 74.8 (2.8) | 67.5 (2.2) | 61.9 | 26.1 (5.6) | 13.8 | 66.5 | ||||||
| Undefined | 10.4 (2.1) | 9.0 (1.6) | 6.1 | 7.0 (2.0) | 7.4 | 7.9 | ||||||
| n | 3 | 6 | 1 | 4 | 1 | 2 | ||||||
Gold particles were counted over cryosections of GLUT-3–labeled platelets, in resting state and after activation by thrombin or ADP, and were attributed to the items indicated. Number represent percentages (± SEM). The number of experiments (n) is indicated at the bottom of the table. In each experiment at least 1,000 gold particles were counted in ∼50 randomly photographed platelet profiles.
Effects of Thrombin and ADP on GLUT-3 Localization
In isolated control platelets that were fixed after 10-min incubation in Hepes buffer, we found evidence for mild activation. This is a well-established phenomenon and is hard to avoid during the washing steps of these fragile cells, in spite of all precautions taken. However, the general morphology of the washed platelets used in our experiments resembled that in resting state fairly well, containing still numerous α-granules, and they did not exhibit the characteristic shape change of an activated platelet. Under these conditions, 67% of the total immunogold labeling was associated with the α-granules and 23% was found on OCS and surface membranes (Table I). In response to 0.05 U/ml of thrombin, the platelets underwent the shape changes characteristic for a low stimulatory response (i.e., centralization of α-granules and the beginning of cell surface ruffling). No substantial redistribution of GLUT-3 was observed under these conditions. Incubation with 0.2 and 0.5 U/ml thrombin caused a marked activation of the platelets, concomitant with a substantial redistribution of GLUT-3 to the platelet cell surface membranes. A majority of the α-granules had fused with membranes of the cell surface and the OCS, leaving large vacuoles with released contents in the center (Fig. 3, a and b). A dose of 0.05, 0.2, or 0.5 U/ml thrombin caused a 1.4-, 2.8-, or 3.3-fold increase in GLUT-3 labeling at the plasma membrane, concomitant with a 1.3-, 1.9-, or 2.8-fold increase in 2-DG uptake, when compared with controls.
Figure 3.
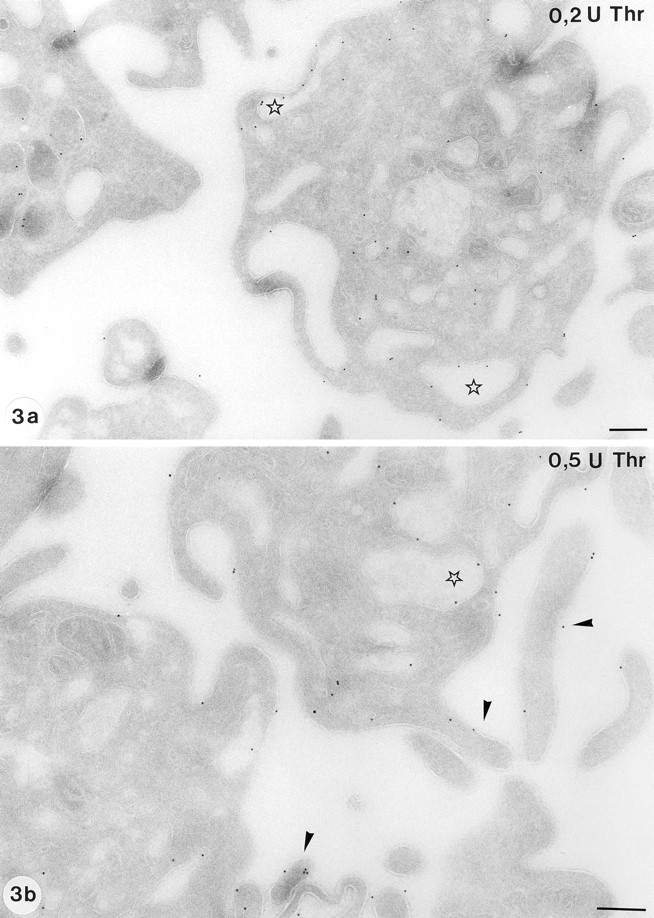
Distribution of GLUT-3 in activated platelets. Activation with both 0.2 U/ml thrombin (a) and 0.5 U/ml thrombin (b) results in a significant loss of the α-granules and a remarkable shape change to a ruffled cell surface. Some platelets had retained α-granules, a finding which was more often encountered at the lower dose of thrombin (a). The translocation of GLUT-3 to the OCS (stars) and to the cell surface is obvious, particularly to the pseudopods. (b, arrowheads). Bar, 250 nm.
To determine if the changes in 2-DG uptake were triggered by changes in cell shape or activation of the platelet contractile apparatus, the effect of ADP on GLUT-3 localization was measured. In agreement with previous findings, ADP caused characteristic changes in platelet shape, i.e., centralization of α-granules and the formation of pseudopodal extensions (Fig. 4). Under the experimental conditions, ADP had no effect on the number of intracellular α-granules, as well as on the distribution of GLUT-3 in platelets (Fig. 4; Table I), which is consistent with the inability of ADP to trigger a change in 2-DG uptake (Fig. 1).
Figure 4.
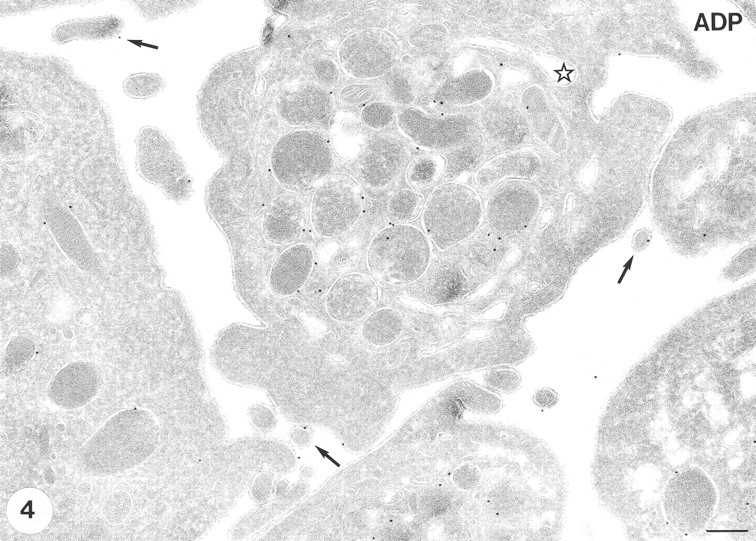
GLUT-3 expression after stimulation with 10 μM ADP. The shape changes characteristic for ADP stimulation are observed (i.e., centralization of α-granules and some cell surface ruffling ). A similar morphology is observed when the low dose of 0.05 U/ml thrombin is used (not shown). The majority of the label is still associated with the membranes of the α-granules. Some label is observed at developing pseudopods. Bar, 200 nm.
Discussion
In the present study we show that activation of human platelets with thrombin causes a rapid stimulation of glucose uptake, suggesting that this may be a key metabolic step for the generation of ATP to sustain the increased energy demands during thrombus formation. This acute elevation in transport is precisely coupled to platelet activation via the efficient targeting of the major facilitative transporter found in this cell, GLUT-3, from the intracellular α-granules toward the cell surface. As these granules fuse with the cell surface, there is a significant increase in surface levels of GLUT-3, accompanied by an increased glucose transport activity, which was of similar magnitude at the different levels of activation (Fig. 1 vs Table I) We could not measure glucose uptake in circulating human blood platelets, but our immunocytochemical data indicate that, compared with the very low levels (∼15%) in resting, directly fixed platelets, a five- to sixfold increase in GLUT-3 surface expression occurs during full platelet activation. The parallel course of increases in GLUT-3 surface expression and glucose uptake that we measured in isolated platelet preparations suggests that glucose transport likewise can be upregulated five- to sixfold when circulating platelets are activated in vivo.
Previous studies have shown that extracellular glucose is an important source of energy for human platelets (Akkerman, 1978, 1987). Moreover, much of the metabolic flux in platelets appears to be directed toward lactate production. In view of the relatively low energy yield of this pathway (∼2 mol ATP per mol glucose), compared with the TCA cycle (∼36 mol ATP per mol glucose), it is likely that the flux through this pathway must increase substantially during platelet activation when the ATP requirements to maintain contraction of the actomyosin system as well as other functions are likely to be quite significant. However, the rate-determining steps in the glycolytic pathway that facilitate these changes in platelet metabolism have not been defined. The free intracellular glucose concentration is very low in platelets, suggesting that the rate of flux through this step is not in equilibrium with other steps in the glycolytic pathway (Akkerman, 1978, 1987). This provided a clue that the glucose transport step could play an important role as a rate-determining step in this cell. Until recently, however, very little was known concerning the platelet glucose transport system.
Over the past decade a family of facilitative glucose transporters have been identified in mammals (Mueckler, 1994). Each member of this family, referred to as GLUTs 1–7, displays a unique tissue distribution, as well as characteristic kinetic properties that appear to enable different cell types to respond appropriately to a particular metabolic situation. For example, GLUT-2, which is found in liver and pancreatic B cells, has a very low affinity for glucose (Colville et al., 1993). This is probably advantageous for these particular cells, enabling them to transmit changes in the circulation to the cell interior. Using antibodies specific for each glucose transporter isoform, it was recently reported that platelets predominantly express GLUT-3 (Craik et al., 1995), which was confirmed by the present immunolocalization study. GLUT-3 has a particularly low K m for glucose, allowing it to transport efficiently even when blood glucose levels are very low (Gould et al., 1991). Since the internal environment of a platelet plug is deprived of blood flow, this may explain the selective advantage of using the GLUT-3 isotype. The irreversible surface expression of GLUT-3 may be a desirable feature to ensure that, after the secretory process is complete, high cell surface levels of GLUT-3 are maintained. It enables the platelets to maintain prolonged access to extracellular glucose throughout the course of hemostatic plug formation, thereby providing a continuous source of energy for platelet contraction.
The major tissues in which GLUT-3 expression has been reported are brain (Kayano et al., 1988), mouse embryos (Pantaleon et al., 1997), sperm (Burant and Davidson, 1994), and polarized epithelial cells derived from human tumors (Harris et al., 1992). The subcellular distribution of GLUT-3 in each of these tissues differs from that of other transporters, thus providing an unique feature of this isoform. In mouse embryos, MDCK cells (Pascoe et al., 1996), and CaCo2 cells (Harris et al., 1992), GLUT-3 is targeted to the apical cell surface. In neurons, GLUT-3 appears to be targeted to axons (James, D.E., unpublished data), whereas in sperm it is found predominantly in the tail (Slot, J.W., and D.E. James, unpublished observations). Hence, it will be of interest to determine if similar motifs are responsible for maintaining each of these unique targeting phenotypes, and if there is some link between each of these targeting events and the localization of GLUT-3 to the limiting membrane of the α-granules in platelets. A targeting motif that regulates the sequestration of P-selectin in secretory granules has been identified (Koedam et al., 1992), but we have been unable to identify a motif resembling this in GLUT-3.
In all cell types studied, GLUT-3 molecules are almost exclusively confined to the plasma membrane (Pascoe et al., 1996; Harris et al., 1992), in agreement with the absence of a consensus internalization signal in the cytosolic domains of GLUT-3 (Asano et al., 1992). In contrast, platelets harbor most of their GLUT-3 intracellularly in α-granule membranes. These are most likely biosynthetic GLUT-3 molecules that are stored in the α-granules before their translocation to the cell surface by stimulated α-granule release. This is an irreversible process and occurs only once in the platelet's life time, during activation. The only glucose transporter normally stored intracellularly in resting cells is GLUT-4, which resides in an endosome-related compartment until it becomes transiently expressed at the cell surface after stimulation (James et al., 1994). Interestingly, we have recently observed a major pool of intracellular GLUT-4 molecules residing in the membranes of regulated secretory granules in atrial myocytes (Slot et al., 1997). Several lines of evidence indicated that these GLUT-4 molecules derive from endocytosis rather than from the biosynthetic pathway. Thus, while atrial myocytes seem to exploit secretion of their storage granules to ensure stimulus-dependent repetitive endocytotic and translocation cycles of GLUT-4, platelets store biosynthetic GLUT-3 in α-granules during differentiation from megakaryocytes until the platelets are stimulated to release the α-granules before disintegration during clot formation.
Abbreviations used in this paper
- 2-DG
2-deoxyglucose
- GLUT
glucose transporter
- OCS
open canalicular system
Footnotes
Please address all correspondence to Harry F.G. Heijnen, Department of Hematology, University Hospital, P.O. Box 85500, 3508 GA Utrecht, The Netherlands. Tel.: (31) 30-2507769. Fax: (31) 30-2511893. e-mail: H.F.G.Heijnen@lab.azu.nl
References
- Akkerman JWN. Regulation of carbohydrate metabolism in platelets. A Review. Thromb Haemostasis. 1978;39:712–724. [PubMed] [Google Scholar]
- Akkerman, J.W.N. 1987. Carbohydrate metabolism. In Platelet Responses and Metabolism. H. Holmsen, editor. CRC Press, Inc., Boca Raton, FL. 189–213.
- Asano T, Katagiri H, Taakata K, Tsukuda K, Lin JL, Ishihara H, Inukai K, Hirano H, Yazaki Y, Oka Y. Characterization of GLUT3 protein expressed in Chinese hamster ovary cells. Biochem J. 1992;288:189–193. doi: 10.1042/bj2880189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behnke O. Microtubules and microfilaments. Triangle. 1974;13:7–15. [PubMed] [Google Scholar]
- Bettex-Galland M, Luscher EF. Extraction of an actomyosin-like protein from human thrombocytes. Nature (Lond) 1959;184:276–277. doi: 10.1038/184276b0. [DOI] [PubMed] [Google Scholar]
- Burant CF, Davidson NO. GLUT3 glucose transporter isoform in rat testis: localization, effect of diabetes mellites, and comparison to human testis. Am J Physiol. 1994;267:R1488–R1495. doi: 10.1152/ajpregu.1994.267.6.R1488. [DOI] [PubMed] [Google Scholar]
- Burant CF, Takeda J, Brot-Laroche LE, Bell GI, Davidson NO. Fructose transporter in human spermatozoa and small intestine is GLUT5. J Biol Chem. 1992;267:14523–14526. [PubMed] [Google Scholar]
- Cohen I, Gerrard JM, White JG. Ultrastructure of clots during isometric contraction. J Cell Biol. 1982;93:775–787. doi: 10.1083/jcb.93.3.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colville CA, Seatter MJ, Jess TJ, Gould GW, Thomas HM. Kinetic analysis of the liver-type (GLUT2) and brain-type (GLUT3) glucose transporters in Xenopus oocytes: substrate specifities and effects of transport inhibitors. Biochem J. 1993;290:701–706. doi: 10.1042/bj2900701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craik JD, Stewart M, Cheeseman CI. GLUT-3 (brain-type) glucose transporter polypeptides in human blood platelets. Thromb Res. 1995;79:461–469. doi: 10.1016/0049-3848(95)00136-f. [DOI] [PubMed] [Google Scholar]
- Cushman SW, Wardzala LJ. Potential mechanism of insulin action on glucose transport in the isolated rat adipose cell. Apparent translocation of intracellular transport systems to the plasma membrane. J Biol Chem. 1980;255:4758–4762. [PubMed] [Google Scholar]
- Fox JE, Reynolds CC, Boyles JK. Studying the platelet cytoskeleton in Triton X-100 lysates. Methods Enzymol. 1992;215:42–58. doi: 10.1016/0076-6879(92)15052-e. [DOI] [PubMed] [Google Scholar]
- Gould GW, Thomas HM, Jess TJ, Bell GI. Expression of human glucose transporters in Xenopus oocytes: kinetic characterization and substrate specificities of the erythrocyte, liver, and brain isoforms. Biochemistry. 1991;30:5139–5145. doi: 10.1021/bi00235a004. [DOI] [PubMed] [Google Scholar]
- Harris DS, Slot JW, Geuze HJ, James DE. Polarized distribution of glucose transporter isoforms in Caco-2 cells. Proc Natl Acad Sci USA. 1992;89:7556–7560. doi: 10.1073/pnas.89.16.7556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwig JH. Mechanisms of actin rearrangements mediating platelet activation. J Cell Biol. 1992;118:1421–1442. doi: 10.1083/jcb.118.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James DE, Piper RC, Slot JW. Insulin stimulation of GLUT-4 translocation: a model for regulated recycling. Trends Cell Biol. 1994;4:120–126. doi: 10.1016/0962-8924(94)90066-3. [DOI] [PubMed] [Google Scholar]
- Jung CY, Rampal AL. Cytochalasin B binding sites and glucose transport carrier in human erythrocyte ghosts. J Biol Chem. 1977;252:5456–5463. [PubMed] [Google Scholar]
- Kaplan KL, Broekman MJ, Chernoff A, Lesznik GR, Drillings M. Platelet alpha-granule proteins: studies on release and subcellular localization. Blood. 1979;53:604–618. [PubMed] [Google Scholar]
- Kayano T, Fukumoto H, Eddy RL, Fan F-S, Byers MG, Shows TB, Bell GI. Evidence for a family of human glucose transporter-like proteins. J Biol Chem. 1988;263:15245–15248. [PubMed] [Google Scholar]
- Koedam JA, Cramer EM, Briend E, Furie B, Furie BC, Wagner DD. P-selectin, a granule membrane protein of platelets and endothelial cells, follows the regulated secretory pathway in AtT-20 cells. J Cell Biol. 1992;116:617–625. doi: 10.1083/jcb.116.3.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder BL, Chernoff A, Kaplan KL, Goodman DS. Release of platelet-derived growth factor from human platelets by arachidonic acid. Proc Natl Acad Sci USA. 1979;76:4107–4111. doi: 10.1073/pnas.76.8.4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou W, Geuze HJ, Slot JW. Improving structural integrity of cryosections for immunogold labeling. Histochem Cell Biol. 1996;106:41–58. doi: 10.1007/BF02473201. [DOI] [PubMed] [Google Scholar]
- Mueckler M. Facilitative glucose transporters. Eur J Biochem. 1994;219:713–725. doi: 10.1111/j.1432-1033.1994.tb18550.x. [DOI] [PubMed] [Google Scholar]
- Pantaleon M, Harvey MB, Pascoe WS, James DE, Kaye PL. GLUT3: ontogeny, targeting and role in the mouse blastocyst. Proc Natl Acad Sci USA. 1997;94:3795–3800. doi: 10.1073/pnas.94.8.3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascoe WS, Inukai K, Oka Y, Slot JW, James DE. Differential targeting of facilitative glucose transporters in polarized epithelial cells. Am J Physiol. 1996;271:C547–C554. doi: 10.1152/ajpcell.1996.271.2.C547. [DOI] [PubMed] [Google Scholar]
- Roth J, Bendayan M, Orci L. Ultrastructural localization of intracellular antigens by the use of protein-A gold complex. J Histochem Cytochem. 1978;26:1074–1081. doi: 10.1177/26.12.366014. [DOI] [PubMed] [Google Scholar]
- Sander HJ, Slot JW, Bouma BN, Bolhuis PA, Pepper DS, Sixma JJ. Immunocytochemical localization of fibrinogen, platelet factor 4, and beta-thromboglobulin in thin frozen sections of human blood platelets. J Clin Invest. 1983;72:1277–1287. doi: 10.1172/JCI111084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sixma JJ, Nievelstein PF, Houdijk WP, Van Breugel H, Hindriks G. Adhesion of blood platelets to isolated components of the vessel wall. Ann NY Acad Sci. 1987a;509:103–117. doi: 10.1111/j.1749-6632.1987.tb30988.x. [DOI] [PubMed] [Google Scholar]
- Sixma JJ, Nievelstein PF, Zwaginga JJ, de Groot PG. Adhesion of blood platelets to the extracellular matrix of cultured human endothelial cells. Ann NY Acad Sci. 1987b;516:39–51. doi: 10.1111/j.1749-6632.1987.tb33028.x. [DOI] [PubMed] [Google Scholar]
- Sixma JJ, Slot JW, Geuze HJ. Immunocytochemical localization of platelet granule proteins. Methods Enzymol. 1989;169:301–311. doi: 10.1016/0076-6879(89)69070-2. [DOI] [PubMed] [Google Scholar]
- Slot JW, Geuze HJ. A new method of preparing gold probes for multiple-labeling cytochemistry. Eur J Cell Biol. 1985;38:87–93. [PubMed] [Google Scholar]
- Slot JW, Geuze HJ, Gigengack S, Lienhard GE, James DE. Immunolocalization of the insulin regulatable glucose transporter in brown adipose tissue of the rat. J Cell Biol. 1991;113:123–135. doi: 10.1083/jcb.113.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slot JW, Garruti G, Martin S, Oorschot V, Posthuma G, Kraegen EW, Laybutt R, Thibault G, James DE. Glucose transporter (GLUT-4) is targeted to secretory granules in rat atrial cardiomyocytes. J Cell Biol. 1997;137:1243–1254. doi: 10.1083/jcb.137.6.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki I, Kono T. Evidence that insulin causes translocation of glucose transport activity to the plasma membrane from an intracellular storage site. Proc Natl Acad Sci USA. 1980;77:2542–2545. doi: 10.1073/pnas.77.5.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziel FH, Venkatesan N, Davidson MB. Glucose transport is rate limiting for skeletal muscle glucose metabolism in normal and STZ-induced diabetic rats. Diabetes. 1988;37:885–890. doi: 10.2337/diab.37.7.885. [DOI] [PubMed] [Google Scholar]