Abstract
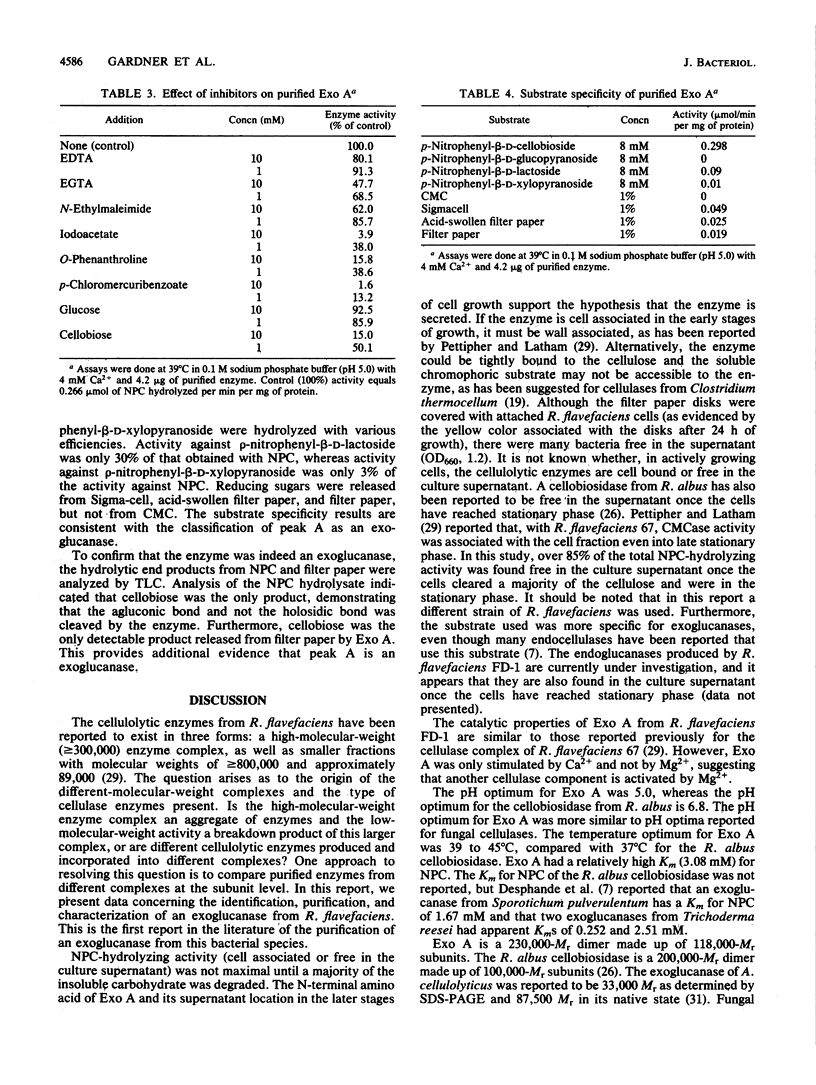
An exo-beta-1,4-glucanase (Exo A) from Ruminococcus flavefaciens FD-1 was purified to homogeneity and characterized. Enzyme activity was monitored during purification by using the substrate p-nitrophenyl-beta-D-cellobioside (NPC). Over 85% of the NPC activity was found to be extracellular once the filter paper was degraded (7 days). Culture supernatant was harvested, and the protein was concentrated by ultrafiltration. The retentate (greater than or equal to 300,000 Mr), containing most of the activity against NPC, was then fractionated with a TSK DEAE-5PW column. This yielded a sharp major peak of NPC enzyme activity, followed by a broader, less active area that appeared to contain at least six minor peaks of lower enzymatic activity. Further purification was achieved by chromatography with a hydroxylapatite column. Finally, gel filtration chromatography yielded a homogeneous enzyme (Exo A) as determined by silver stains of both sodium dodecyl sulfate- and nondenaturing electrophoresis gels. Substrate specificity experiments and the products of cellulose digestion indicate that the enzyme was an exo-beta-1,4-glucanase. Exo A required Ca2+ for maximal activity and had an apparent Km of 3.08 mM for NPC, with a Vmax of 0.298 mumol/min per mg of protein. The enzyme had an Mr of 230,000, as determined by gel filtration chromatography, and was a dimer of 118,000-Mr subunits. The N-terminal amino acid sequence of the enzyme is presented.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bryant M. P. Commentary on the Hungate technique for culture of anaerobic bacteria. Am J Clin Nutr. 1972 Dec;25(12):1324–1328. doi: 10.1093/ajcn/25.12.1324. [DOI] [PubMed] [Google Scholar]
- Bryant M. P. Nutritional requirements of the predominant rumen cellulolytic bacteria. Fed Proc. 1973 Jul;32(7):1809–1813. [PubMed] [Google Scholar]
- Deshpande M. V., Eriksson K. E., Pettersson L. G. An assay for selective determination of exo-1,4,-beta-glucanases in a mixture of cellulolytic enzymes. Anal Biochem. 1984 May 1;138(2):481–487. doi: 10.1016/0003-2697(84)90843-1. [DOI] [PubMed] [Google Scholar]
- Eriksson K. E., Hollmark B. H. Kinetic studies of the action of cellulase upon sodium carboxymethyl cellulose. Arch Biochem Biophys. 1969 Sep;133(2):233–237. doi: 10.1016/0003-9861(69)90450-0. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Genthner B. R., Davis C. L., Bryant M. P. Features of rumen and sewage sludge strains of Eubacterium limosum, a methanol- and H2-CO2-utilizing species. Appl Environ Microbiol. 1981 Jul;42(1):12–19. doi: 10.1128/aem.42.1.12-19.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALLIWELL G., BRYANT M. P. THE CELLULOLYTIC ACTIVITY OF PURE STRAINS OF BACTERIA FROM THE RUMEN OF CATTLE. J Gen Microbiol. 1963 Sep;32:441–448. doi: 10.1099/00221287-32-3-441. [DOI] [PubMed] [Google Scholar]
- HUNGATE R. E. The anaerobic mesophilic cellulolytic bacteria. Bacteriol Rev. 1950 Mar;14(1):1–49. doi: 10.1128/br.14.1.1-49.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalb V. F., Jr, Bernlohr R. W. A new spectrophotometric assay for protein in cell extracts. Anal Biochem. 1977 Oct;82(2):362–371. doi: 10.1016/0003-2697(77)90173-7. [DOI] [PubMed] [Google Scholar]
- Kanda T., Nakakubo S., Wakabayashi K., Nisizawa K. Purification and properties of an exo-cellulase of Avicelase type from a wood-rotting fungus, Irpex lacteus (Polyporus tulipiferae). J Biochem. 1978 Nov;84(5):1217–1226. doi: 10.1093/oxfordjournals.jbchem.a132239. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- MacKenzie C. R., Bilous D., Johnson K. G. Purification and characterization of an exoglucanase from Streptomyces flavogriseus. Can J Microbiol. 1984 Sep;30(9):1171–1178. doi: 10.1139/m84-183. [DOI] [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Sedman S. A., Ebert M. H. Ultrasensitive stain for proteins in polyacrylamide gels shows regional variation in cerebrospinal fluid proteins. Science. 1981 Mar 27;211(4489):1437–1438. doi: 10.1126/science.6162199. [DOI] [PubMed] [Google Scholar]
- Ohmiya K., Shimizu M., Taya M., Shimizu S. Purification and properties of cellobiosidase from Ruminococcus albus. J Bacteriol. 1982 Apr;150(1):407–409. doi: 10.1128/jb.150.1.407-409.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmiya K., Shirai M., Kurachi Y., Shimizu S. Isolation and properties of beta-glucosidase from Ruminococcus albus. J Bacteriol. 1985 Jan;161(1):432–434. doi: 10.1128/jb.161.1.432-434.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARK J. T., JOHNSON M. J. A submicrodetermination of glucose. J Biol Chem. 1949 Nov;181(1):149–151. [PubMed] [Google Scholar]
- Russell J. B. Fermentation of cellodextrins by cellulolytic and noncellulolytic rumen bacteria. Appl Environ Microbiol. 1985 Mar;49(3):572–576. doi: 10.1128/aem.49.3.572-576.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saddler J. N., Khan A. W. Cellulolytic enzyme system of Acetivibrio cellulolyticus. Can J Microbiol. 1981 Mar;27(3):288–294. doi: 10.1139/m81-045. [DOI] [PubMed] [Google Scholar]
- Selby K., Maitland C. C. The cellulase of Trichoderma viride. Separation of the components involved in the solubilization of cotton. Biochem J. 1967 Sep;104(3):716–724. doi: 10.1042/bj1040716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umezurike G. M. The purification and properties of extracellular beta-glucosidase from Botryodiplodia theobromae Pat. Biochim Biophys Acta. 1971 Feb 10;227(2):419–428. doi: 10.1016/0005-2744(71)90073-8. [DOI] [PubMed] [Google Scholar]
- Wardi A. H., Allen W. S. Alcian blue staining of glycoproteins. Anal Biochem. 1972 Aug;48(2):621–623. doi: 10.1016/0003-2697(72)90118-2. [DOI] [PubMed] [Google Scholar]
- Wood T. M., McCrae S. I. The purification and properties of the C 1 component of Trichoderma koningii cellulase. Biochem J. 1972 Aug;128(5):1183–1192. doi: 10.1042/bj1281183. [DOI] [PMC free article] [PubMed] [Google Scholar]