Abstract
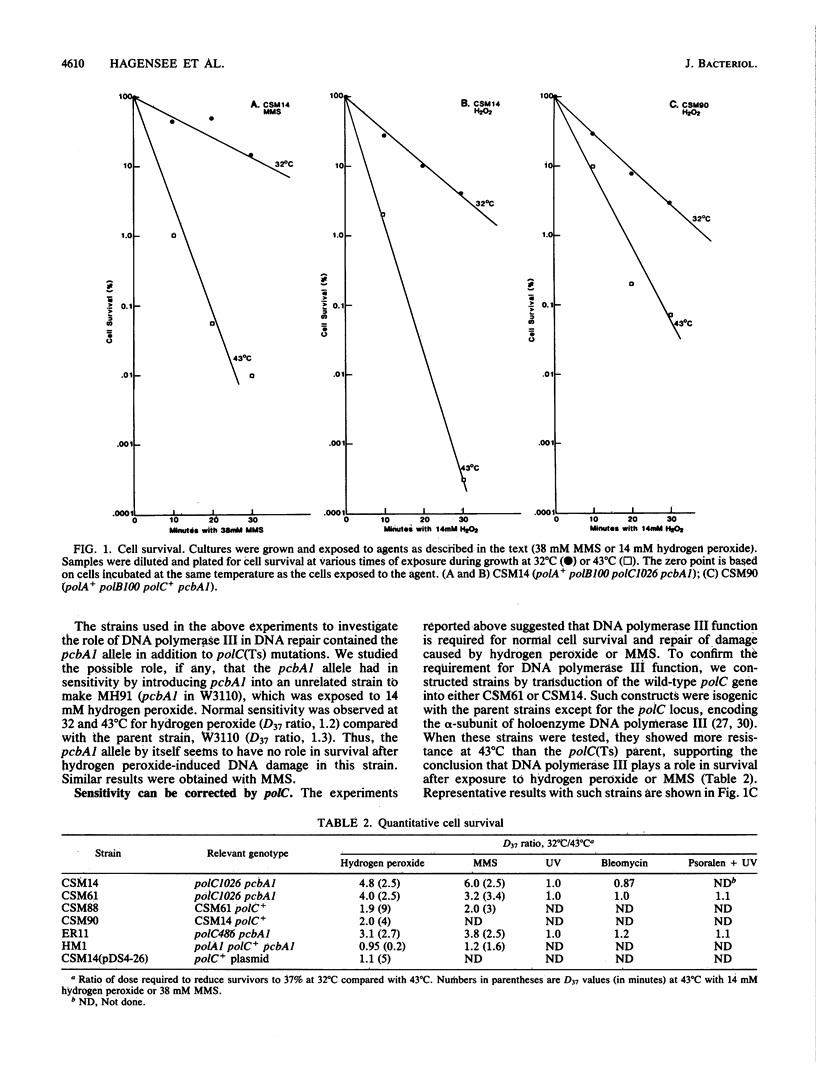
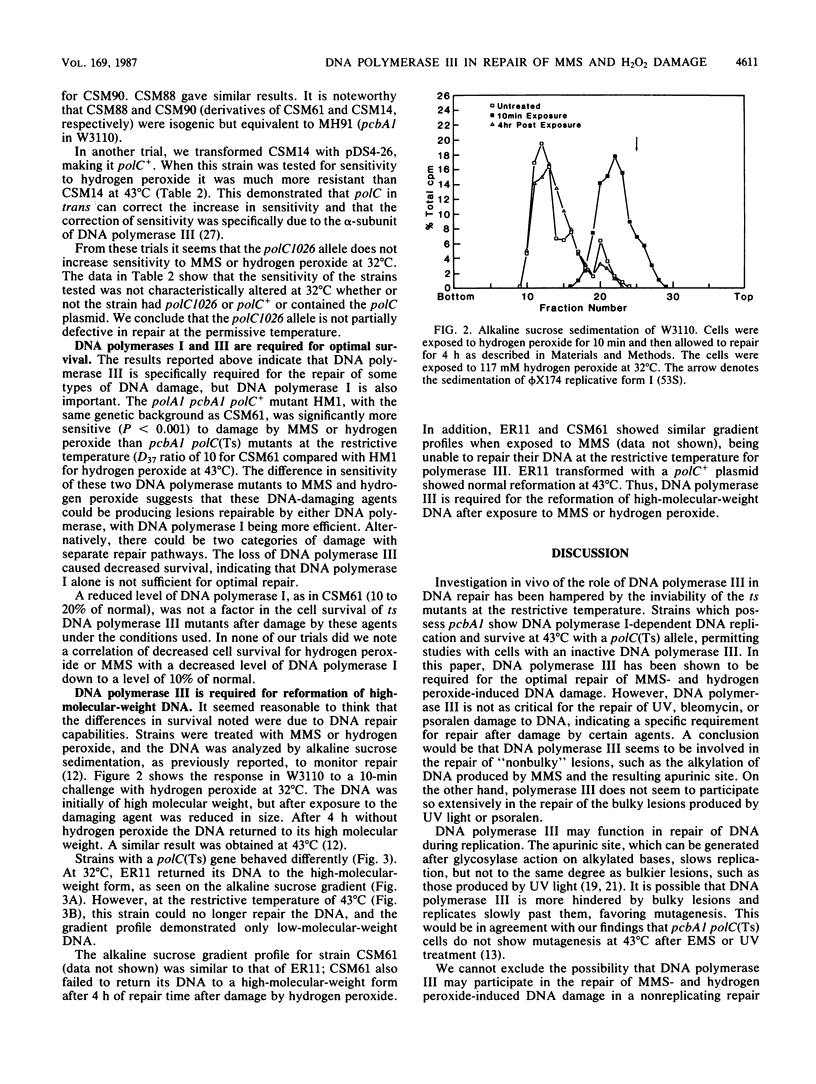
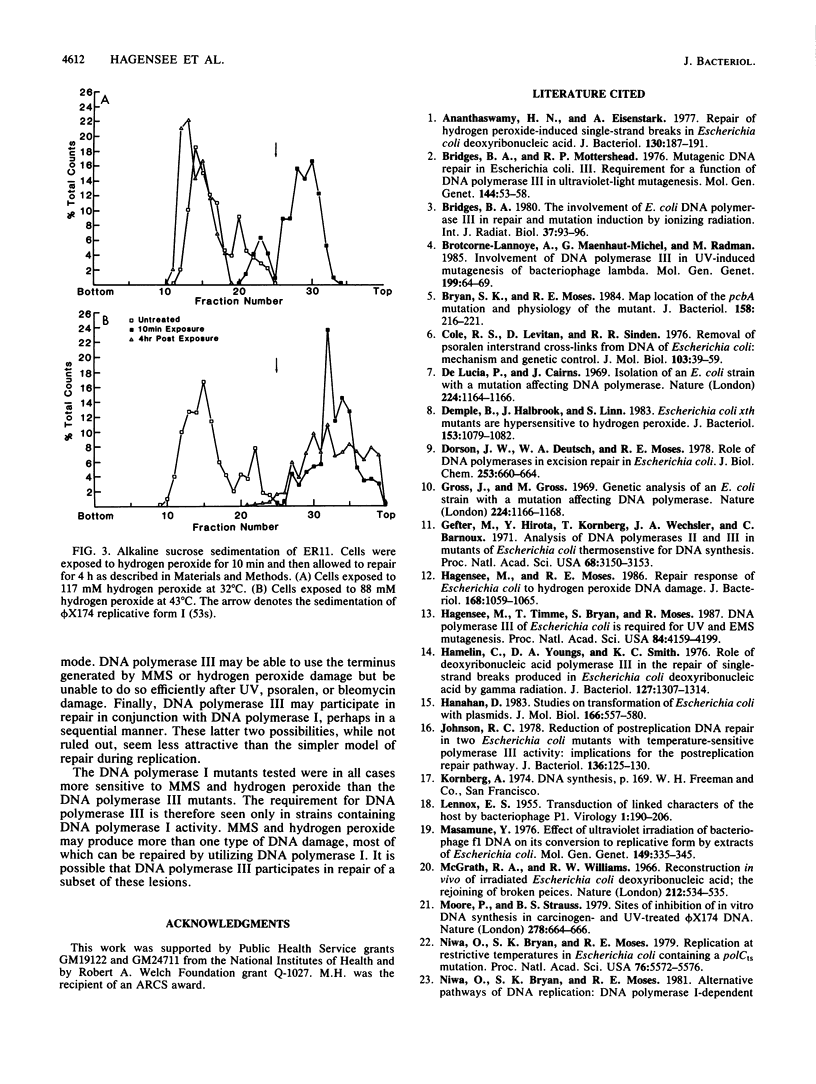
The pcbA1 mutation allows DNA replication dependent on DNA polymerase I at the restrictive temperature in polC(Ts) strains. Cells which carry pcbA1, a functional DNA polymerase I, and a temperature-sensitive DNA polymerase III gene were used to study the role of DNA polymerase III in DNA repair. At the restrictive temperature for DNA polymerase III, these strains were more sensitive to the alkylating agent methyl methanesulfonate (MMS) and hydrogen peroxide than normal cells. The same strains showed no increase in sensitivity to bleomycin, UV light, or psoralen at the restrictive temperature. The sensitivity of these strains to MMS and hydrogen peroxide was not due to the pcbAl allele, and normal sensitivity was restored by the introduction of a chromosomal or cloned DNA polymerase III gene, verifying that the sensitivity was due to loss of DNA polymerase III alpha-subunit activity. A functional DNA polymerase III is required for the reformation of high-molecular-weight DNA after treatment of cells with MMS or hydrogen peroxide, as demonstrated by alkaline sucrose sedimentation results. Thus, it appears that a functional DNA polymerase III is required for the optimal repair of DNA damage by MMS or hydrogen peroxide.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ananthaswamy H. N., Eisenstark A. Repair of hydrogen peroxide-induced single-strand breaks in Escherichia coli deoxyribonucleic acid. J Bacteriol. 1977 Apr;130(1):187–191. doi: 10.1128/jb.130.1.187-191.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges B. A., Mottershead R. P. Mutagenic DNA repair in Escherichia coli. III. Requirement for a function of DNA polymerase III in ultraviolet-light mutagenesis. Mol Gen Genet. 1976 Feb 27;144(1):53–58. doi: 10.1007/BF00277304. [DOI] [PubMed] [Google Scholar]
- Bridges B. A. The involvement of E. coli DNA polymerase III in repair and mutation induction by ionizing radiation. Int J Radiat Biol Relat Stud Phys Chem Med. 1980 Jan;37(1):93–96. doi: 10.1080/09553008014550111. [DOI] [PubMed] [Google Scholar]
- Brotcorne-Lannoye A., Maenhaut-Michel G., Radman M. Involvement of DNA polymerase III in UV-induced mutagenesis of bacteriophage lambda. Mol Gen Genet. 1985;199(1):64–69. doi: 10.1007/BF00327511. [DOI] [PubMed] [Google Scholar]
- Bryan S. K., Moses R. E. Map location of the pcbA mutation and physiology of the mutant. J Bacteriol. 1984 Apr;158(1):216–221. doi: 10.1128/jb.158.1.216-221.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole R. S., Levitan D., Sinden R. R. Removal of psoralen interstrand cross-links from DNA of Escherichia coli: mechanism and genetic control. J Mol Biol. 1976 May 5;103(1):39–59. doi: 10.1016/0022-2836(76)90051-6. [DOI] [PubMed] [Google Scholar]
- De Lucia P., Cairns J. Isolation of an E. coli strain with a mutation affecting DNA polymerase. Nature. 1969 Dec 20;224(5225):1164–1166. doi: 10.1038/2241164a0. [DOI] [PubMed] [Google Scholar]
- Demple B., Halbrook J., Linn S. Escherichia coli xth mutants are hypersensitive to hydrogen peroxide. J Bacteriol. 1983 Feb;153(2):1079–1082. doi: 10.1128/jb.153.2.1079-1082.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorson J. W., Deutsch W. A., Moses R. E. Role of DNA polymerases in excision repair in Escherichia coli. J Biol Chem. 1978 Feb 10;253(3):660–664. [PubMed] [Google Scholar]
- Gefter M. L., Hirota Y., Kornberg T., Wechsler J. A., Barnoux C. Analysis of DNA polymerases II and 3 in mutants of Escherichia coli thermosensitive for DNA synthesis. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3150–3153. doi: 10.1073/pnas.68.12.3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J., Gross M. Genetic analysis of an E. coli strain with a mutation affecting DNA polymerase. Nature. 1969 Dec 20;224(5225):1166–1168. doi: 10.1038/2241166a0. [DOI] [PubMed] [Google Scholar]
- Hagensee M. E., Moses R. E. Repair response of Escherichia coli to hydrogen peroxide DNA damage. J Bacteriol. 1986 Dec;168(3):1059–1065. doi: 10.1128/jb.168.3.1059-1065.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagensee M. E., Timme T. L., Bryan S. K., Moses R. E. DNA polymerase III of Escherichia coli is required for UV and ethyl methanesulfonate mutagenesis. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4195–4199. doi: 10.1073/pnas.84.12.4195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamelin C., Youngs D. A., Smith K. C. Role of deoxyribonucleic acid polymerase III in the repair of single-strand breaks produced in Escherichia coli deoxyribonucleic acid by gamma radiation. J Bacteriol. 1976 Sep;127(3):1307–1314. doi: 10.1128/jb.127.3.1307-1314.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Johnson R. C. Reduction of postreplication DNA repair in two Escherichia coli mutants with temperature-sensitive polymerase III activity: implications for the postreplication repair pathway. J Bacteriol. 1978 Oct;136(1):125–130. doi: 10.1128/jb.136.1.125-130.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- Masamune Y. Effect of ultraviolet irradiation of bacteriophage f1 DNA on its conversion to replicative form by extracts of Escherichia coli. Mol Gen Genet. 1976 Dec 22;149(3):335–345. doi: 10.1007/BF00268536. [DOI] [PubMed] [Google Scholar]
- McGrath R. A., Williams R. W. Reconstruction in vivo of irradiated Escherichia coli deoxyribonucleic acid; the rejoining of broken pieces. Nature. 1966 Oct 29;212(5061):534–535. doi: 10.1038/212534a0. [DOI] [PubMed] [Google Scholar]
- Moore P., Strauss B. S. Sites of inhibition of in vitro DNA synthesis in carcinogen- and UV-treated phi X174 DNA. Nature. 1979 Apr 12;278(5705):664–666. doi: 10.1038/278664a0. [DOI] [PubMed] [Google Scholar]
- Niwa O., Bryan S. K., Moses R. E. Alternate pathways of DNA replication: DNA polymerase I-dependent replication. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7024–7027. doi: 10.1073/pnas.78.11.7024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa O., Bryan S. K., Moses R. E. Replication at restrictive temperatures in Escherichia coli containing a polCts mutation. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5572–5576. doi: 10.1073/pnas.76.11.5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross S. L., Moses R. E. Two actions of bleomycin on superhelical DNA. Biochemistry. 1978 Feb 21;17(4):581–586. doi: 10.1021/bi00597a004. [DOI] [PubMed] [Google Scholar]
- Ross S. L., Sharma S., Moses R. E. DNA polymerase III-dependent repair synthesis in response to bleomycin in toluene-treated Escherichia coli. Mol Gen Genet. 1980;179(3):595–605. doi: 10.1007/BF00271750. [DOI] [PubMed] [Google Scholar]
- Sedgwick S. G., Bridges B. A. Requirement for either DNA polymerase I or DNA polymerase 3 in post-replication repair in excision-proficient Escherichia coli. Nature. 1974 May 24;249(455):348–349. doi: 10.1038/249348a0. [DOI] [PubMed] [Google Scholar]
- Shepard D., Oberfelder R. W., Welch M. M., McHenry C. S. Determination of the precise location and orientation of the Escherichia coli dnaE gene. J Bacteriol. 1984 May;158(2):455–459. doi: 10.1128/jb.158.2.455-459.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer B. The chemical effects of nucleic acid alkylation and their relation to mutagenesis and carcinogenesis. Prog Nucleic Acid Res Mol Biol. 1975;15(0):219–284. [PubMed] [Google Scholar]
- Welch M. M., McHenry C. S. Cloning and identification of the product of the dnaE gene of Escherichia coli. J Bacteriol. 1982 Oct;152(1):351–356. doi: 10.1128/jb.152.1.351-356.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngs D. A., Smith K. C. Involvement of DNA polymerase 3 in excision repair after ultraviolet irradiation. Nat New Biol. 1973 Aug 22;244(138):240–241. doi: 10.1038/newbio244240a0. [DOI] [PubMed] [Google Scholar]



