Abstract
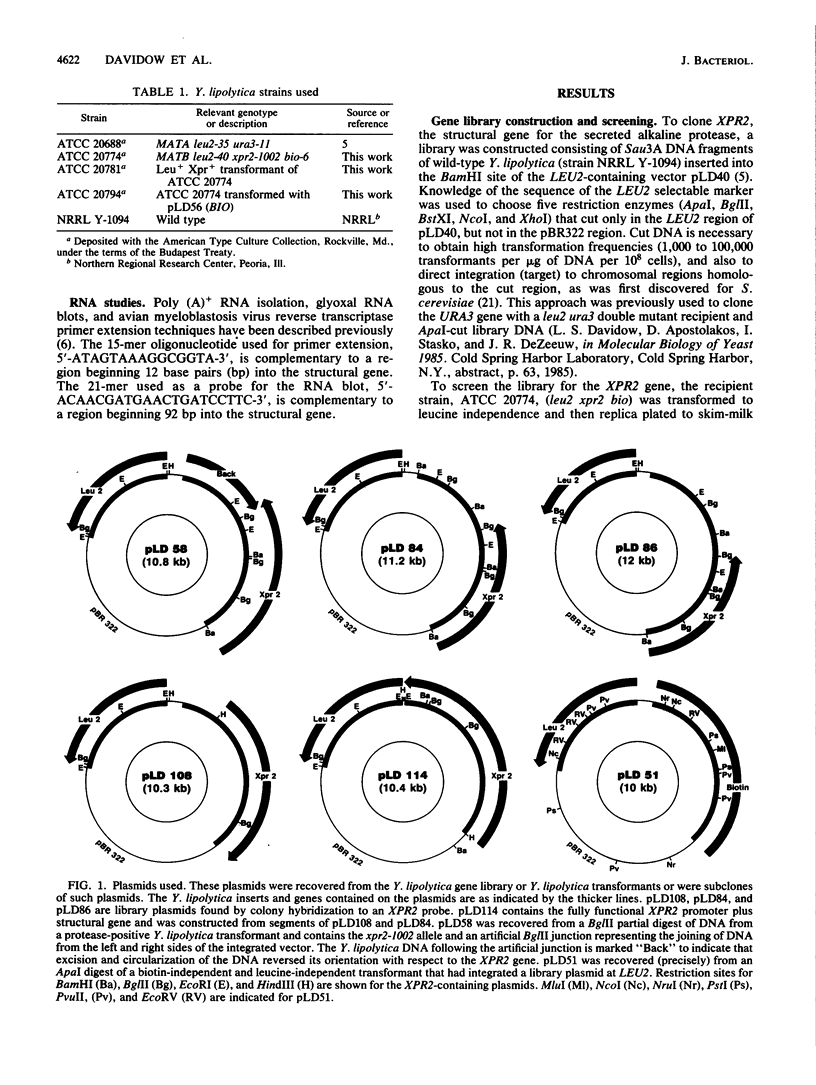
The XPR2 gene encoding an alkaline extracellular protease (AEP) from Yarrowia lipolytica was cloned, and its complete nucleotide sequence was determined. The amino acid sequence deduced from the nucleotide sequence reveals that the mature AEP consists of 297 amino acids with a relative molecular weight of 30,559. The gene codes for a putative 22-amino-acid prepeptide (signal sequence) followed by an additional 135-amino-acid propeptide containing a possible N-linked glycosylation site and two Lys-Arg peptidase-processing sites. The final Lys-Arg site occurs at the junction with the mature, extracellular form. The mature protease contains two potential glycosylation sites. AEP is a member of the subtilisin family of serine proteases, with 42.6% homology to the fungal proteinase K. The functional promoter is more than 700 base pairs long, allowing for the observed complex regulation of this gene. The 5' and 3' flanking regions of the XPR2 gene have structural features in common with other yeast genes.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ammerer G. Expression of genes in yeast using the ADCI promoter. Methods Enzymol. 1983;101:192–201. doi: 10.1016/0076-6879(83)01014-9. [DOI] [PubMed] [Google Scholar]
- Bennetzen J. L., Hall B. D. Codon selection in yeast. J Biol Chem. 1982 Mar 25;257(6):3026–3031. [PubMed] [Google Scholar]
- Cheng S. C., Ogrydziak D. M. Extracellular RNase produced by Yarrowia lipolytica. J Bacteriol. 1986 Nov;168(2):581–589. doi: 10.1128/jb.168.2.581-589.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidow L. S., Kaczmarek F. S., DeZeeuw J. R., Conlon S. W., Lauth M. R., Pereira D. A., Franke A. E. The Yarrowia lipolytica LEU2 gene. Curr Genet. 1987;11(5):377–383. doi: 10.1007/BF00378180. [DOI] [PubMed] [Google Scholar]
- Hubbard S. C., Ivatt R. J. Synthesis and processing of asparagine-linked oligosaccharides. Annu Rev Biochem. 1981;50:555–583. doi: 10.1146/annurev.bi.50.070181.003011. [DOI] [PubMed] [Google Scholar]
- Julius D., Brake A., Blair L., Kunisawa R., Thorner J. Isolation of the putative structural gene for the lysine-arginine-cleaving endopeptidase required for processing of yeast prepro-alpha-factor. Cell. 1984 Jul;37(3):1075–1089. doi: 10.1016/0092-8674(84)90442-2. [DOI] [PubMed] [Google Scholar]
- Kozak M. Comparison of initiation of protein synthesis in procaryotes, eucaryotes, and organelles. Microbiol Rev. 1983 Mar;47(1):1–45. doi: 10.1128/mr.47.1.1-45.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Nasmyth K. At least 1400 base pairs of 5'-flanking DNA is required for the correct expression of the HO gene in yeast. Cell. 1985 Aug;42(1):213–223. doi: 10.1016/s0092-8674(85)80117-3. [DOI] [PubMed] [Google Scholar]
- Nedkov P., Oberthür W., Braunitzer G. Die Primärstruktur von Subtilisin DY. Hoppe Seylers Z Physiol Chem. 1983 Nov;364(11):1537–1540. doi: 10.1515/bchm2.1983.364.2.1537. [DOI] [PubMed] [Google Scholar]
- Ogrydziak D. M., Demain A. L., Tannenbaum S. R. Regulation of extracellular protease production in Candida lipolytica. Biochim Biophys Acta. 1977 Apr 27;497(2):525–538. doi: 10.1016/0304-4165(77)90209-4. [DOI] [PubMed] [Google Scholar]
- Ogrydziak D. M., Mortimer R. K. Genetics of Extracellular Protease Production in SACCHAROMYCOPSIS LIPOLYTICA. Genetics. 1977 Dec;87(4):621–632. doi: 10.1093/genetics/87.4.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogrydziak D. M., Scharf S. J. Alkaline extracellular protease produced by Saccharomycopsis lipolytica CX161-1B. J Gen Microbiol. 1982 Jun;128(6):1225–1234. doi: 10.1099/00221287-128-6-1225. [DOI] [PubMed] [Google Scholar]
- Orr-Weaver T. L., Szostak J. W., Rothstein R. J. Yeast transformation: a model system for the study of recombination. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6354–6358. doi: 10.1073/pnas.78.10.6354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker R. C., Watson R. M., Vinograd J. Mapping of closed circular DNAs by cleavage with restriction endonucleases and calibration by agarose gel electrophoresis. Proc Natl Acad Sci U S A. 1977 Mar;74(3):851–855. doi: 10.1073/pnas.74.3.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman D., Halvorson H. O. A putative signal peptidase recognition site and sequence in eukaryotic and prokaryotic signal peptides. J Mol Biol. 1983 Jun 25;167(2):391–409. doi: 10.1016/s0022-2836(83)80341-6. [DOI] [PubMed] [Google Scholar]
- Rholam M., Nicolas P., Cohen P. Precursors for peptide hormones share common secondary structures forming features at the proteolytic processing sites. FEBS Lett. 1986 Oct 20;207(1):1–6. doi: 10.1016/0014-5793(86)80002-3. [DOI] [PubMed] [Google Scholar]
- Russell D. W., Jensen R., Zoller M. J., Burke J., Errede B., Smith M., Herskowitz I. Structure of the Saccharomyces cerevisiae HO gene and analysis of its upstream regulatory region. Mol Cell Biol. 1986 Dec;6(12):4281–4294. doi: 10.1128/mcb.6.12.4281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simms P. C., Ogrydziak D. M. Structural gene for the alkaline extracellular protease of Saccharomycopsis lipolytica. J Bacteriol. 1981 Jan;145(1):404–409. doi: 10.1128/jb.145.1.404-409.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells J. A., Ferrari E., Henner D. J., Estell D. A., Chen E. Y. Cloning, sequencing, and secretion of Bacillus amyloliquefaciens subtilisin in Bacillus subtilis. Nucleic Acids Res. 1983 Nov 25;11(22):7911–7925. doi: 10.1093/nar/11.22.7911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T., Ogrydziak D. M. Extracellular acid proteases produced by Saccharomycopsis lipolytica. J Bacteriol. 1983 Apr;154(1):23–31. doi: 10.1128/jb.154.1.23-31.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaret K. S., Sherman F. DNA sequence required for efficient transcription termination in yeast. Cell. 1982 Mar;28(3):563–573. doi: 10.1016/0092-8674(82)90211-2. [DOI] [PubMed] [Google Scholar]



