Abstract
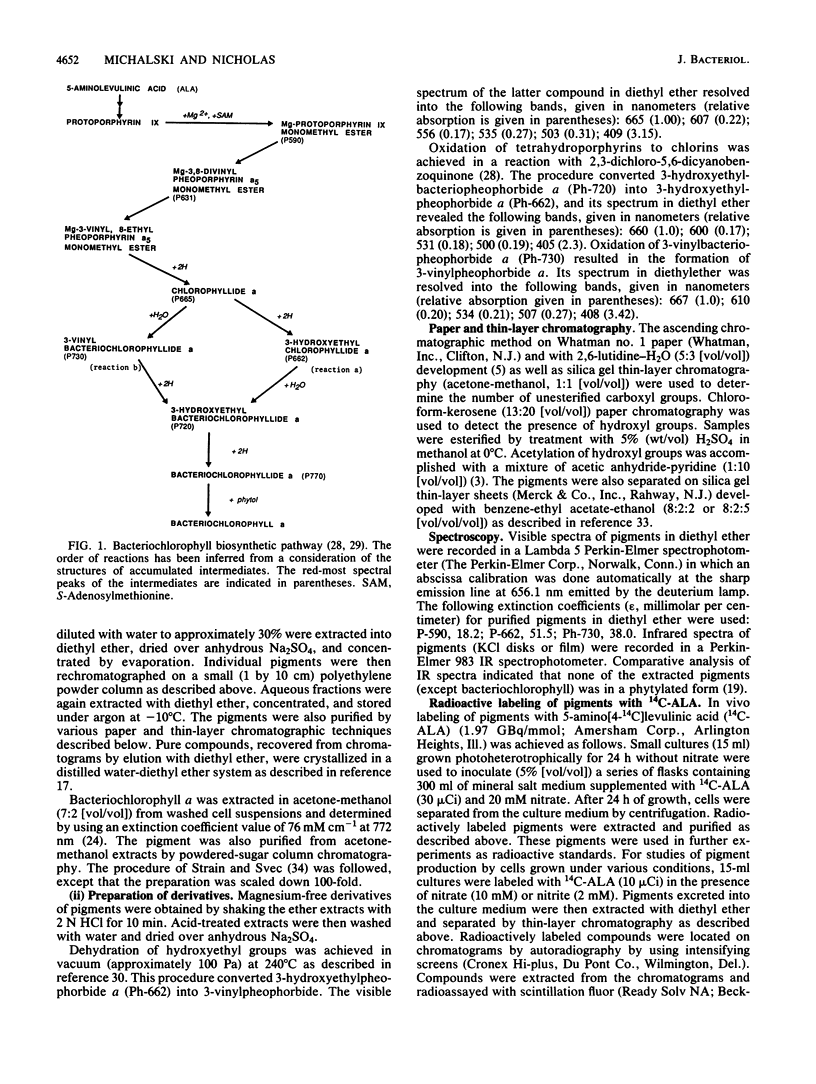
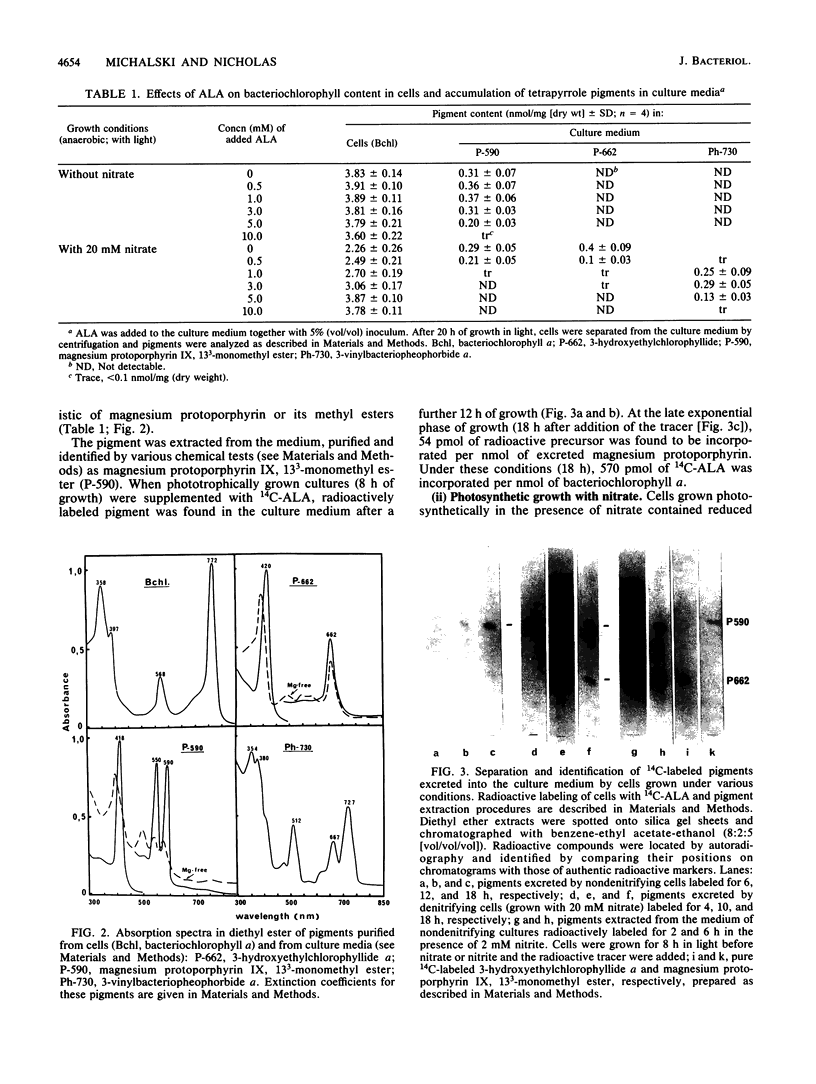
The inclusion of nitrate or nitrite in cultures of Rhodobacter spaeroides subsp. denitrificans grown heterotrophically in light depressed the formation of bacteriochlorophyll a. The pigment biosynthesis was inhibited at the stage of the reduction of chlorophyllide (chlorin) to bacteriochlorophyllide (tetrahydroporphyrin) since 3-hydroxyethylchlorophyllide a accumulated in the culture medium. The addition of exogenous 5-aminolevulinic acid to these cultures resulted in a complete restoration of bacteriochlorophyll synthesis accompanied by the accumulation of 3-vinylbacteriopheophorbide. This indicates that under these conditions bacteriochlorophyll was formed via an alternative route, in which the reduction of chlorins to tetrahydroporphyrins precedes modifications of the C-3 side chain. The multiple forms of 5-aminolevulinic acid synthase were purified from cells grown with and without nitrate. Antibodies against these proteins were raised in rabbits and used in enzyme-linked immunosorbent assays for various forms of 5-aminolevulinic acid synthase. In denitrifying cells, the amount and activity of fraction I of the enzyme was reduced by approximately 40 and 30%, respectively. Partly active enzymes from both types of cells were activated by cystine trisulfide.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSON A. F., CALVIN M. An improved method for the separation and purification of chlorophyll a. Nature. 1962 Apr 21;194:285–286. doi: 10.1038/194285a0. [DOI] [PubMed] [Google Scholar]
- BARRETT J. Detection of hydroxyl groups in porphyrins and chlorins. Nature. 1959 Apr 25;183(4669):1185–1186. doi: 10.1038/1831185a0. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- CHU T. C., SISTER A A GREEN, CHU E. J. Paper chromatography of methyl esters of porphyrins. J Biol Chem. 1951 Jun;190(2):643–646. [PubMed] [Google Scholar]
- Cleland W. W. The statistical analysis of enzyme kinetic data. Adv Enzymol Relat Areas Mol Biol. 1967;29:1–32. doi: 10.1002/9780470122747.ch1. [DOI] [PubMed] [Google Scholar]
- Connelly J. L., Jones O. T., Saunders V. A., Yates D. W. Kinetic and thermodynamic properties of membrane-bound cytochromes of aerobically and photosynthetically grown Rhodopseudomonas spheroides. Biochim Biophys Acta. 1973 Apr 5;292(3):644–653. doi: 10.1016/0005-2728(73)90012-1. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Fletcher J. C., Robson A. The occurrence of bis-(2-amino-2-carboxyethyl) trisulphide in hydrolysates of wool and other proteins. Biochem J. 1963 Jun;87(3):553–559. doi: 10.1042/bj0870553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallenbeck P. C., Meyer C. M., Vignais P. M. Regulation of nitrogenase in the photosynthetic bacterium Rhodopseudomonas capsulata as studied by two-dimensional gel electrophoresis. J Bacteriol. 1982 Sep;151(3):1612–1616. doi: 10.1128/jb.151.3.1612-1616.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harboe N., Ingild A. Immunization, isolation of immunoglobulins, estimation of antibody titre. Scand J Immunol Suppl. 1973;1:161–164. doi: 10.1111/j.1365-3083.1973.tb03798.x. [DOI] [PubMed] [Google Scholar]
- Hayasaka S., Tuboi S. Control of delta-aminolevulinate synthetase activity in Rhodopseudomonas spheroides. III. Partial purification of the fraction I activating enzyme and the occurrence of two forms of fraction II. J Biochem. 1974 Jul;76(1):157–168. doi: 10.1093/oxfordjournals.jbchem.a130541. [DOI] [PubMed] [Google Scholar]
- Inoue I., Oyama H., Tuboi S. On the nature of the activating enzyme of the inactive form of delta-aminolevulinate synthetase in Rhodopseudomonas spheroides. J Biochem. 1979 Aug;86(2):477–482. doi: 10.1093/oxfordjournals.jbchem.a132547. [DOI] [PubMed] [Google Scholar]
- JACOBS E. E., VATTER A. E., HOLT A. S. Crystalline chlorophyll and bacteriochlorophyll. Arch Biochem Biophys. 1954 Nov;53(1):228–238. doi: 10.1016/0003-9861(54)90248-9. [DOI] [PubMed] [Google Scholar]
- NASON A., EVANS H. J. Triphosphopyridine nucleotide-nitrate reductase in Neurospora. J Biol Chem. 1953 Jun;202(2):655–673. [PubMed] [Google Scholar]
- Nandi D. L., Shemin D. Quaternary structure of delta-aminolevulinic acid synthase from Rhodopseudomonas spheroides. J Biol Chem. 1977 Apr 10;252(7):2278–2280. [PubMed] [Google Scholar]
- Pudek M. R., Richards W. R. A possible alternate pathway of bacteriochlorophyll biosynthesis in a mutant of Rhodopseudomonas sphaeroides. Biochemistry. 1975 Jul 15;14(14):3132–3137. doi: 10.1021/bi00685a015. [DOI] [PubMed] [Google Scholar]
- Richards W. R., Lascelles J. The biosynthesis of bacteriochlorophyll. The characterization of latter stage intermediates from mutants of Rhodopseudomonas spheroides. Biochemistry. 1969 Aug;8(8):3473–3482. doi: 10.1021/bi00836a051. [DOI] [PubMed] [Google Scholar]
- Satoh T. Light-activated, -inhibited and -independent denitrification by a denitrifying phototrophic bacterium. Arch Microbiol. 1977 Dec 15;115(3):293–298. doi: 10.1007/BF00446455. [DOI] [PubMed] [Google Scholar]
- Seliskar C. J. Separation of phytylated and non-phytylated chlorophylls by thin-layer chromatography. Anal Biochem. 1966 Oct;17(1):174–177. doi: 10.1016/0003-2697(66)90021-2. [DOI] [PubMed] [Google Scholar]
- Tuboi S., Hayasaka S. Control of -aminolevulinate synthetase activity in Rhodopseudomonas spheroides. II. Requirement of a disulfide compound for the conversion of the inactive form of fraction I to the active form. Arch Biochem Biophys. 1972 Jun;150(2):690–697. doi: 10.1016/0003-9861(72)90087-2. [DOI] [PubMed] [Google Scholar]
- Tuboi S., Kim H. J., Kikuchi G. Occurrence and properes of two types of delta-aminolevulinate synthetase in Rhodopseudomonas spheroides. Arch Biochem Biophys. 1970 May;138(1):147–154. doi: 10.1016/0003-9861(70)90293-6. [DOI] [PubMed] [Google Scholar]
- Wider de Xifra E. A., Sandy J. D., Davies R. C., Neuberger A. Control of 5-aminolaevulinate synthetase activity in Rhodopseudomonas spheroides. Philos Trans R Soc Lond B Biol Sci. 1976 Feb 5;273(924):79–98. doi: 10.1098/rstb.1976.0002. [DOI] [PubMed] [Google Scholar]