Abstract
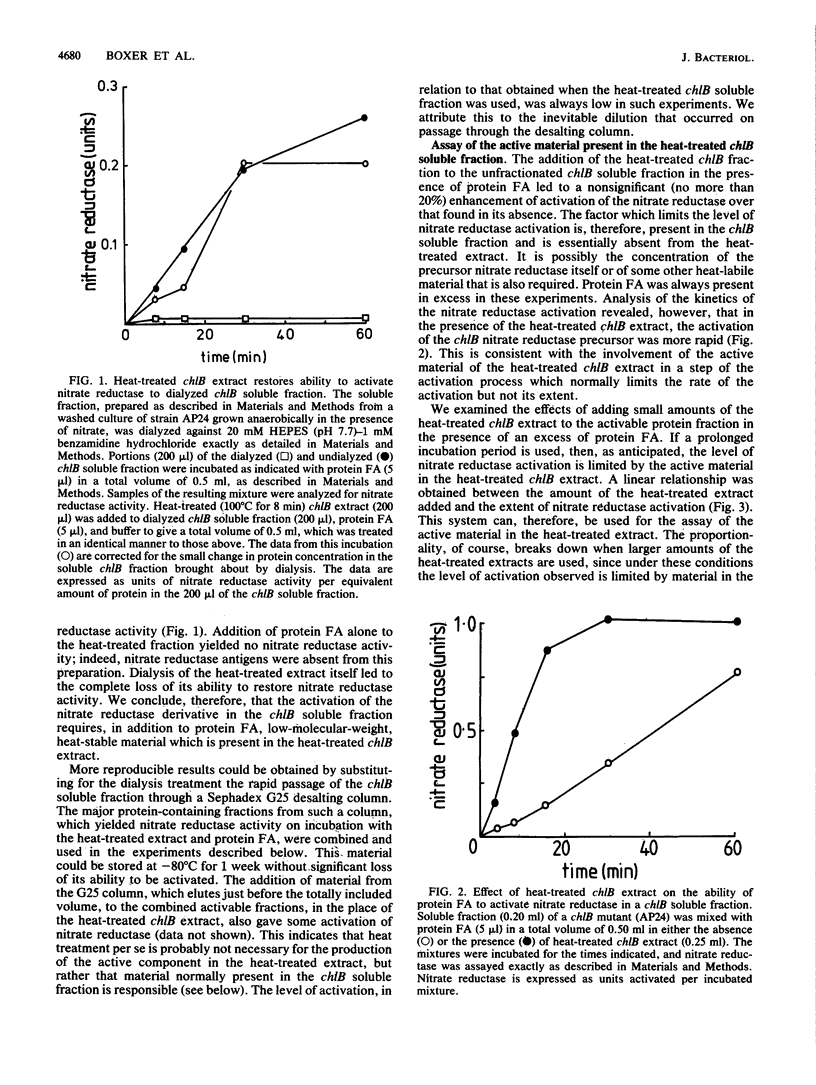
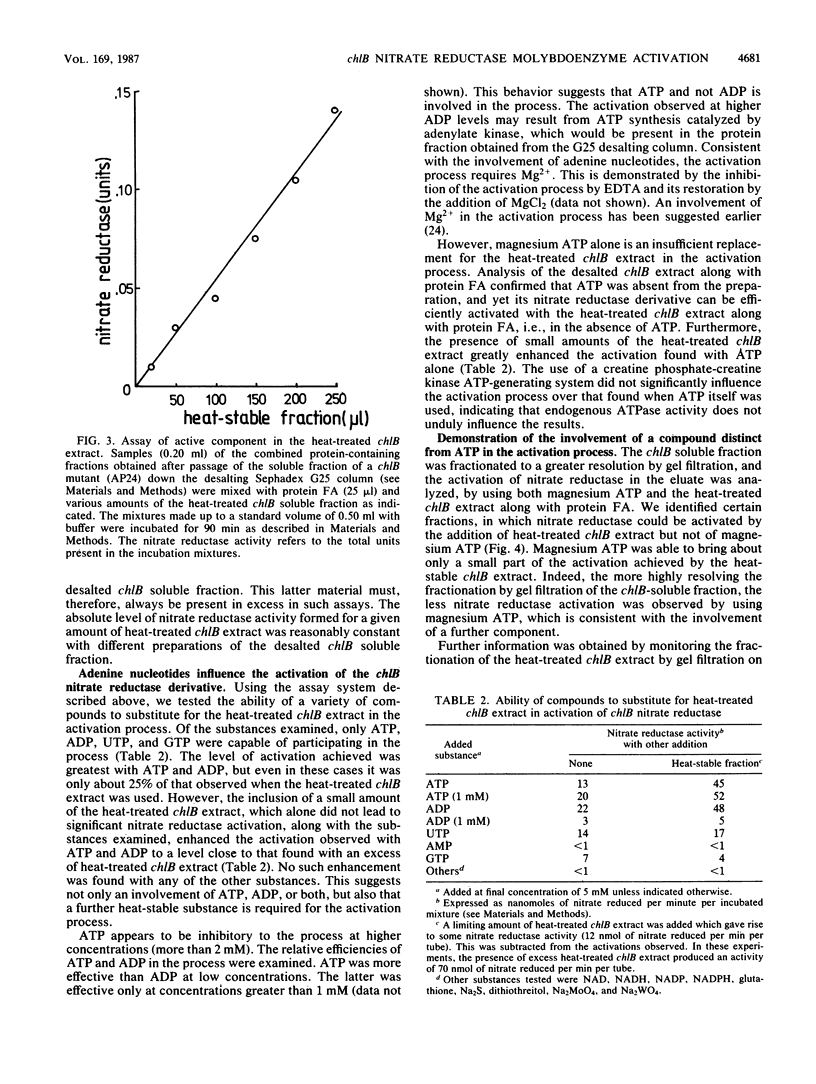
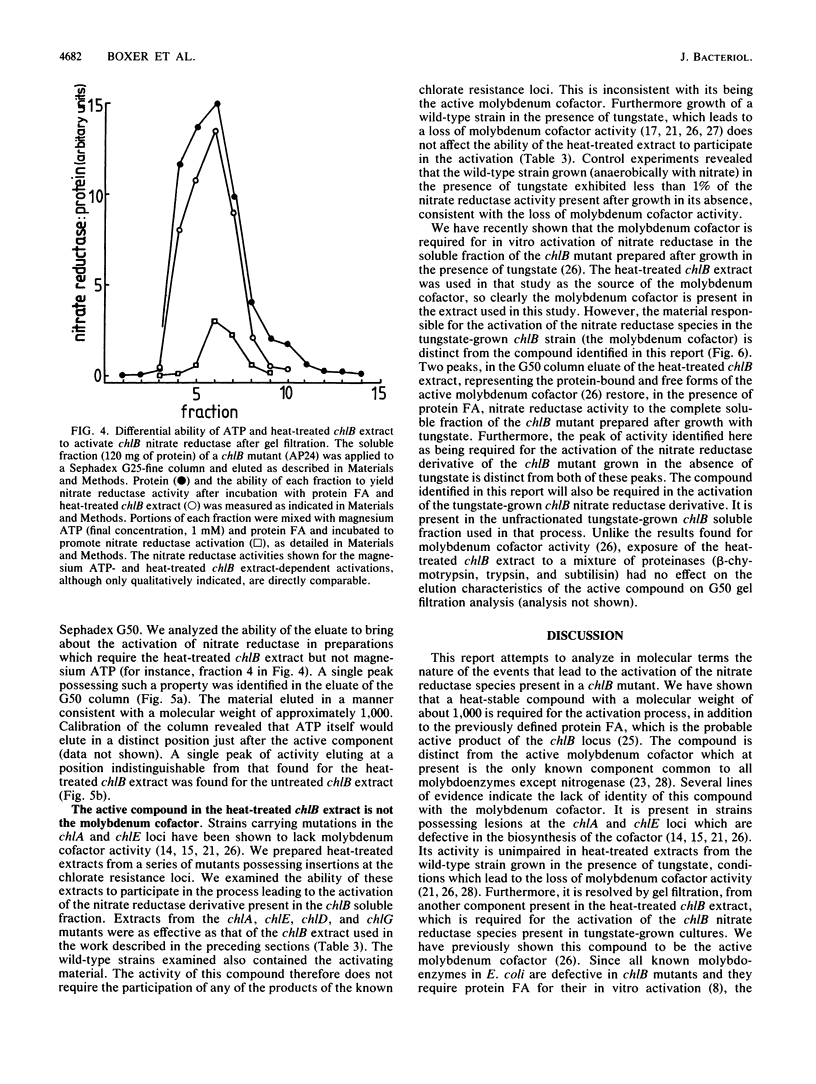
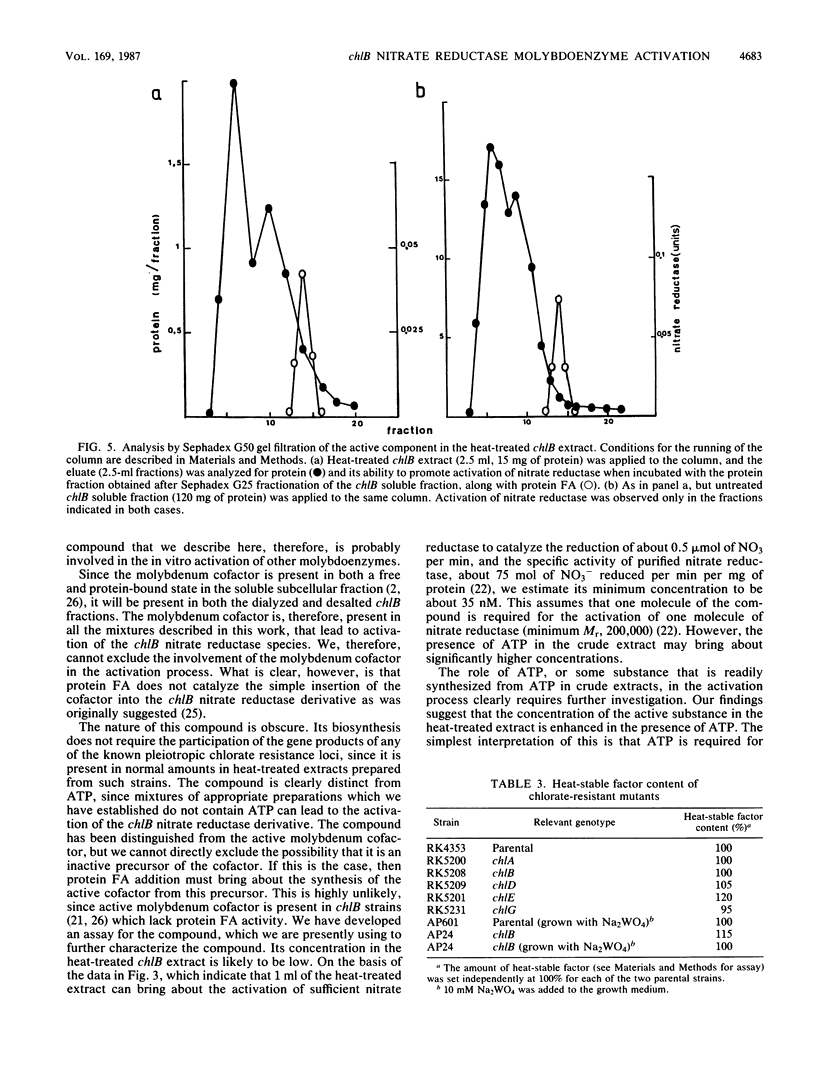
The soluble subcellular fraction of a chlB mutant contains an inactive precursor form of the molybdoenzyme nitrate reductase, which can be activated by the addition to the soluble fraction of protein FA, which is thought to be the active product of the chlB locus. Dialysis or desalting of the chlB soluble fraction leads to the loss of nitrate reductase activation, indicating that some low-molecular-weight material is required for the activation. The protein FA-dependent activation of nitrate reductase can be restored to the desalted chlB soluble fraction by the addition of a clarified extract obtained after heating the chlB soluble fraction at 100 degrees C for 8 min. The heat-stable substance present in this preparation has a molecular weight of approximately 1,000. This substance is distinct from the active molybdenum cofactor since its activity is unimpaired in heat-treated extracts prepared from the organism grown in the presence of tungstate, which leads to loss of cofactor activity. Mutations at the chlA or chlE locus, which are required for molybdenum cofactor biosynthesis, similarly do not affect the activity of the heat-treated extract in the in vitro activation process. Moreover, the active material can be separated from the molybdenum cofactor activity by gel filtration. None of the other known pleiotropic chlorate resistance loci (chlD, chlG) are required for the expression of its activity. Magnesium ATP appears to have a role in the formation of the active substance. We conclude that a low-molecular-weight substance, distinct from the active molybdenum cofactor, is required to bestow activity on the molybdoenzyme nitrate reductase during its biosynthesis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amy N. K. Identification of the molybdenum cofactor in chlorate-resistant mutants of Escherichia coli. J Bacteriol. 1981 Oct;148(1):274–282. doi: 10.1128/jb.148.1.274-282.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amy N. K., Rajagopalan K. V. Characterization of molybdenum cofactor from Escherichia coli. J Bacteriol. 1979 Oct;140(1):114–124. doi: 10.1128/jb.140.1.114-124.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azoulay E., Puig J. Reconstitution of enzymatically active particles from inactive soluble elements in Escherichia coli K 12. Biochem Biophys Res Commun. 1968 Dec 30;33(6):1019–1024. doi: 10.1016/0006-291x(68)90415-4. [DOI] [PubMed] [Google Scholar]
- Chaudhry G. R., MacGregor C. H. Escherichia coli nitrate reductase subunit A: its role as the catalytic site and evidence for its modification. J Bacteriol. 1983 Apr;154(1):387–394. doi: 10.1128/jb.154.1.387-394.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George G. N., Bray R. C., Morpeth F. F., Boxer D. H. Complexes with halide and other anions of the molybdenum centre of nitrate reductase from Escherichia coli. Biochem J. 1985 May 1;227(3):925–931. doi: 10.1042/bj2270925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano G., Graham A., Boxer D. H., Haddock B. A., Azoulay E. Characterization of the membrane-bound nitrate reductase activity of aerobically grown chlorate-sensitive mutants of Escherichia coli K12. FEBS Lett. 1978 Nov 15;95(2):290–294. doi: 10.1016/0014-5793(78)81013-8. [DOI] [PubMed] [Google Scholar]
- Giordano G., Grillet L., Pommier J., Terriere C., Haddock B. A., Azoulay E. Precursor forms of the subunits of nitrate reductase in chlA and chlB mutants of Escherichia coli K12. Eur J Biochem. 1980 Apr;105(2):297–306. doi: 10.1111/j.1432-1033.1980.tb04501.x. [DOI] [PubMed] [Google Scholar]
- Giordano G., Violet M., Medani C. L., Pommier J. A common pathway for the activation of several molybdoenzymes in Escherichia coli K12. Biochim Biophys Acta. 1984 Apr 10;798(2):216–225. doi: 10.1016/0304-4165(84)90307-6. [DOI] [PubMed] [Google Scholar]
- Glaser J. H., DeMoss J. A. Comparison of nitrate reductase mutants of Escherichia coli selected by alternative procedures. Mol Gen Genet. 1972;116(1):1–10. doi: 10.1007/BF00334254. [DOI] [PubMed] [Google Scholar]
- Graham A., Boxer D. H. Arrangement of respiratory nitrate reductase in the cytoplasmic membrane of Escherichia coli. Location of beta subunit. FEBS Lett. 1980 Apr 21;113(1):15–20. doi: 10.1016/0014-5793(80)80484-4. [DOI] [PubMed] [Google Scholar]
- Grillet L., Giordano G. Identification and purification of a protein involved in the activation of nitrate reductase in the soluble fraction of a chlA mutant of Escherichia coli K12. Biochim Biophys Acta. 1983 Nov 28;749(1):115–124. doi: 10.1016/0167-4838(83)90158-9. [DOI] [PubMed] [Google Scholar]
- Johann S., Hinton S. M. Cloning and nucleotide sequence of the chlD locus. J Bacteriol. 1987 May;169(5):1911–1916. doi: 10.1128/jb.169.5.1911-1916.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M. E., Rajagopalan K. V. In vitro system for molybdopterin biosynthesis. J Bacteriol. 1987 Jan;169(1):110–116. doi: 10.1128/jb.169.1.110-116.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M. E., Rajagopalan K. V. Involvement of chlA, E, M, and N loci in Escherichia coli molybdopterin biosynthesis. J Bacteriol. 1987 Jan;169(1):117–125. doi: 10.1128/jb.169.1.117-125.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. W., Garland P. B. Sites and specificity of the reaction of bipyridylium compounds with anaerobic respiratory enzymes of Escherichia coli. Effects of permeability barriers imposed by the cytoplasmic membrane. Biochem J. 1977 Apr 15;164(1):199–211. doi: 10.1042/bj1640199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lester R. L., DeMoss J. A. Effects of molybdate and selenite on formate and nitrate metabolism in Escherichia coli. J Bacteriol. 1971 Mar;105(3):1006–1014. doi: 10.1128/jb.105.3.1006-1014.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGregor C. H., Schnaitman C. A. Reconstitution of nitrate reductase activity and formation of membrane particles from cytoplasmic extracts of chlorate-resistant mutants of Escherichia coli. J Bacteriol. 1973 Jun;114(3):1164–1176. doi: 10.1128/jb.114.3.1164-1176.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGregor C. H. Synthesis of nitrate reductase components in chlorate-resistant mutants of Escherichia coli. J Bacteriol. 1975 Mar;121(3):1117–1121. doi: 10.1128/jb.121.3.1117-1121.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. B., Amy N. K. Molybdenum cofactor in chlorate-resistant and nitrate reductase-deficient insertion mutants of Escherichia coli. J Bacteriol. 1983 Aug;155(2):793–801. doi: 10.1128/jb.155.2.793-801.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morpeth F. F., Boxer D. H. Kinetic analysis of respiratory nitrate reductase from Escherichia coli K12. Biochemistry. 1985 Jan 1;24(1):40–46. doi: 10.1021/bi00322a007. [DOI] [PubMed] [Google Scholar]
- Pienkos P. T., Shah V. K., Brill W. J. Molybdenum cofactors from molybdoenzymes and in vitro reconstitution of nitrogenase and nitrate reductase. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5468–5471. doi: 10.1073/pnas.74.12.5468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riviere C., Azoulay E. Reconstitution in vitro of membranous particles by complementation between extracts of chl-r mutants in Escherichia coli K12. Biochem Biophys Res Commun. 1971 Dec 17;45(6):1608–1614. doi: 10.1016/0006-291x(71)90205-1. [DOI] [PubMed] [Google Scholar]
- Riviere C., Giordano G., Pommier J., Azoulay E. Membrane reconstitution in chl-r mutants of Escherichia coli K 12. VIII. Purification and properties of the FA factor, the product of the chl B gene. Biochim Biophys Acta. 1975 May 6;389(2):219–235. doi: 10.1016/0005-2736(75)90317-x. [DOI] [PubMed] [Google Scholar]
- Saracino L., Violet M., Boxer D. H., Giordano G. Activation in vitro of respiratory nitrate reductase of Escherichia coli K12 grown in the presence of tungstate. Involvement of molybdenum cofactor. Eur J Biochem. 1986 Aug 1;158(3):483–490. doi: 10.1111/j.1432-1033.1986.tb09780.x. [DOI] [PubMed] [Google Scholar]
- Scott R. H., Sperl G. T., DeMoss J. A. In vitro incorporation of molybdate into demolybdoproteins in Escherichia coli. J Bacteriol. 1979 Feb;137(2):719–726. doi: 10.1128/jb.137.2.719-726.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah V. K., Brill W. J. Isolation of an iron-molybdenum cofactor from nitrogenase. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3249–3253. doi: 10.1073/pnas.74.8.3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart V., MacGregor C. H. Nitrate reductase in Escherichia coli K-12: involvement of chlC, chlE, and chlG loci. J Bacteriol. 1982 Aug;151(2):788–799. doi: 10.1128/jb.151.2.788-799.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]



