Abstract
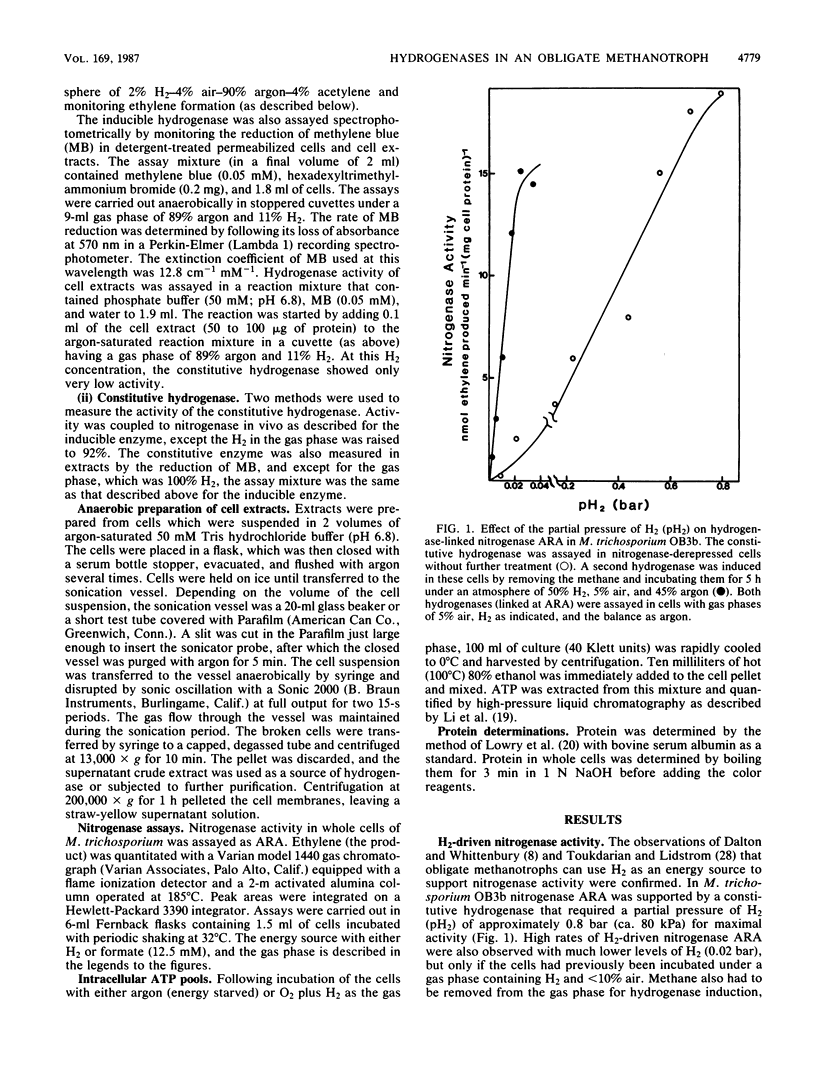
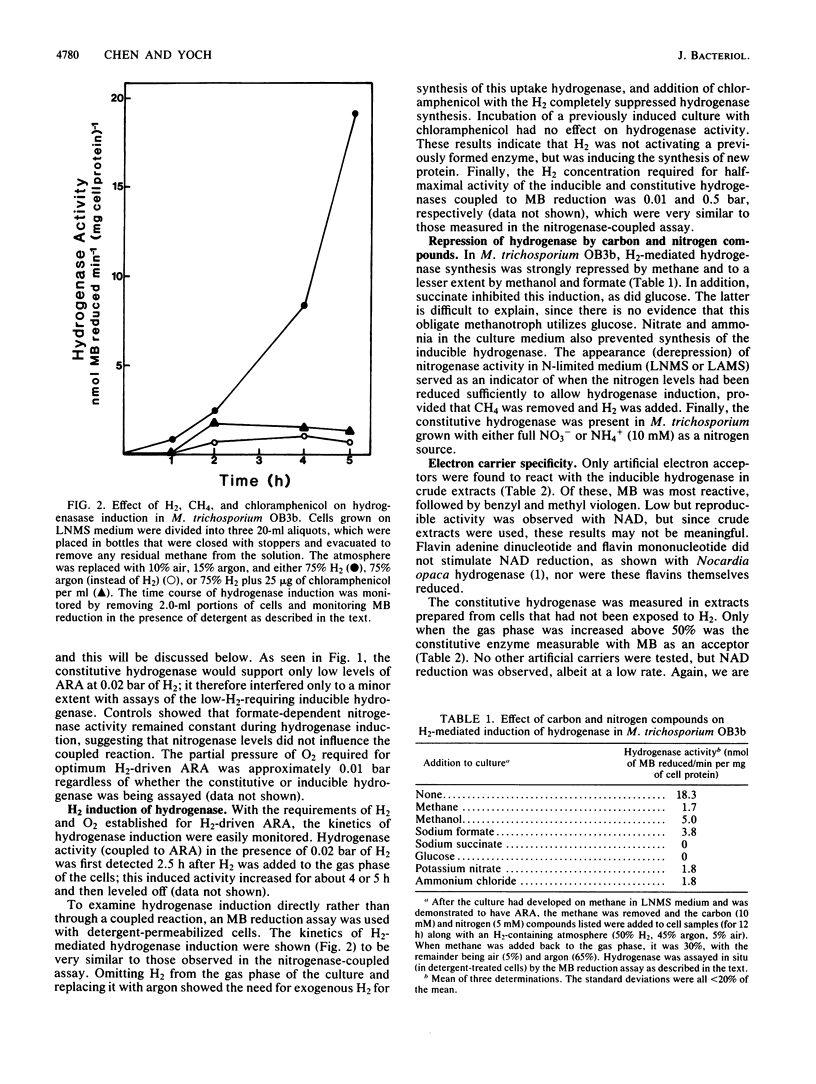
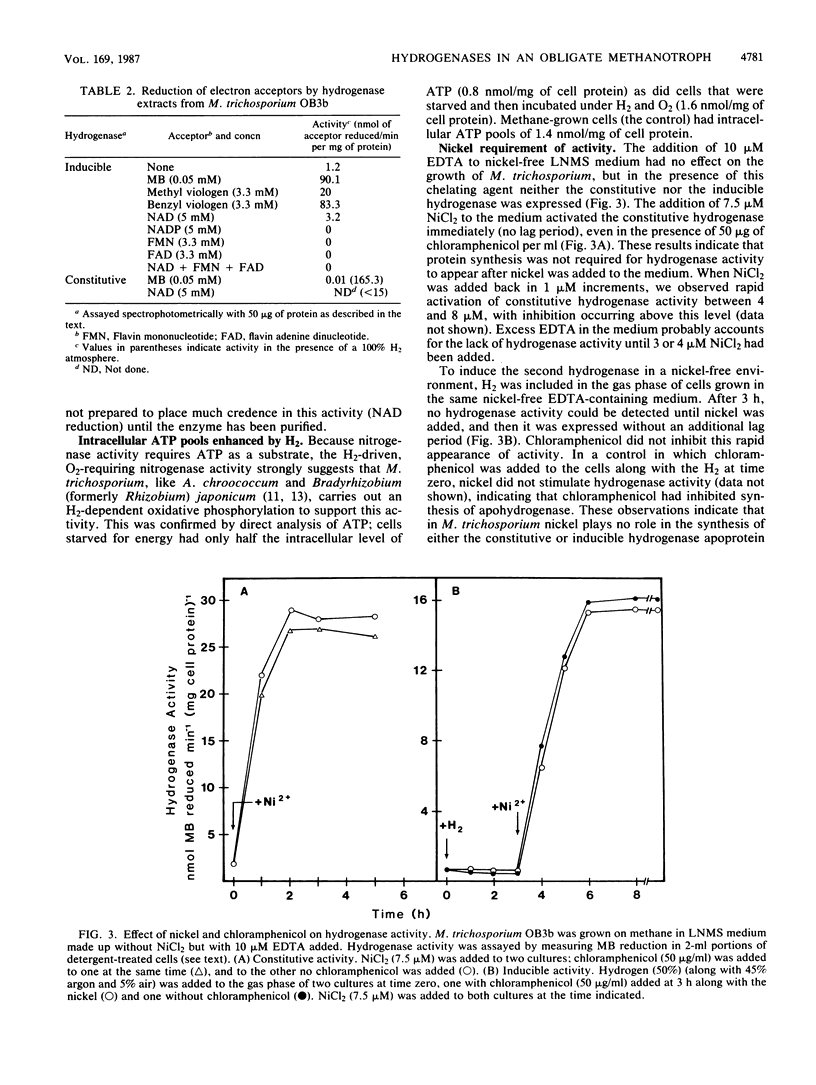
Two uptake hydrogenases were found in the obligate methanotroph Methylosinus trichosporium OB3b; one was constitutive, and a second was induced by H2. Both hydrogenases could be assayed by measuring methylene blue reduction anaerobically or by coupling their activity to nitrogenase acetylene reduction activity in vivo in an O2-dependent reaction. The H2 concentration for half-maximal activity of the inducible and constitutive hydrogenases in both assays was 0.01 and 0.5 bar (1 and 50 kPa), respectively, making it easy to distinguish these enzymes from one another both in vivo and in vitro. Hydrogen uptake was shown to be coupled to ATP synthesis in methane-starved cells. Methane, methanol, formate, succinate, and glucose all repressed the H2-mediated synthesis of the inducible hydrogenase. Furthermore, this enzyme was only expressed in N-starved cultures and was repressed by NH4+ and NO3-; synthesis of the constitutive hydrogenase was not affected by excess N in the growth medium. In nickel-free, EDTA-containing medium, the activities of these two enzymes were negligible; however, both enzyme activities appeared rapidly following the addition of nickel to the culture. Chloramphenicol, when added along with nickel, had no effect on the rapid appearance of either the constitutive or inducible activity, indicating that nickel is not required for synthesis of the hydrogenase apoproteins. These observations all suggest that these hydrogenases are nickel-containing enzymes. Finally, both hydrogenases were soluble and could be fractionated by 20% ammonium sulfate; the constitutive enzyme remained in the supernatant solution, while the inducible enzyme was precipitated under these conditions.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aggag M., Schlegel H. G. Studies on a gram-positive hydrogen bacterium, Nocardia opaca 1 b. III. Purification, stability and some properties of the soluble hydrogen dehydrogenase. Arch Microbiol. 1974;100(1):25–39. doi: 10.1007/BF00446303. [DOI] [PubMed] [Google Scholar]
- Bont J. A. Hydrogenase activity in nitrogen-fixing methane-oxidizing bacteria. Antonie Van Leeuwenhoek. 1976;42(3):255–259. doi: 10.1007/BF00394122. [DOI] [PubMed] [Google Scholar]
- Bothe H., Tennigkeit J., Eisbrenner G. The utilization of molecular hydrogen by the blue-green alga Anabaena cylindrica. Arch Microbiol. 1977 Jul 26;114(1):43–49. doi: 10.1007/BF00429628. [DOI] [PubMed] [Google Scholar]
- Colbeau A., Kelley B. C., Vignais P. M. Hydrogenase activity in Rhodopseudomonas capsulata: relationship with nitrogenase activity. J Bacteriol. 1980 Oct;144(1):141–148. doi: 10.1128/jb.144.1.141-148.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon R. O. Hydrogenase in legume root nodule bacteroids: occurrence and properties. Arch Mikrobiol. 1972;85(3):193–201. doi: 10.1007/BF00408844. [DOI] [PubMed] [Google Scholar]
- Emerich D. W., Ruiz-Argüeso T., Ching T. M., Evans H. J. Hydrogen-dependent nitrogenase activity and ATP formation in Rhizobium japonicum bacteroids. J Bacteriol. 1979 Jan;137(1):153–160. doi: 10.1128/jb.137.1.153-160.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich B., Heine E., Finck A., Friedrich C. G. Nickel requirement for active hydrogenase formation in Alcaligenes eutrophus. J Bacteriol. 1981 Mar;145(3):1144–1149. doi: 10.1128/jb.145.3.1144-1149.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich C. G. Depression of hydrogenase during limitation of electron donors and derepression of ribulosebisphosphate carboxylase during carbon limitation of Alcaligenes eutrophus. J Bacteriol. 1982 Jan;149(1):203–210. doi: 10.1128/jb.149.1.203-210.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausinger R. P. Nickel utilization by microorganisms. Microbiol Rev. 1987 Mar;51(1):22–42. doi: 10.1128/mr.51.1.22-42.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klucas R. V., Hanus F. J., Russell S. A., Evans H. J. Nickel: A micronutrient element for hydrogen-dependent growth of Rhizobium japonicum and for expression of urease activity in soybean leaves. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2253–2257. doi: 10.1073/pnas.80.8.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Li J. D., Hu C. Z., Yoch D. C. Changes in amino acid and nucleotide pools of Rhodospirillum rubrum during switch-off of nitrogenase activity initiated by NH4+ or darkness. J Bacteriol. 1987 Jan;169(1):231–237. doi: 10.1128/jb.169.1.231-237.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier R. J., Hanus F. J., Evans H. J. Regulation of hydrogenase in Rhizobium japonicum. J Bacteriol. 1979 Feb;137(2):825–829. doi: 10.1128/jb.137.2.825-829.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge C. D., Yates M. G. Effect of chelating agents on hydrogenase in Azotobacter chroococcum. Evidence that nickel is required for hydrogenase synthesis. Biochem J. 1982 Apr 15;204(1):339–344. doi: 10.1042/bj2040339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siefert E., Pfennig N. Hydrogen metabolism and nitrogen fixation in wild type and Nif- mutants of Rhodopseudomonas acidophila. Biochimie. 1978;60(3):261–265. doi: 10.1016/s0300-9084(78)80822-0. [DOI] [PubMed] [Google Scholar]
- Stults L. W., O'Hara E. B., Maier R. J. Nickel is a component of hydrogenase in Rhizobium japonicum. J Bacteriol. 1984 Jul;159(1):153–158. doi: 10.1128/jb.159.1.153-158.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toukdarian A. E., Lidstrom M. E. Nitrogen metabolism in a new obligate methanotroph, 'Methylosinus' strain 6. J Gen Microbiol. 1984 Jul;130(7):1827–1837. doi: 10.1099/00221287-130-7-1827. [DOI] [PubMed] [Google Scholar]
- Vignais P. M., Colbeau A., Willison J. C., Jouanneau Y. Hydrogenase, nitrogenase, and hydrogen metabolism in the photosynthetic bacteria. Adv Microb Physiol. 1985;26:155–234. doi: 10.1016/s0065-2911(08)60397-5. [DOI] [PubMed] [Google Scholar]
- Walker C. C., Yates M. G. The hydrogen cycle in nitrogen-fixing Azotobacter chroococcum. Biochimie. 1978;60(3):225–231. doi: 10.1016/s0300-9084(78)80818-9. [DOI] [PubMed] [Google Scholar]



