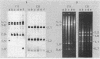
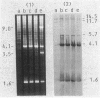
Abstract
Streptomyces lividans 66 exhibits genetic instability, involving sequential loss of resistance to chloramphenicol (Cams) and subsequent mutation of argG. Associated with this instability is the amplification of a 5.7-kilobase (kb) amplified DNA sequence (ADS). We have characterized a second, independent pathway of genetic instability, involving sequential loss of resistance to tetracycline (Tets) followed by mutation in nitrogen assimilation (Ntr). We detected DNA amplification in many of these mutant strains, as well as other reiterations coresident with the 5.7-kb ADS in Cams Arg mutants. However, in contrast to the 5.7-kb ADS, none of the novel elements were observed to amplify at high frequency. The mutation of argG is due to a deletion, one endpoint of which is defined by the 5.7-kb ADS. This amplification derives from a structure, the tandemly duplicated amplifiable unit of DNA (AUD), present in the wild-type genome. We found that progenitor strains containing just a single-copy AUD failed to reproducibly generate amplification of this element in Cams argG mutants, and DNA deletion endpoints proximal to the element were found to be unspecific. These results suggest that a duplicated AUD structure is required for high-frequency amplification and that this reiteration can subsequently buffer the extent of deletion formation in the relevant chromosomal region.
Full text
PDF

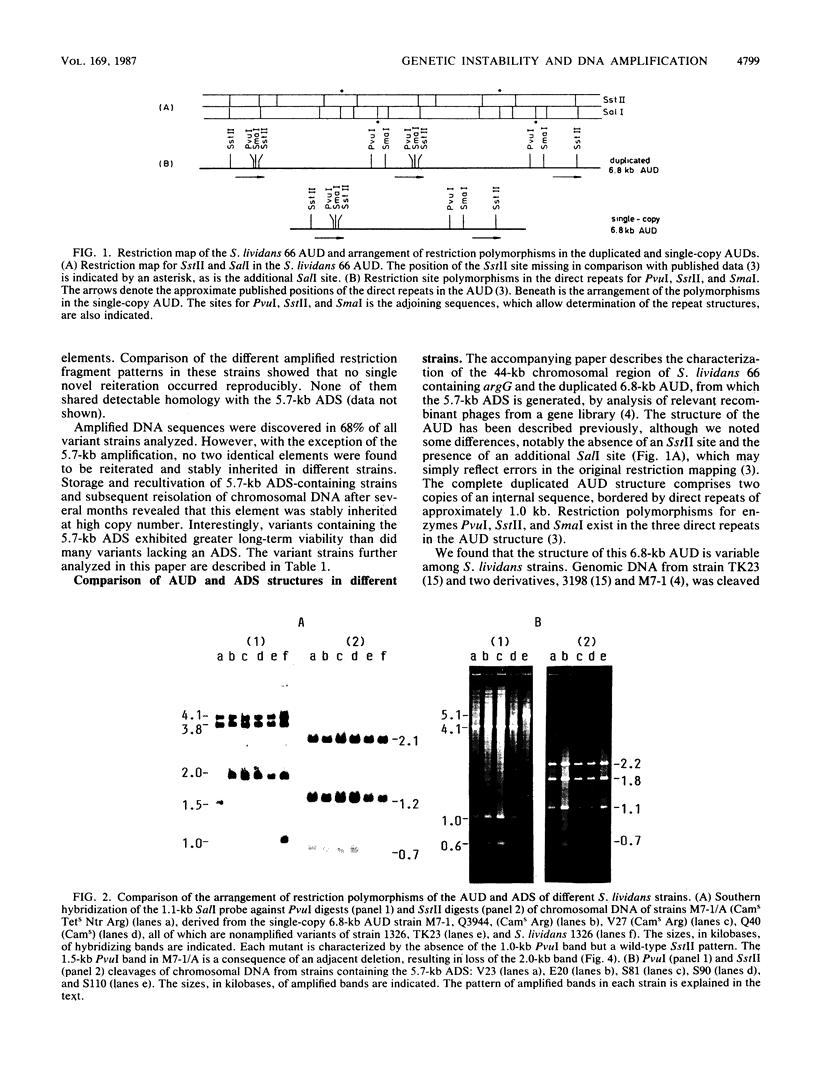




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akagawa H., Okanishi M., Umezawa H. Genetics and biochemical studies of chloramphenicol-nonproducing mutants of Streptomyces venezuelae carrying plasmid. J Antibiot (Tokyo) 1979 Jun;32(6):610–620. doi: 10.7164/antibiotics.32.610. [DOI] [PubMed] [Google Scholar]
- Altenbuchner J., Cullum J. DNA amplification and an unstable arginine gene in Streptomyces lividans 66. Mol Gen Genet. 1984;195(1-2):134–138. doi: 10.1007/BF00332735. [DOI] [PubMed] [Google Scholar]
- Altenbuchner J., Cullum J. Structure of an amplifiable DNA sequence in Streptomyces lividans 66. Mol Gen Genet. 1985;201(2):192–197. doi: 10.1007/BF00425659. [DOI] [PubMed] [Google Scholar]
- Betzler M., Dyson P., Schrempf H. Relationship of an unstable argG gene to a 5.7-kilobase amplifiable DNA sequence in Streptomyces lividans 66. J Bacteriol. 1987 Oct;169(10):4804–4810. doi: 10.1128/jb.169.10.4804-4810.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crameri R., Davies J. E., Hütter R. Plasmid curing and generation of mutations induced with ethidium bromide in streptomycetes. J Gen Microbiol. 1986 Mar;132(3):819–824. doi: 10.1099/00221287-132-3-819. [DOI] [PubMed] [Google Scholar]
- Edlund T., Normark S. Recombination between short DNA homologies causes tandem duplication. Nature. 1981 Jul 16;292(5820):269–271. doi: 10.1038/292269a0. [DOI] [PubMed] [Google Scholar]
- Fedorenko V. A., Danilenko V. N. Nestabil'nost' prirodnoi mnozhestvennoi lekarstvennoi ustoichivosti u aktinomitsetov. Antibiotiki. 1980 Mar;25(3):170–174. [PubMed] [Google Scholar]
- Fishman S. E., Hershberger C. L. Amplified DNA in Streptomyces fradiae. J Bacteriol. 1983 Aug;155(2):459–466. doi: 10.1128/jb.155.2.459-466.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman S. E., Rosteck P. R., Jr, Hershberger C. L. A 2.2-kilobase repeated DNA segment is associated with DNA amplification in Streptomyces fradiae. J Bacteriol. 1985 Jan;161(1):199–206. doi: 10.1128/jb.161.1.199-206.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREGORY K. F., HUANG J. C. TYROSINASE INHERITANCE IN STREPTOMYCES SCABIES. I. GENETIC RECOMBINATION. J Bacteriol. 1964 Jun;87:1281–1286. doi: 10.1128/jb.87.6.1281-1286.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa M., Hintermann G., Simonet J. M., Crameri R., Piret J., Hütter R. Certain chromosomal regions in Streptomyces glaucescens tend to carry amplifications and deletions. Mol Gen Genet. 1985;200(3):375–384. doi: 10.1007/BF00425720. [DOI] [PubMed] [Google Scholar]
- Hashimoto H., Rownd R. H. Transition of the R factor NR1 and Proteus mirabilis: level of drug resistance of nontransitioned and transitioned cells. J Bacteriol. 1975 Jul;123(1):56–68. doi: 10.1128/jb.123.1.56-68.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopwood D. A., Kieser T., Wright H. M., Bibb M. J. Plasmids, recombination and chromosome mapping in Streptomyces lividans 66. J Gen Microbiol. 1983 Jul;129(7):2257–2269. doi: 10.1099/00221287-129-7-2257. [DOI] [PubMed] [Google Scholar]
- Horinouchi S., Kumada Y., Beppu T. Unstable genetic determinant of A-factor biosynthesis in streptomycin-producing organisms: cloning and characterization. J Bacteriol. 1984 May;158(2):481–487. doi: 10.1128/jb.158.2.481-487.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda H., Tanaka H., Omura S. Genetic and biochemical features of spiramycin biosynthesis in Streptomyces ambofaciens--curing, protoplast regeneration and plasmid transfer. J Antibiot (Tokyo) 1982 Apr;35(4):507–516. doi: 10.7164/antibiotics.35.507. [DOI] [PubMed] [Google Scholar]
- Jannière L., Niaudet B., Pierre E., Ehrlich S. D. Stable gene amplification in the chromosome of Bacillus subtilis. Gene. 1985;40(1):47–55. doi: 10.1016/0378-1119(85)90023-x. [DOI] [PubMed] [Google Scholar]
- Langer P. R., Waldrop A. A., Ward D. C. Enzymatic synthesis of biotin-labeled polynucleotides: novel nucleic acid affinity probes. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6633–6637. doi: 10.1073/pnas.78.11.6633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano M. M., Ogawara H. Multiple effects induced by unstable mutation in Streptomyces lavendulae. J Antibiot (Tokyo) 1980 Apr;33(4):420–425. doi: 10.7164/antibiotics.33.420. [DOI] [PubMed] [Google Scholar]
- Peterson B. C., Rownd R. H. Drug resistance gene amplification of plasmid NR1 derivatives with various amounts of resistance determinant DNA. J Bacteriol. 1985 Mar;161(3):1042–1048. doi: 10.1128/jb.161.3.1042-1048.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redshaw P. A., McCann P. A., Pentella M. A., Pogell B. M. Simultaneous loss of multiple differentiated functions in aerial mycelium-negative isolates of streptomycetes. J Bacteriol. 1979 Feb;137(2):891–899. doi: 10.1128/jb.137.2.891-899.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rownd R., Mickel S. Dissociation and reassociation of RTF and r-determinants of the R-factor NR1 in Proteus mirabilis. Nat New Biol. 1971 Nov 10;234(45):40–43. doi: 10.1038/newbio234040a0. [DOI] [PubMed] [Google Scholar]
- Schrempf H. Deletion and amplification of DNA sequences in melanin-negative variants of Streptomyces reticuli. Mol Gen Genet. 1983;189(3):501–505. doi: 10.1007/BF00325917. [DOI] [PubMed] [Google Scholar]
- Sermonti G., Petris A., Micheli M., Lanfaloni L. Chloramphenicol resistance in Streptomyces coelicolor A3(2): possible involvement of a transposable element. Mol Gen Genet. 1978 Aug 4;164(1):99–103. doi: 10.1007/BF00267604. [DOI] [PubMed] [Google Scholar]
- Singer R. H., Ward D. C. Actin gene expression visualized in chicken muscle tissue culture by using in situ hybridization with a biotinated nucleotide analog. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7331–7335. doi: 10.1073/pnas.79.23.7331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. Gel electrophoresis of restriction fragments. Methods Enzymol. 1979;68:152–176. doi: 10.1016/0076-6879(79)68011-4. [DOI] [PubMed] [Google Scholar]
- Stark G. R., Wahl G. M. Gene amplification. Annu Rev Biochem. 1984;53:447–491. doi: 10.1146/annurev.bi.53.070184.002311. [DOI] [PubMed] [Google Scholar]
- Tlsty T. D., Albertini A. M., Miller J. H. Gene amplification in the lac region of E. coli. Cell. 1984 May;37(1):217–224. doi: 10.1016/0092-8674(84)90317-9. [DOI] [PubMed] [Google Scholar]
- Usdin K., Christians K. M., de Wet C. A., Potgieter T. D., Shaw C. B., Kirby R. The loss of a large DNA fragment is associated with an aerial mycelium negative (Amy-) phenotype of Streptomyces cattleya. J Gen Microbiol. 1985 Apr;131(4):979–981. doi: 10.1099/00221287-131-4-979. [DOI] [PubMed] [Google Scholar]