Abstract
Recent studies have established that neurotrophin synthesis and secretion are regulated by activity and that these factors are involved in activity-dependent processes in the nervous system. Neurotrophins also are known to induce increases in intracellular calcium, a trigger for regulated secretion. This finding raises the possibility that neurotrophins themselves may stimulate regulated secretion of neurotrophins. To address this question, we studied the release of neurotrophins from transfected PC12 cells, a widely used model for neuronal secretion and neurotrophin signal transduction. We found that neurotrophins induced the regulated secretion of brain-derived neurotrophic factor, neurotrophin-3 (NT-3), and neurotrophin-4/5. The effect of brain-derived neurotrophic factor on release of NT-3 could be abolished by REX, a p75 blocking antibody, but not by K252a, an inhibitor of neurotrophin tyrosine kinase receptor (Trk) signaling. The nerve growth factor effect on release of NT-3 could be blocked only by simultaneous application of REX and K252a, suggesting that they are mediated by TrkA as well as p75. Our data show that neurotrophins are able to induce the regulated secretion of neurotrophins and suggest a signal-transducing role for both TrkA and p75 in this process. The neurotrophin-induced release of neurotrophins may be relevant for activity-dependent processes such as synaptic plasticity and memory formation.
Nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NT-3), and neurotrophin-4/5 (NT-4/5) comprise the mammalian neurotrophins, a family of structurally related, dimeric proteins that play a critical role in the survival, differentiation, and maintenance of specific neuronal populations in the developing peripheral nervous system (1). Neurotrophins and their receptors also are widely expressed in the central nervous system where they are involved in activity-related processes such as the enhancement of synaptic transmission (2, 3), formation of ocular dominance columns (4–7), or long-term potentiation (8–11). There appears to be a complex interplay between neuronal activity and neurotrophins in that activity increases expression of neurotrophins whereas neurotrophins induce expression of ion channels or modulate their activity posttranslationally (12).
We and others recently have found that neurotrophins undergo not only constitutive but also activity-dependent regulated secretion, therefore suggesting a possible mechanism by which more active synapses might locally and rapidly be rewarded, resulting in an enhancement in synaptic transmission (13–15). In view of these findings it appears important that neurotrophins are sorted not only to dendrites (15, 16) and therefore postsynaptic locations, but also anterogradely along the axon to presynaptic locations (17–19). The release of neurotrophins therefore might take place from the presynaptic as well as from the postsynaptic part of a synapse.
The regulated secretion of neurotrophins has been shown to depend on increases in intracellular calcium concentrations (15, 16). Moreover the interaction of neurotrophins with their receptors activates signal transduction pathways, leading to increases in intracellular calcium concentration (20). This finding suggested that neurotrophins might be able to induce regulated secretion of neurotrophins.
Indeed, the release of NGF and BDNF induced by neurotrophins recently has been found in hippocampal slices and neurons in culture as well as in PC12 cells (21); the process was described as solely depending on neurotrophin-induced activation of neurotrophin tyrosine kinase receptors (Trk) with subsequent mobilization of calcium from cytoplasmic stores. In parallel studies (22), we observed a similar neurotrophin-induced release of BDNF, NT-3, and NT-4/5 in PC12 cells, but noted that in addition to TrkA, p75 is able to mediate this process.
MATERIALS AND METHODS
Cell Culture and Transfection.
The construction and characterization of the plasmids pBJ-5-NT-3, pBJ-5-NT-3 myc, and pBJ-5-BDNF has been described elsewhere (13, 23). pBJ-5-NT-4/5 was constructed by subcloning a EcoRI fragment derived from pKS-NT-4/5 (a gift of G. Yancopoulos, Regeneron Pharmaceuticals, Tarrytown, NY) containing the NT-4/5 cDNA into the pBJ-5 expression vector. PC12 cells were maintained at 5% CO2 in DMEM containing 6% bovine calf serum and 6% horse serum. Transient and stable transfections were performed as previously described (13) by using LipofectAmine (GIBCO/BRL) according to the manufacturer’s recommendations. After selection with G418, NT-3 with a myc-epitope (NT-3 myc) expressing clones were determined by ELISA. Four clones were selected for further analysis (clones 4, 12, 13, and 22). Their phenotype appeared normal, and they showed robust neurite outgrowth in response to NGF but not to BDNF. Cells were kept in culture for not longer than 15 passages.
Antibodies and Reagents.
The REX antibodies directed against extracellular domain of p75 and the RTA antibodies against TrkA were a generous gift of Louis Reichardt (University of California, San Francisco) and were purified by using Protein A columns (Pierce). The polyclonal anti-p75 antibody, polyclonal anti-BDNF antiserum, and anti-chicken horseradish peroxidase (HRP) were purchased from Promega. The PY20 and pan-Trk antibodies were obtained from Santa Cruz Biotechnology. TrkB-Fc receptor bodies, recombinant BDNF, NT-3, NT-4/5, and the polyclonal anti-NT-4/5 antiserum were generous gifts of Regeneron Pharmaceuticals. Recombinant NGF was purchased from Harlan Bioproducts (Indianapolis, IN). All other chemicals and secondary antibodies were purchased from Sigma unless otherwise specified.
ELISAs.
Blocking solution, wash buffer, and tetramethyl-benzidine peroxidase-developing substrate were purchased from Kirkegaard and Perry Laboratories. The BDNF ELISA was performed as follows: ELISA plates (Immobilon, Nunc) were coated with TrkB-Fc receptor body as “capture” layer. The ELISA was developed by using a polyclonal chicken anti-BDNF antiserum and anti-chicken-HRP for detection. We found no crossreactivity with NGF, NT-3, and NT-4/5 at 1 μg/ml. The NT-4/5 ELISA was performed by using a similar strategy. Plates were coated with TrkB-Fc and developed with a polyclonal chicken anti-NT-4/5 antiserum and anti-chicken-HRP.
The two-site NT-3 ELISA was a gift of Regeneron Pharmaceuticals. We observed no crossreactivity with NGF, BDNF, or NT-4/5 at concentrations of 1 μg/ml.
To perform the NT-3 myc ELISA, plates were coated with a polyclonal anti-myc antibody (Santa Cruz Biotechnology) followed by incubation of the sample and detection of NT-3 myc by using a biotinylated monoclonal anti-NT-3 antibody (Regeneron Pharmaceuticals) and Streptavidin-HRP. No crossreactivity with native NT-3 was observed at concentrations up to 0.5 μg/ml. The NT-3 myc protein used as standard in this ELISA was produced as follows: COS-7 cells were transiently transfected with the expression plasmid pBJ-5-NT-3 myc by using the DEAE-dextran/chloroquine method as described (24). After 3 days, conditioned media were taken and cleared by high-speed centrifugation, and the concentration was determined by the above-mentioned NT-3 ELISA. The bioactivity of NT-3 myc and its ability to undergo regulated secretion was equivalent to the wild-type counterpart (23).
Western Blot Analysis.
The Trk-phosphotyrosine assay was performed as previously described (24). Briefly, PC12 cells and PC12 cells stable-transfected with pBJ-5-NT-3 myc were incubated with control media or media containing NGF, BDNF, and NT-3 (100 ng/ml each) for 5 min and immunoprecipitated with anti-panTrk (Santa Cruz Biotechnology). After SDS/PAGE (7.5%) and transfer to nitrocellulose membranes (Schleicher and Schuell), the blot was probed with PY20 antiphosphotyrosine (1:1,000, Santa Cruz Biotechnology) and anti-mouse HRP (1:4,000, Sigma).
Secretion Experiments.
Cells were plated on poly-l-lysine-coated 6-well plates 1 day before the stimulation experiment. Experiments were performed in a modification of the previously described protocol (13). Cells at a density of ≈90% were first rinsed two times with 2 ml of prewarmed “physiological buffer” (125 mM NaCl/5 mM KCl/1.2 mM NaH2PO4/1 mM CaCl2/1.2 mM MgCl2/1 μM ZnCl2/10 mM glucose/25 mM Hepes/0.25% BSA, pH 7.4) (14) and subsequently preincubated for 45 min with physiological buffer. In case of blocking experiments, purified REX was added at 20 μg/ml or K252a at 100 nM (13). After preincubation, supernatants were removed and prewarmed media (0.7 ml) containing stimuli and/or blocking reagents were added. As positive control, high potassium buffer (physiological buffer containing 55 mM KCl and 75 mM NaCl) was used (14). After 30-min incubation at 37°C, supernatants were taken, cleared from debris by centrifugation (5 sec at 14,000 rpm) and subjected to ELISA in triplicate.
RESULTS
Neurotrophins Induce Regulated Secretion of NT-3, NT-4/5, or BDNF in Transiently Transfected PC12 Cells.
To determine whether neurotrophins are capable of inducing the regulated secretion of other neurotrophins, we used PC12 cells transfected with neurotrophin expression plasmids as a model system. This rat pheochromocytoma cell line expresses TrkA as well as the pan-neurotrophin receptor p75 and has been widely used to study neurotrophin signal transduction (20). Furthermore, because these neuroendocrine cells contain vesicle populations that resemble large dense core and synaptic vesicles in neurons, they also have been widely used as a model for neuronal secretion (25). We and others recently found that neurotrophins expressed in PC12 cells undergo regulated secretion in response to depolarization (13, 15) and that they are stored in large dense core vesicles (23).
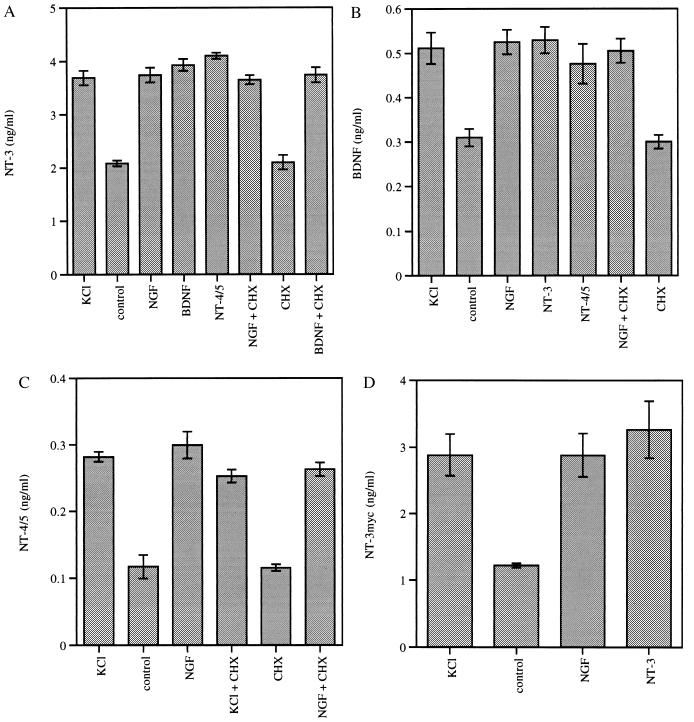
PC12 cells were transiently transfected with the expression plasmid pBJ-5-NT-3, and NT-3 secretion was assayed by ELISA 3 days after transfection as previously described (13). In agreement with previous results (13), depolarization with high potassium buffer stimulated the regulated secretion of NT-3, whereas cycloheximide, an inhibitor of protein synthesis, had no effect (Fig. 1A). NGF, BDNF, and NT-4/5 all stimulated release of NT-3 to a similar extent as high potassium buffer (Fig. 1A).
Figure 1.
Regulated secretion of neurotrophins from PC12 cells transiently transfected with neurotrophin expression plasmids. Three days after transient transfection with neurotrophin expression plasmids using Lipofectamine, PC12 cells were washed twice, preincubated for 45 min in physiological buffer, and subsequently stimulated for 30 min at 37°C with high potassium buffer (KCl), physiological buffer alone (control), or buffer containing the indicated neurotrophin at 100 ng/ml. In some experiments, new protein synthesis was blocked by adding cycloheximide (CHX) during the 45-min preincubation and the 30-min stimulation period. After stimulation, supernatants were removed, cleared by centrifugation, and subjected to neurotrophin-specific ELISA. PC12 cells were transiently transfected with pBJ-5-NT-3 (A), pBJ-5-BDNF (B), pBJ-5-NT-4/5 (C), or pBJ-5-NT-3 myc (D). Experiments were performed in triplicate; error bars indicate ±SD.
To assay BDNF secretion from PC12 cells, a sensitive BDNF ELISA was established by using an approach to circumvent the lack of commercially available monoclonal anti-BDNF antibodies. Instead of coating the ELISA plate with a specific monoclonal anti-BDNF antibody, we used a TrkB-Fc fusion protein (26) as “capture antibody.” In general, this strategy could allow development of ELISAs for other growth factors, for which the high affinity receptors are cloned but for which sensitive or specific mAbs are not available.
When PC12 cells transiently transfected with pBJ-5-BDNF were assayed as described above, NGF, NT-3, and NT-4/5 all were able to induce regulated secretion of BDNF to a similar extent as high potassium buffer (Fig. 1B). Furthermore, we noted that NT-4/5 was able to undergo regulated secretion in transiently transfected PC12 cells and that this process is induced by NGF as well as depolarization (Fig. 1C).
NT-3 Induces Regulated Secretion of NT-3 myc in Transiently Transfected PC12 Cells.
To determine whether one neurotrophin might be able to induce the regulated secretion of the same neurotrophin, we established an ELISA for the measurement of NT-3 myc by using an anti-myc mAb as capture antibody. This ELISA showed no crossreactivity to wild-type NT-3 up to 500 ng/ml (five times higher than the concentration of NT-3 contained in the stimulation media). PC12 cells were transiently transfected with pBJ-5-NT-3 myc and subsequently subjected to secretion experiments. As shown in Fig. 1D, NT-3 indeed stimulated the regulated secretion of NT-3 myc.
The results obtained by transient transfections of PC12 cells with NT-3 expression plasmids were confirmed in four different stably transfected PC12 clones expressing NT-3 myc with different expression levels ranging from 0.1 to 0.7 ng/ml. In each of these lines NGF and BDNF induced the secretion of NT-3 myc to a similar extent as high potassium buffer (data not shown). This finding indicates that our observed effect is independent of the NT-3 myc expression level and argues against a clonal artifact. The highest expressing stable cell line (clone 13) was used in subsequent experiments.
The Neurotrophin-Induced Regulated Secretion of Neurotrophins Can Be Mediated by Both p75 and TrkA.
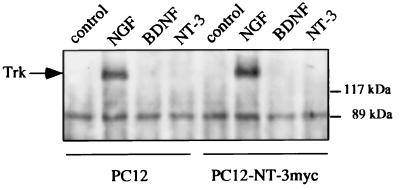
To study the role of the two neurotrophin receptors we first assayed TrkA tyrosine phosphorylation in response to NGF and BDNF (at 100 ng/ml, i.e., the concentration used in secretion experiments) in wild-type or NT-3 myc-expressing stable transfected PC12 cells. As shown in Fig. 2, NGF, but not BDNF or NT-3, induced Trk tyrosine phosphorylation. This finding demonstrates that the BDNF effect is not mediated by Trk tyrosine kinase activation and suggests a possible participation of p75 in this process.
Figure 2.
Western blot analysis of neurotrophin-induced Trk activation. PC12 cells or PC12 cells stably transfected with pBJ-5-NT-3 myc were incubated with DMEM/0.1% BSA (control) or DMEM/0.1% BSA containing NGF, BDNF, or NT-3 (100 ng/ml each) for 5 min, lysed, and subjected to immunoprecipitation by using a polyclonal anti-panTrk antiserum. After SDS/PAGE and transfer to nitrocellulose membranes, Western analysis was performed by using antiphosphotyrosine antibodies. The arrow indicates tyrosine phosphorylated TrkA.
To further examine the roles of TrkA and p75, we used two different well-established approaches. The binding of neurotrophins to p75 can be blocked by REX, a polyclonal antiserum raised against the extracellular domain of this receptor (27). Additionally, tyrosine autophosphorylation of Trks and their downstream signal transduction can be blocked by use of the compound K252a (28–30).
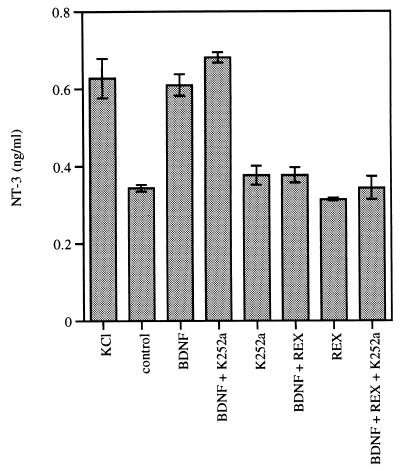
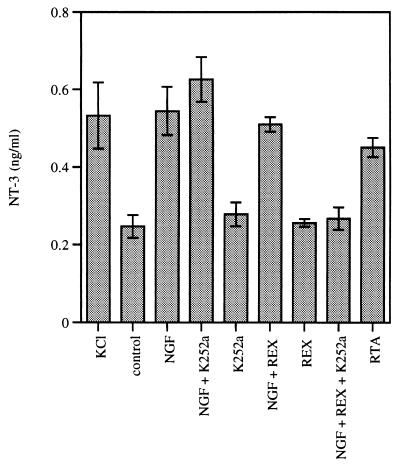
As shown in Fig. 3, the BDNF-induced regulated secretion of NT-3 myc was blocked by addition of REX, but not by K252a, providing additional evidence that the BDNF signal is transduced by p75 and not by TrkA. Control rabbit IgG had no effect whereas K252a blocked NGF-induced TrkA tyrosine phosphorylation in these cells (data not shown). In contrast to these findings, however, NGF-induced regulated secretion was not blocked by either K252a or REX alone (Fig. 4). Only a combination of both compounds led to blockade of the NGF effect. These results suggest that NGF can transduce its signal in PC12 cells by using TrkA as well as p75.
Figure 3.
The BDNF-induced release of NT-3 is mediated by p75. PC12 cells stably transfected with pBJ-5-NT-3 myc were washed twice, preincubated for 45 min in physiological buffer, and subsequently stimulated for 30 min at 37°C with high potassium buffer (KCl), physiological buffer (control), or purified neurotrophins at 100 ng/ml each in physiological buffer. The p75 blocking antibodies (REX) were used at a concentration of 20 μg/ml, whereas the Trk tyrosine kinase blocker K252a (K252a) was applied at a concentration of 100 nM. Experiments were performed in triplicate; error bars indicate ±SD.
Figure 4.
The NGF-induced release of NT-3 can be mediated by p75 and TrkA. PC12 cells stably transfected with pBJ-5-NT-3 myc were washed twice, preincubated for 45 min in physiological buffer, and subsequently stimulated for 30 min at 37°C with high potassium buffer (KCl), physiological buffer (control), or purified neurotrophins at 100 ng/ml each in physiological buffer. The p75 blocking antibodies (REX) were used at a concentration of 20 μg/ml, the RTA antibodies were used at 200 μg/ml, and the Trk tyrosine kinase blocker K252a was applied at a concentration of 100 nM. Experiments were performed in triplicate; error bars indicate ±SD.
In addition, we found that RTA antibodies induced release of NT-3 myc (Fig. 4). This polyclonal antiserum raised against the extracellular domain of rat TrkA has been shown to crosslink TrkA receptors on the surface of PC12 cells, inducing receptor tyrosine autophosphorylation and thereby mimicking NGF-induced TrkA activation (31). Control rabbit IgG had no effect (data not shown). This finding further supports a role for TrkA in mediating NGF-induced regulated secretion of neurotrophins.
DISCUSSION
Neurotrophins, once regarded solely as target-derived survival factors (32), now are known to serve diverse roles in shaping and modulating synaptic connections throughout the nervous system (2, 3, 12). With growing appreciation of the complexities in neurotrophin function, greater attention has been focused on understanding neurotrophin release and the factors that trigger it. Recent studies from neuroendocrine cell lines, primary neuronal cultures, and hippocampal slices provide evidence that in at least some cell types, NGF, BDNF, and NT-3 undergo regulated secretion in response to depolarization by high potassium or compounds such as glutamate or carbachol (14–16, 21, 33, 34). We found that NT-4/5, a neurotrophin capable of potentiating synaptic transmission at neuromuscular synapses (35), undergoes depolarization-induced secretion in a manner similar to the other neurotrophins.
The identity of the neurotrophin storage compartment within neurons is a matter of debate. Evidence has been presented that BDNF colocalizes with markers of dense core vesicles in AtT-20 cells (15) as do myc-tagged forms of NGF and NT-3 transfected into PC12 cells (23) and NGF in hippocampal neurons (16). At the electron microscopy level, BDNF was found in dense core vesicles of rat sensory neurons (36) as was myc-tagged NGF transfected into PC12 cells (23). Based on observations that neurotrophin secretion depends on calcium release from intracellular stores it also has been proposed that neurotrophins are released from a subcompartment of the endoplasmatic reticulum located near the dendritic plasma membrane (21).
In the current study, we found that neurotrophins are able to induce regulated secretion of neurotrophins from transfected PC12 cells. NGF induced release of BDNF, NT-3, and NT-4/5 to a similar extent as depolarization. BDNF induced NT-3 release and NT-4/5 led to secretion of NT-3 and BDNF. When cells were subjected to depolarization and neurotrophin simultaneously, their effects were not additive (data not shown), suggesting that both treatments induce secretion from a single pool of stored neurotrophins. The finding, using an epitope-tagged form of NT-3, that NT-3 was able to induce its own secretion ties in with a similar observation made with BDNF in hippocampal neurons (21). This finding is of interest in regard to the previously observed coexpression of neurotrophins and their receptors within neurons (37, 38) and the subsequent demonstration of functionally important autocrine loops (39, 40).
Neurotrophin-Induced Neurotrophin Release Is Mediated by p75 and TrkA.
An additional finding was that neurotrophins that bind the pan-neurotrophin-receptor p75, but not TrkA, also were able to stimulate release of neurotrophins. Several lines of evidence support the conclusion that p75 can mediate this effect in the absence of TrkA activation. BDNF, which binds to p75 but not TrkA (41), stimulated NT-3 and NT-3 myc secretion in transiently transfected PC12 cells as well as in four stably tranfected cell lines with different expression levels. Furthermore, the BDNF-mediated effect was not caused by activation of Trk receptors as no Trk tyrosine autophosphorylation was detectable in response to BDNF, nor did the Trk inhibitor K252a block the BDNF-induced release of NT-3. In contrast, the BDNF effect was abolished by the REX polyclonal antibody, which is known to block interactions of neurotrophins with p75 (27, 42, 43).
The action of NGF in mediating neurotrophin release was more complex. Neither inhibition of TrkA activation by K252a nor blocking of NGF binding to p75 by REX alone abolished NGF-induced regulated secretion of NT-3. However, simultaneous inhibition of both receptors blocked the NT-3 release completely, suggesting that both TrkA and p75 can mediate this process.
These findings are consistent with reports describing p75-mediated release of dopamine from mesencephalic primary neurons (44) and PC12 cells (45) as well as p75-mediated increases of intracellular calcium (46) in p75-expressing fibroblasts. On the other hand, other investigators failed to find a p75-mediated effect on intracellular calcium in a glioma tumor cell line expressing p75 (47) and, in particular, on neurotrophin secretion from hippocampal neurons or PC12 cells (21).
In the latter study, NGF failed to induce BDNF secretion from adenovirus-infected hippocampal neurons whereas NT-3 and NT-4/5 did. In addition, NGF was found to be the only neurotrophin effective in induction of BDNF release from PC12 cells infected with adenoviruses coding for BDNF. Furthermore, all of the described neurotrophin-induced release of neurotrophins was blocked by addition of K252a.
It is possible that the cellular context is responsible for these differences. Most of the experiments reported by Canossa et al. (21) were performed with hippocampal neurons. Those authors did, however, use PC12 cells for some experiments and found that only NGF, but not NT-3 or NT-4/5, elicited BDNF release with the same effectiveness as high potassium. No NGF-induced secretion of BDNF was observed in the mutant PC12 cell line nnr5, known to express normal levels of p75 and greatly reduced levels of TrkA (48). Whether differences in the stimulation paradigms or the expression method (adenovirus-mediated infection versus plasmid-mediated transfection) used in the two studies are responsible for the observed discrepancy remains to be determined. It also is known that different PC12 clones vary in terms of parameters of neurotrophin-influenced neuronal excitability and ion-channel expression (49), and perhaps this variance applies to neurotrophin release, too. But given that a p75-mediated effect on release of dopamine has been described for primary neurons (44), it appears possible that our present observation of p75-mediated regulated secretion in PC12 cells may be applicable to at least some neuronal populations in vivo.
One potential explanation for neurotrophin-induced release of neurotrophins could be competition-induced release of surface-bound secreted neurotrophins by the addition of exogenous neurotrophin. Two observations argue against this possibility. First, the levels of released neurotrophins induced by depolarization using isotonic high potassium buffer are very similar to the levels induced by exogenous added neurotrophins. Second, we found that another growth factor, epidermal growth factor, which does not belong to the neurotrophin family, also is able to induce neurotrophin release from transfected PC12 cells (data not shown).
The specific signal transduction mechanisms responsible for neurotrophin-induced neurotrophin secretion were not addressed in this work. Recent studies suggest a role for the ceramide pathway in p75-mediated signals (44, 50–53). Trk effects may be mediated by PLC-γ- and IP-3-induced release of calcium from intracellular stores (54, 55), consistent with the recent report that chelators of intracellular, but not extracellular, calcium blocked neurotrophin-induced neurotrophin secretion (21). Indirect mechanisms also may be at work; for example, neurotrophins may trigger neurotransmitter release, which in turn causes depolarization and neurotrophin release (56). The specific signal transduction mechanisms used in neurons may depend not only on the cellular context, but on the subcellular location of the released neurotrophins and activated receptors as well. Trk receptors have been reported at postsynaptic densities (57), and preliminary reports suggest that both Trk and p75 receptors are present in synaptosomes prepared from ferret visual cortex (T. Pizzorusso, personal communication), potentially positioning both receptors to exert local effects at active synapses.
Implications of Neurotrophin-Induced Neurotrophin Secretion.
One critical question raised by these and other studies is whether neurotrophins stimulate the secretion of molecules other that neurotrophins. As noted, earlier studies have shown that neurotrophins facilitate dopamine release by mesencephalic primary cultures (44). Both dopamine and neurotrophins can be stored in dense core vesicles, suggesting that the release of a particular vesicle type (as opposed to only neurotrophin-containing vesicles) may be stimulated. Alternatively, secretion may be increased in general, perhaps by modification of the secretory machinery (58) or ion channels (59, 60).
By inducing their own secretion, neurotrophins may trigger a positive feedback loop that amplifies small signals or focuses neurotrophin release at subcellular locations where receptors are present. Furthermore, this mechanism might select for neurotrophin release by Trk or p75-bearing cells, therefore potentially promoting autocrine interactions that are known to be critical for the survival of some neuronal populations (39, 40). Further investigations of neurotrophin receptor localization, sites of neurotrophin storage and release, and the signal transduction mechanisms underlying neurotrophin-induced neurotrophin secretion should provide insights into the significance of this phenomena in the development, maintenance, and plasticity of the nervous system.
Acknowledgments
We are grateful to Regeneron Pharmaceuticals (Tarrytown, NY) for TrkB-Fc-receptor bodies, the NT-3 ELISA, pKS-NT-4/5, anti-NT-4/5, as well as purified recombinant BDNF, NT-3, and NT-4/5, and to Dr. Louis F. Reichardt (University of California, San Francisco) for providing the REX and RTA antibodies. We also thank Dr. Jose Cosgaya and Dr. Jouni Vesa for stimulating discussions. This research was supported by grants from the National Institute of Neurological Disorders and Stroke (NS04270), the Alzheimer’s Association (RG2-96-059), the McGowan Charitable Trust, and the Deutsche Forschungsgemeinschaft (A.K. and J.C.M.).
ABBREVIATIONS
- NGF
nerve growth factor: BDNF, brain-derived neurotrophic factor
- NT-3
neurotrophin-3
- NT-4/5
neurotrophin-4/5
- NT-3 myc
NT-3 with a myc-epitope
- Trk
neurotrophin tyrosine kinase receptors
- HRP
horseradish peroxidase
References
- 1.Lewin G R, Barde Y A. Annu Rev Neurosci. 1996;19:289–317. doi: 10.1146/annurev.ne.19.030196.001445. [DOI] [PubMed] [Google Scholar]
- 2.Lo D C. Neuron. 1995;15:979–981. doi: 10.1016/0896-6273(95)90085-3. [DOI] [PubMed] [Google Scholar]
- 3.Thoenen H. Science. 1995;270:593–598. doi: 10.1126/science.270.5236.593. [DOI] [PubMed] [Google Scholar]
- 4.Berardi N, Cellerino A, Domenici L, Fagiolini M, Pizzorusso T, Cattaneo A, Maffei L. Proc Natl Acad Sci USA. 1994;91:684–688. doi: 10.1073/pnas.91.2.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cabelli R J, Hohn A, Shatz C J. Science. 1995;267:1662–1666. doi: 10.1126/science.7886458. [DOI] [PubMed] [Google Scholar]
- 6.Cabelli R J, Shelton D L, Segal R A, Shatz C J. Neuron. 1997;19:63–76. doi: 10.1016/s0896-6273(00)80348-7. [DOI] [PubMed] [Google Scholar]
- 7.Maffei L, Berardi N, Domenici L, Parisi V, Pizzorusso T. J Neurosci. 1992;12:4651–4662. doi: 10.1523/JNEUROSCI.12-12-04651.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patterson S L, Grover L M, Schwartzkroin P A, Bothwell M. Neuron. 1992;9:1081–1088. doi: 10.1016/0896-6273(92)90067-n. [DOI] [PubMed] [Google Scholar]
- 9.Kang H, Schuman E M. Science. 1995;267:1658–1662. doi: 10.1126/science.7886457. [DOI] [PubMed] [Google Scholar]
- 10.Korte M, Carroll P, Wolf E, Brem G, Thoenen H, Bonhoeffer T. Proc Natl Acad Sci USA. 1995;92:8856–8860. doi: 10.1073/pnas.92.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patterson S L, Abel T, Deuel T A S, Martin K C, Rose J C, Kandel E R. Neuron. 1996;16:1137–1145. doi: 10.1016/s0896-6273(00)80140-3. [DOI] [PubMed] [Google Scholar]
- 12.Snider W D, Lichtman J W. Mol Cell Neurosci. 1996;7:433–442. doi: 10.1006/mcne.1996.0031. [DOI] [PubMed] [Google Scholar]
- 13.Heymach J V, Kruttgen A, Suter U, Shooter E M. J Biol Chem. 1996;271:25430–25437. doi: 10.1074/jbc.271.41.25430. [DOI] [PubMed] [Google Scholar]
- 14.Blochl A, Thoenen H. Eur J Neurosci. 1995;7:1220–1228. doi: 10.1111/j.1460-9568.1995.tb01112.x. [DOI] [PubMed] [Google Scholar]
- 15.Goodman L J, Valverde J, Lim F, Geschwind M D, Federoff H J, Geller A I, Hefti F. Mol Cell Neurosci. 1996;7:222–238. doi: 10.1006/mcne.1996.0017. [DOI] [PubMed] [Google Scholar]
- 16.Blochl A, Thoenen H. Mol Cell Neurosci. 1996;7:173–190. doi: 10.1006/mcne.1996.0014. [DOI] [PubMed] [Google Scholar]
- 17.Zhou X F, Rush R A. Neuroscience. 1996;74:945–953. doi: 10.1016/0306-4522(96)00237-0. [DOI] [PubMed] [Google Scholar]
- 18.Von Bartheld C S, Byers M R, Williams R, Bothwell M. Nature (London) 1996;379:830–833. doi: 10.1038/379830a0. [DOI] [PubMed] [Google Scholar]
- 19.Altar C A, Cai N, Bliven T, Juhasz M, Conner J M, Acheson A L, Lindsay R M, Wiegand S J. Nature (London) 1997;389:856–860. doi: 10.1038/39885. [DOI] [PubMed] [Google Scholar]
- 20.Kaplan D R, Miller F D. Curr Opin Cell Biol. 1997;9:213–221. doi: 10.1016/s0955-0674(97)80065-8. [DOI] [PubMed] [Google Scholar]
- 21.Canossa M, Griesbeck O, Berninger B, Campana G, Kolbeck R, Thoenen H. Proc Natl Acad Sci USA. 1997;94:13279–13286. doi: 10.1073/pnas.94.24.13279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kruttgen A, Moller J C, Heymach J C, Shooter E M. Soc Neurosci Abstr. 1997;23:1704. (Abstr.). [Google Scholar]
- 23.Moller J C, Kruttgen A, Heymach J V J, Ghori N, Shooter E M. J Neurosci Res. 1998;51:463–472. doi: 10.1002/(SICI)1097-4547(19980215)51:4<463::AID-JNR6>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 24.Kruttgen A, Heymach J V, Jr, Kahle P J, Shooter E M. J Biol Chem. 1997;272:29222–29228. doi: 10.1074/jbc.272.46.29222. [DOI] [PubMed] [Google Scholar]
- 25.Kelly R B, Grote E. Annu Rev Neurosci. 1993;16:95–127. doi: 10.1146/annurev.ne.16.030193.000523. [DOI] [PubMed] [Google Scholar]
- 26.Shelton D L, Sutherland J, Gripp J, Camerato T, Armanini M P, Phillips H S, Carroll K, Spencer S D, Levinson A D. J Neurosci. 1995;15:477–491. doi: 10.1523/JNEUROSCI.15-01-00477.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weskamp G, Reichardt L F. Neuron. 1991;6:649–663. doi: 10.1016/0896-6273(91)90067-a. [DOI] [PubMed] [Google Scholar]
- 28.Borasio G D. Neurosci Lett. 1990;108:207–212. doi: 10.1016/0304-3940(90)90732-o. [DOI] [PubMed] [Google Scholar]
- 29.Berg M M, Sternberg D W, Parada L F, Chao M V. J Biol Chem. 1992;267:13–16. [PubMed] [Google Scholar]
- 30.Nye S H, Squinto S P, Glass D J, Stitt T N, Hantzopoulos P, Macchi M J, Lindsay N S, Ip N Y, Yancopoulos G D. Mol Biol Cell. 1992;3:677–686. doi: 10.1091/mbc.3.6.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clary D O, Weskamp G, Austin L R, Reichardt L F. Mol Biol Cell. 1994;5:549–563. doi: 10.1091/mbc.5.5.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levi-Montalcini R. Science. 1987;237:1154–1162. doi: 10.1126/science.3306916. [DOI] [PubMed] [Google Scholar]
- 33.Edwards R H, Selby M J, Mobley W C, Weinrich S L, Hruby D E, Rutter W J. Mol Cell Biol. 1988;8:2456–2464. doi: 10.1128/mcb.8.6.2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heymach J V, Jr, Shooter E M. J Biol Chem. 1995;270:12297–12304. doi: 10.1074/jbc.270.20.12297. [DOI] [PubMed] [Google Scholar]
- 35.Wang X H, Poo M M. Neuron. 1997;19:825–835. doi: 10.1016/s0896-6273(00)80964-2. [DOI] [PubMed] [Google Scholar]
- 36.Michael G J, Averill S, Nitkunan A, Rattray M, Bennett D L H, Yan Q, Priestley J V. J Neurosci. 1997;17:8476–8490. doi: 10.1523/JNEUROSCI.17-21-08476.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schecterson L C, Bothwell M. Neuron. 1992;9:449–463. doi: 10.1016/0896-6273(92)90183-e. [DOI] [PubMed] [Google Scholar]
- 38.Snider W D. Cell. 1994;77:627–638. doi: 10.1016/0092-8674(94)90048-5. [DOI] [PubMed] [Google Scholar]
- 39.Ghosh A, Carnahan J, Greenberg M E. Science. 1994;263:1618–1623. doi: 10.1126/science.7907431. [DOI] [PubMed] [Google Scholar]
- 40.Acheson A, Conover J C, Fandl J P, DeChiara T M, Russell M, Thadani A, Squinto S P, Yancopoulos G D, Lindsay R M. Nature (London) 1995;374:450–453. doi: 10.1038/374450a0. [DOI] [PubMed] [Google Scholar]
- 41.Bothwell M. Annu Rev Neurosci. 1995;18:223–253. doi: 10.1146/annurev.ne.18.030195.001255. [DOI] [PubMed] [Google Scholar]
- 42.DiStefano P S, Friedman B, Radziejewski C, Alexander C, Boland P, Schick C M, Lindsay R M, Wiegand S J. Neuron. 1992;8:983–993. doi: 10.1016/0896-6273(92)90213-w. [DOI] [PubMed] [Google Scholar]
- 43.von Bartheld C S, Williams R, Lefcort F, Clary D O, Reichardt L F, Bothwell M. J Neurosci. 1996;16:2995–3008. doi: 10.1523/JNEUROSCI.16-09-02995.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blochl A, Sirrenberg C. J Biol Chem. 1996;271:21100–21107. doi: 10.1074/jbc.271.35.21100. [DOI] [PubMed] [Google Scholar]
- 45.Jiang H, Dickens G, Chabuck A, Guroff G. Soc Neurosci Abstr. 1997;23:1147. (Abstr.). [Google Scholar]
- 46.Jiang H, Ulme D S, Dickens G, Chabuk A, Lavarreda M, Lazarovici P, Guroff G. J Biol Chem. 1997;272:6835–6837. doi: 10.1074/jbc.272.11.6835. [DOI] [PubMed] [Google Scholar]
- 47.De Bernardi M A, Rabins S J, Colangelo A M, Brooker G, Mocchetti I. J Biol Chem. 1996;271:6092–6098. doi: 10.1074/jbc.271.11.6092. [DOI] [PubMed] [Google Scholar]
- 48.Loeb D M, Maragos J, Martin-Zanca D, Chao M V, Parada L F, Greene L A. Cell. 1991;66:961–966. doi: 10.1016/0092-8674(91)90441-z. [DOI] [PubMed] [Google Scholar]
- 49.Sherwood N T, Lesser S S, Lo D C. Proc Natl Acad Sci USA. 1997;94:5917–5922. doi: 10.1073/pnas.94.11.5917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Casaccia-Bonnefil P, Carter B D, Dobrowsky R T, Chao M V. Nature (London) 1996;383:716–720. doi: 10.1038/383716a0. [DOI] [PubMed] [Google Scholar]
- 51.Dobrowsky R T, Jenkins G M, Hannun Y A. J Biol Chem. 1995;270:22135–22142. doi: 10.1074/jbc.270.38.22135. [DOI] [PubMed] [Google Scholar]
- 52.Carter B D, Lewin G R. Neuron. 1997;18:187–190. doi: 10.1016/s0896-6273(00)80259-7. [DOI] [PubMed] [Google Scholar]
- 53.MacPhee I J, Barker P A. J Biol Chem. 1997;272:23547–23551. doi: 10.1074/jbc.272.38.23547. [DOI] [PubMed] [Google Scholar]
- 54.Vetter M L, Martin-Zanca D, Parada L F, Bishop J M, Kaplan D R. Proc Natl Acad Sci USA. 1991;88:5650–5654. doi: 10.1073/pnas.88.13.5650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Finkbeiner S, Tavazoie S F, Maloratsky A, Jacobs K M, Harris K M, Greenberg M E. Neuron. 1997;19:1031–1047. doi: 10.1016/s0896-6273(00)80395-5. [DOI] [PubMed] [Google Scholar]
- 56.Missale C, Boroni F, Sigala S, Buriani A, Fabris M, Leon A, Dal Toso R, Spano P F. Proc Natl Acad Sci USA. 1996;93:4240–4245. doi: 10.1073/pnas.93.9.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wu K, Xu J L, Suen P C, Levine E, Huang Y Y, Mount H T, Lin S Y, Black I B. Brain Res Mol Brain Res. 1996;43:286–290. doi: 10.1016/s0169-328x(96)00211-2. [DOI] [PubMed] [Google Scholar]
- 58.Jovanovic J N, Benfenati F, Siow Y L, Sihra T S, Sanghera J S, Pelech S L, Greengard P, Czernik A J. Proc Natl Acad Sci USA. 1996;93:3679–3683. doi: 10.1073/pnas.93.8.3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Holm N R, Christophersen P, Olesen S P, Gammeltoft S. Proc Natl Acad Sci USA. 1997;94:1002–1006. doi: 10.1073/pnas.94.3.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Suen P C, Wu K, Levine E S, Mount H T, Xu J L, Lin S Y, Black I B. Proc Natl Acad Sci USA. 1997;94:8191–8195. doi: 10.1073/pnas.94.15.8191. [DOI] [PMC free article] [PubMed] [Google Scholar]