Abstract
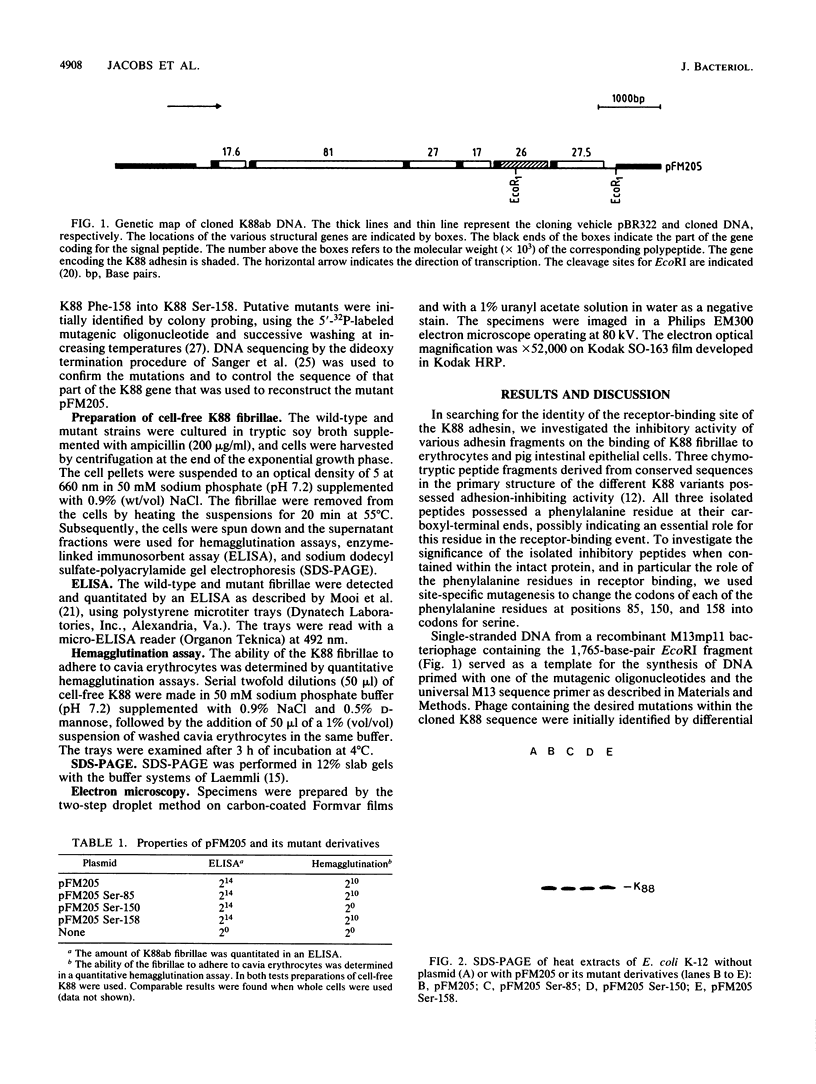
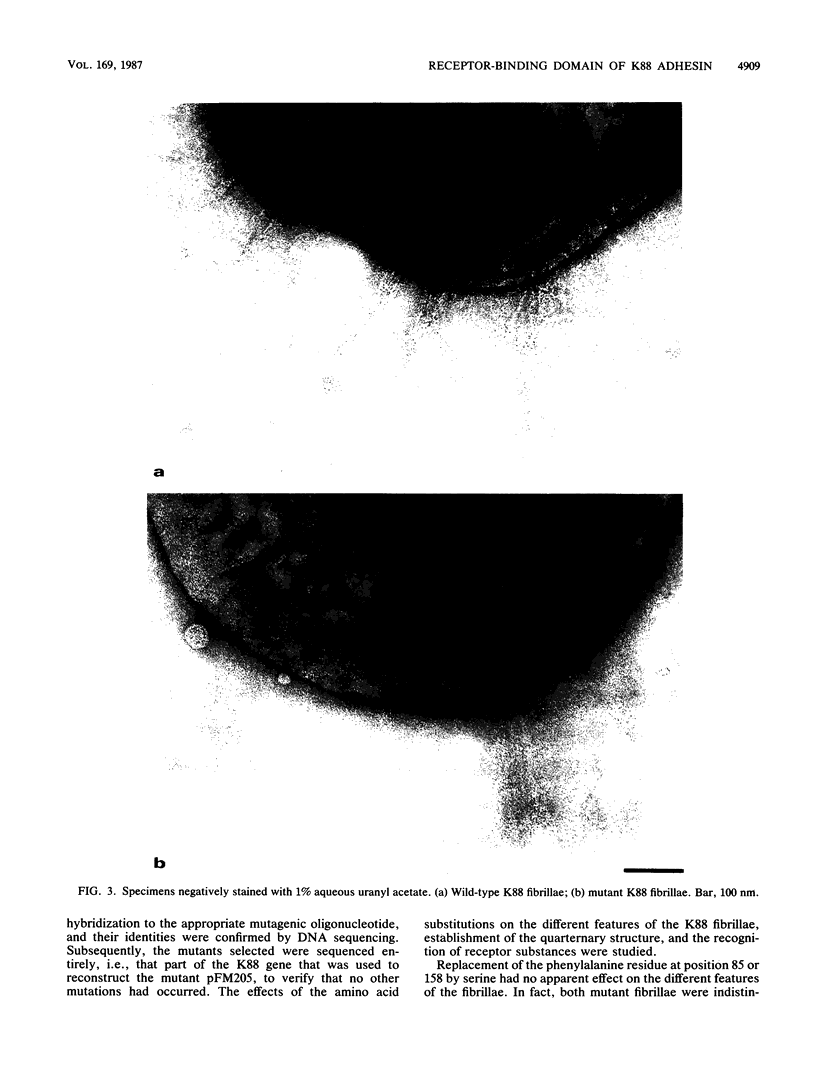
Recently, we reported the isolation of three peptides, Ile-83-Ala-Phe-85, Ser-148-Leu-Phe-150, and Ala-156-Ile-Phe-158, derived from the K88 fibrillar subunit and found to inhibit the binding of K88 fibrillae to cavia erythrocytes or pig intestinal epithelial cells (A. A. C. Jacobs, J. Venema, R. Leeven, H. van Pelt-Heerschap, and F. K. de Graaf, J. Bacteriol. 169:735-741, 1987). The gene encoding the K88 fibrillar adhesin was modified by oligonucleotide-directed site-specific mutagenesis such that each of the phenylalanine residues at positions 85, 150, and 158 were replaced by serine. Replacement of phenylalanine 85 or 158 had no apparent effect on the biosynthesis of the fibrillae or on their adhesive capacity. In contrast, substitution of phenylalanine 150 with serine resulted in a dramatic decrease in adhesive capacity of the K88 fibrillae. Apparently, phenylalanine 150 plays an essential role in the interaction of the adhesin with receptor molecules present on eucaryotic cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson M. J., Whitehead J. S., Kim Y. S. Interaction of Escherichia coli K88 antigen with porcine intestinal brush border membranes. Infect Immun. 1980 Sep;29(3):897–901. doi: 10.1128/iai.29.3.897-901.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagert M., Ehrlich S. D. Prolonged incubation in calcium chloride improves the competence of Escherichia coli cells. Gene. 1979 May;6(1):23–28. doi: 10.1016/0378-1119(79)90082-9. [DOI] [PubMed] [Google Scholar]
- Falmagne P., Capiau C., Lambotte P., Zanen J., Cabiaux V., Ruysschaert J. M. The complete amino acid sequence of diphtheria toxin fragment B. Correlation with its lipid-binding properties. Biochim Biophys Acta. 1985 Jan 21;827(1):45–50. doi: 10.1016/0167-4838(85)90099-8. [DOI] [PubMed] [Google Scholar]
- Gaastra W., de Graaf F. K. Host-specific fimbrial adhesins of noninvasive enterotoxigenic Escherichia coli strains. Microbiol Rev. 1982 Jun;46(2):129–161. doi: 10.1128/mr.46.2.129-161.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinée P. A., Jansen W. H. Behavior of Escherichia coli K antigens K88ab, K88ac, and K88ad in immunoelectrophoresis, double diffusion, and hemagglutination. Infect Immun. 1979 Mar;23(3):700–705. doi: 10.1128/iai.23.3.700-705.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacker J., Schmidt G., Hughes C., Knapp S., Marget M., Goebel W. Cloning and characterization of genes involved in production of mannose-resistant, neuraminidase-susceptible (X) fimbriae from a uropathogenic O6:K15:H31 Escherichia coli strain. Infect Immun. 1985 Feb;47(2):434–440. doi: 10.1128/iai.47.2.434-440.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagblom P., Segal E., Billyard E., So M. Intragenic recombination leads to pilus antigenic variation in Neisseria gonorrhoeae. Nature. 1985 May 9;315(6015):156–158. doi: 10.1038/315156a0. [DOI] [PubMed] [Google Scholar]
- Jacobs A. A., Venema J., Leeven R., van Pelt-Heerschap H., de Graaf F. K. Inhibition of adhesive activity of K88 fibrillae by peptides derived from the K88 adhesin. J Bacteriol. 1987 Feb;169(2):735–741. doi: 10.1128/jb.169.2.735-741.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs A. A., van Mechelen J. R., de Graaf F. K. Effect of chemical modifications on the K99 and K88ab fibrillar adhesins of Escherichia coli. Biochim Biophys Acta. 1985 Nov 29;832(2):148–155. doi: 10.1016/0167-4838(85)90326-7. [DOI] [PubMed] [Google Scholar]
- Jones G. W., Rutter J. M. Role of the K88 antigen in the pathogenesis of neonatal diarrhea caused by Escherichia coli in piglets. Infect Immun. 1972 Dec;6(6):918–927. doi: 10.1128/iai.6.6.918-927.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lindberg F., Lund B., Johansson L., Normark S. Localization of the receptor-binding protein adhesin at the tip of the bacterial pilus. Nature. 1987 Jul 2;328(6125):84–87. doi: 10.1038/328084a0. [DOI] [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Mooi F. R., de Graaf F. K. Molecular biology of fimbriae of enterotoxigenic Escherichia coli. Curr Top Microbiol Immunol. 1985;118:119–138. doi: 10.1007/978-3-642-70586-1_7. [DOI] [PubMed] [Google Scholar]
- Mooi F. R., de Graaf F. K., van Embden J. D. Cloning, mapping and expression of the genetic determinant that encodes for the K88ab antigen. Nucleic Acids Res. 1979 Mar;6(3):849–865. doi: 10.1093/nar/6.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooi F. R., van Buuren M., Koopman G., Roosendaal B., de Graaf F. K. K88ab gene of Escherichia coli encodes a fimbria-like protein distinct from the K88ab fimbrial adhesin. J Bacteriol. 1984 Aug;159(2):482–487. doi: 10.1128/jb.159.2.482-487.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norgren M., Normark S., Lark D., O'Hanley P., Schoolnik G., Falkow S., Svanborg-Edén C., Båga M., Uhlin B. E. Mutations in E coli cistrons affecting adhesion to human cells do not abolish Pap pili fiber formation. EMBO J. 1984 May;3(5):1159–1165. doi: 10.1002/j.1460-2075.1984.tb01945.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proia R. L., Wray S. K., Hart D. A., Eidels L. Characterization and affinity labeling of the cationic phosphate-binding (nucleotide-binding) peptide located in the receptor-binding region of the B-fragment of diphtheria toxin. J Biol Chem. 1980 Dec 25;255(24):12025–12033. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoolnik G. K., Fernandez R., Tai J. Y., Rothbard J., Gotschlich E. C. Gonococcal pili. Primary structure and receptor binding domain. J Exp Med. 1984 May 1;159(5):1351–1370. doi: 10.1084/jem.159.5.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoller M. J., Smith M. Oligonucleotide-directed mutagenesis: a simple method using two oligonucleotide primers and a single-stranded DNA template. DNA. 1984 Dec;3(6):479–488. doi: 10.1089/dna.1.1984.3.479. [DOI] [PubMed] [Google Scholar]




