Abstract
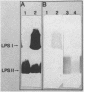
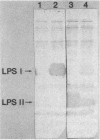
The lipopolysaccharide (LPS) from a Rhizobium phaseoli mutant, CE109, was isolated and compared with that of its wild-type parent, CE3. A previous report has shown that the mutant is defective in infection thread development, and sodium dodecyl sulfate-polyacrylamide gel electrophoresis shows that it has an altered LPS (K. D. Noel, K. A. VandenBosch, and B. Kulpaca, J. Bacteriol. 168:1392-1462, 1986). Mild acid hydrolysis of the CE3 LPS released a polysaccharide and an oligosaccharide, PS1 and PS2, respectively. Mild acid hydrolysis of CE109 LPS released only an oligosaccharide. Chemical and immunochemical analyses showed that CE3-PS1 is the antigenic O chain of this strain and that CE109 LPS does not contain any of the major sugar components of CE3-PS1. CE109 oligosaccharide was identical in composition to CE3-PS2. The lipid A's from both strains were very similar in composition, with only minor quantitative variations. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis of CE3 and CE109 LPSs showed that CE3 LPS separated into two bands, LPS I and LPS II, while CE109 had two bands which migrated to positions similar to that of LPS II. Immunoblotting with anti-CE3 antiserum showed that LPS I contains the antigenic O chain of CE3, PS1. Anti-CE109 antiserum interacted strongly with both CE109 LPS bands and CE3 LPS II and interacted weakly with CE3 LPS I. Mild-acid hydrolysis of CE3 LPS I, extracted from the polyacrylamide gel, showed that it contained both PS1 and PS2. The results in this report showed that CE109 LPS consists of only the lipid A core and is missing the antigenic O chain.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amons R., Schrier P. I. Removal of sodium dodecyl sulfate from proteins and peptides by gel filtration. Anal Biochem. 1981 Sep 15;116(2):439–443. doi: 10.1016/0003-2697(81)90385-7. [DOI] [PubMed] [Google Scholar]
- Blumenkrantz N., Asboe-Hansen G. New method for quantitative determination of uronic acids. Anal Biochem. 1973 Aug;54(2):484–489. doi: 10.1016/0003-2697(73)90377-1. [DOI] [PubMed] [Google Scholar]
- Broughton W. J., van Egeraat A. W., Lie T. A. Dynamics of Rhizobium competition for nodulation of Pisum sativum cv. Afghanistan. Can J Microbiol. 1980 Apr;26(4):562–565. doi: 10.1139/m80-099. [DOI] [PubMed] [Google Scholar]
- Caetano Anollés G., Favelukes G. Host-Symbiont Specificity Expressed during Early Adsorption of Rhizobium meliloti to the Root Surface of Alfalfa. Appl Environ Microbiol. 1986 Aug;52(2):377–382. doi: 10.1128/aem.52.2.377-382.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson R. W. Heterogeneity of Rhizobium lipopolysaccharides. J Bacteriol. 1984 Jun;158(3):1012–1017. doi: 10.1128/jb.158.3.1012-1017.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson R. W., Lee R. P. A Comparison of the Surface Polysaccharides from Rhizobium leguminosarum 128C53 smrif with the Surface Polysaccharides from Its Exo Mutant. Plant Physiol. 1983 Feb;71(2):223–228. doi: 10.1104/pp.71.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson R. W., Sanders R. E., Napoli C., Albersheim P. Host-Symbiont Interactions: III. Purification and Partial Characterization of Rhizobium Lipopolysaccharides. Plant Physiol. 1978 Dec;62(6):912–917. doi: 10.1104/pp.62.6.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson R. W., Shatters R., Duh J. L., Turnbull E., Hanley B., Rolfe B. G., Djordjevic M. A. The Isolation and Partial Characterization of the Lipopolysaccharides from Several Rhizobium trifolii Mutants Affected in Root Hair Infection. Plant Physiol. 1987 Jun;84(2):421–427. doi: 10.1104/pp.84.2.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson R. W., Yadav M. Isolation and partial characterization of the extracellular polysaccharides and lipopolysaccharides from fast-growing Rhizobium japonicum USDA 205 and its Nod- mutant, HC205, which lacks the symbiotic plasmid. Appl Environ Microbiol. 1985 Nov;50(5):1219–1224. doi: 10.1128/aem.50.5.1219-1224.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chart H., Shaw D. H., Ishiguro E. E., Trust T. J. Structural and immunochemical homogeneity of Aeromonas salmonicida lipopolysaccharide. J Bacteriol. 1984 Apr;158(1):16–22. doi: 10.1128/jb.158.1.16-22.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dazzo F. B., Truchet G. L., Sherwood J. E., Hrabak E. M., Abe M., Pankratz S. H. Specific phases of root hair attachment in the Rhizobium trifolii-clover symbiosis. Appl Environ Microbiol. 1984 Dec;48(6):1140–1150. doi: 10.1128/aem.48.6.1140-1150.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrmann J., Wollum A. G. Simplified Enzyme-Linked Immunosorbent Assay for Routine Identification of Rhizobium japonicum Antigens. Appl Environ Microbiol. 1985 Apr;49(4):1010–1013. doi: 10.1128/aem.49.4.1010-1013.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeffner N., Chaby R., Szabó L. Identification of 2-methyl-3-hydroxydecanoic and 2-methyl-3-hydroxytetradecanoic acids in the 'lipid X' fraction of the Bordetella pertussis endotoxin. Eur J Biochem. 1977 Aug 1;77(3):535–544. doi: 10.1111/j.1432-1033.1977.tb11696.x. [DOI] [PubMed] [Google Scholar]
- Hendrick C. A., Sequeira L. Lipopolysaccharide-Defective Mutants of the Wilt Pathogen Pseudomonas solanacearum. Appl Environ Microbiol. 1984 Jul;48(1):94–101. doi: 10.1128/aem.48.1.94-101.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock P. J., Brown T. M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983 Apr;154(1):269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrabak E. M., Urbano M. R., Dazzo F. B. Growth-phase-dependent immunodeterminants of Rhizobium trifolii lipopolysaccharide which bind trifoliin A, a white clover lectin. J Bacteriol. 1981 Nov;148(2):697–711. doi: 10.1128/jb.148.2.697-711.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel K. D., Vandenbosch K. A., Kulpaca B. Mutations in Rhizobium phaseoli that lead to arrested development of infection threads. J Bacteriol. 1986 Dec;168(3):1392–1401. doi: 10.1128/jb.168.3.1392-1401.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pueppke S. G. Adsorption of slow- and fast-growing rhizobia to soybean and cowpea roots. Plant Physiol. 1984 Aug;75(4):924–928. doi: 10.1104/pp.75.4.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rietschel E. T., Gottert H., Lüderitz O., Westphal O. Nature and linkages of the fatty acids present in the lipid-A component of Salmonella lipopolysaccharides. Eur J Biochem. 1972 Jul 13;28(2):166–173. doi: 10.1111/j.1432-1033.1972.tb01899.x. [DOI] [PubMed] [Google Scholar]
- Ryan J. M., Conrad H. E. Structural heterogeneity in the lipopolysaccharide of Salmonella newington. Arch Biochem Biophys. 1974 Jun;162(2):530–535. doi: 10.1016/0003-9861(74)90213-6. [DOI] [PubMed] [Google Scholar]
- WEISSBACH A., HURWITZ J. The formation of 2-keto-3-deoxyheptonic acid in extracts of Escherichia coli B. I. Identification. J Biol Chem. 1959 Apr;234(4):705–709. [PubMed] [Google Scholar]