Abstract
To investigate the role of neurofilaments in motor neuron disease caused by superoxide dismutase (SOD1) mutations, transgenic mice expressing a amyotrophic lateral sclerosis-linked SOD1 mutant (SOD1G37R) were mated with transgenic mice expressing human neurofilament heavy (NF-H) subunits. Unexpectedly, expression of human NF-H transgenes increased by up to 65%, the mean lifespan of SOD1G37R mice. Microscopic examination corroborated the protective effect of NF-H protein against SOD1 toxicity. Although massive neurodegeneration occurred in 1-yr-old mice expressing SOD1G37R alone, spinal root axons and motor neurons were remarkably spared in doubly SOD1G37R;NF-H-transgenic littermates.
Keywords: amyotrophic lateral sclerosis/transgenic mice/intermediate filaments
Mutations in the gene coding for the Cu,Zn superoxide dismutase (SOD1) are responsible for ≈20% of familial amyotrophic lateral sclerosis (ALS) cases (1, 2). This ubiquitous cytosolic enzyme is involved in the conversion of superoxide anion to hydrogen peroxide (3). Many lines of evidence suggest that SOD1 mutations cause ALS through mechanisms involving gain of deleterious activities (4–6). Several hypotheses have been proposed concerning the nature of the adverse activities of SOD1 mutants. One hypothesis suggests that mutations would render the copper in the SOD1 active site more accessible to peroxynitrite, allowing the formation of reactive nitronium-like intermediates having the capacity to nitrate proteins on tyrosine residues (7). Another hypothetical mechanism suggests that the mutations increase the peroxidase activity of SOD1, leading to the formation of more hydroxyl radicals from hydrogen peroxide (8, 9). Both of these hypothetical aberrant activities of mutant SOD1 could contribute to the damage of proteins as well as mitochondria and other organelles, leading to dysfunction and death of motor neurons.
Abnormal accumulations of neurofilaments (10) have been reported in sporadic and in familial ALS caused by SOD1 mutations (11) and in transgenic mice expressing various SOD1 mutants suggesting a possible link between SOD1 and neurofilaments (12–15). Previous studies with transgenic mice overexpressing normal or mutant neurofilament proteins (16–19) and the discovery of very rare neurofilament heavy (NF-H) gene mutations in some ALS cases (6, 20) have provided support for the idea that disorganization of neurofilaments can play a role in motor neuron disease. Of relevance to the present study was the report that overexpression of human NF-H in mice leads to a motor neuronopathy characterized by the formation of perikaryal neurofilamentous accumulations resembling those found in ALS and by the atrophy and slow degeneration of motor axons in old transgenic mice (16).
To clarify the role of neurofilaments in SOD1-mediated disease, we mated transgenic mice expressing a ALS-linked SOD1 mutant (SOD1G37R) with transgenic mice expressing either of the two alleles of human NF-H subunits. We report here that overexpression of NF-H conferred remarkable protection against SOD1-mediated toxicity.
MATERIALS AND METHODS
Generation of Transgenic Mice.
The parental mice expressing the SOD1G37R (line 29) or the human NF-H alleles were predominantly C57BL/6 even though they are not fully inbred. Nonetheless, to circumvent potential difficulties caused by the genetic background of mice, our study was carried out with transgenic littermates. Transgenic mice heterozygous for SOD1G37R were bred with mice heterozygous for each human NF-H transgene to produce offspring constituted of SOD1G37R-transgenic, NF-H-transgenic, doubly SOD1G37R;NF-H, and normal mice.
Protein Analysis.
Cytoskeletal-enriched fractions were prepared from the spinal cord and ventral roots by homogenization of tissue in Triton X-100 buffer (10 mM Tris⋅HCl, pH 7.5/150 mM NaCl/1 mM EDTA/1% Triton X-100) followed by centrifugation at 12,000 × g. Protein extracts were obtained by homogenization of spinal cord in SUB (0.5% SDS/8 M urea/2% 2-mercaptoethanol). Samples were electrophoresed on 7.5% polyacrylamide gels (SDS/PAGE) for the detection of neurofilament proteins, or 15% for detection of SOD1 protein, and transferred on nitrocellulose filter. The NF-H transgene expression product was detected with a human NF-H-specific primary antibody (provided by V. M.-Y. Lee, Univ. of Pennsylvania) and ECL (Amersham) chemoluminescence kit for Western blotting. For the detection of SOD1 protein, a primary antibody from BioDesign (New York) was used.
Histological Methods.
Mice were killed by overdose of chloral hydrate, perfused with PBS, pH 7.4, and then with fixative (2.5% glutaraldehyde in 0.1 M sodium phosphate buffer, pH 7.4). Tissue samples were immersed in fixative for at least 2 hr, rinsed in phosphate buffer, and postfixed in 1% phosphate buffered osmium tetroxide. Each sample was dehydrated in a graded series of ethanol and embedded in Epon from Marivue, Nova Scotia, Canada. The thin sections were stained with toluidine blue from JBS, Canada.
RESULTS
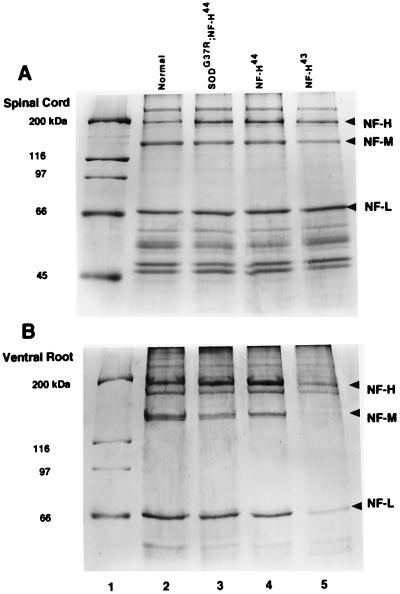
To investigate the effect of increasing the levels of neurofilament proteins in motor neuron disease induced by mutant SOD1, mice heterozygous for a ALS-linked mutant SOD1 transgene (SOD1G37R; line 29) (14) were bred with mice heterozygous for a human NF-H transgene (21). This mating procedure allowed us to produce and analyze transgenic littermates. The NF-H-transgenic lines are referred as the NF-H43 (line 200) (16), coding for 43 Lys-Ser-Pro (KSP) phosphorylation sites, and the NF-H44 (line 398) coding for 44 KSP repeats. These NF-H-transgenic lines were obtained by the microinjection of genomic fragments containing the human NF-H gene, flanked by 5′-promoter region and 3′ sequences. The heterozygous NF-H-transgenic mice from these two lines develop neurofilament accumulations in lower motor neurons and exhibit neurological abnormalities such as fine tremors and limb contraction reflexes during aging. The NF-H-induced pathology progresses with atrophy and slow degeneration of motor axons in old transgenic mice. In heterozygous transgenic mice from these two lines, the NF-H overexpression does not result into significant loss of spinal motor neurons and does not affect lifespan. The total amount of NF-H protein detected in cytoskeletal-enriched preparations of the spinal cord is similar in heterozygous mice from both transgenic lines and corresponds to ≈twofold the level of NF-H detected in normal mice as determined by Coomassie blue after SDS/PAGE (Fig. 1). In both mouse lines, NF-H overexpression resulted in a slight reduction in levels of NF-M protein in the spinal cord. However, the mice from line 200 expressing NF-H43 showed more prominent neurofilamentous swellings in spinal motor neurons and more severe atrophy of ventral and dorsal root axons than mice from line 398 expressing NF-H44 (Fig. 2). As expected, the dramatic reduction in axonal calibers of NF-H43-transgenic mice is accompanied with a marked decrease in the levels of all three neurofilament proteins detected in ventral roots when compared with NF-H44-transgenic or normal mice (Fig. 1). It is unclear whether the distinct phenotypes of transgenic lines 200 and 398 reflect slightly different properties of the two human NF-H alleles or results from variability of NF-H transgene expression in particular neuronal subsets, motor neurons for instance.
Figure 1.
Levels of neurofilament proteins in spinal cord and ventral roots. Coomassie stained SDS/PAGE gel of cytoskeletal-enriched preparations from the spinal cord (A) and lumbar ventral roots (B) of 4-mo-old mice. (A) The level of NF-H protein is increased by ≈twofold in the spinal cord of transgenic mice heterozygous for the NF-H44 (lane 4) or NF-H43 (lane 5) transgenes as compared with normal mice (lane 2). The NF-H overexpression caused a reduction in the levels of NF-M. Expression of SOD1G37R did not affect the levels of neurofilament proteins in the doubly SOD1G37R;NF-H44-transgenic mice (lane 3). (B) The levels of neurofilament proteins were reduced markedly in ventral root axons of NF-H43 mice (lane 5) in contrast to NF-H44 mice (lane 4) or normal mice (lane 2).
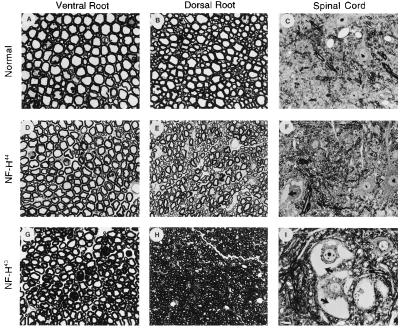
Figure 2.
Light micrographs of ventral roots, dorsal roots and spinal cord from NF-H-transgenic mice. Transverse sections of L5 ventral roots, dorsal roots, and spinal cord from normal mice (A, B, and C), NF-H44 mice (D, E, and F), and NF-H43 mice (G, H, and I) (12-mo-old). Mice overexpressing human NF-H43 showed more prominent neurofilamentous accumulations in spinal motor neurons (I) than mice overexpressing NF-H44 (F). The axonal atrophy in ventral and dorsal roots is also more severe in mice expressing NF-H43 (G and H) than in those expressing NF-H44 (D and E). The arrows point to neurofilament accumulations. (I, Bar = 10 μm.)
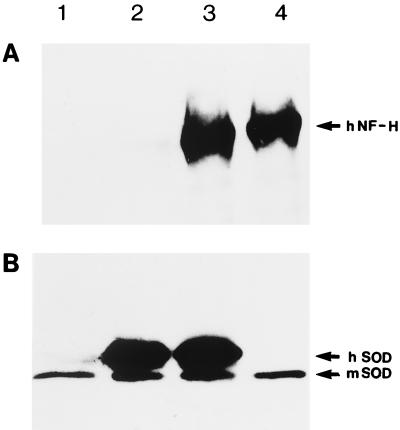
Expression of mutant SOD1 in the doubly SOD1G37R;NF-H-transgenic mice (4 mo old) had no detectable effects on the levels of neurofilament proteins in the spinal cord, including human NF-H protein (Fig. 1A and Fig. 3). Likewise no changes in the levels of SOD1G37R in the spinal cord occurred as a result of NF-H overexpression in doubly SOD1G37R;NF-H-transgenic mice (Fig. 3).
Figure 3.
Expression of human NF-H and SOD1G37R proteins in transgenic mice. (A) Immunodetection of human NF-H protein in spinal cord extracts from normal mice (lane 1), transgenic mice bearing the SOD1G37R (lane 2), doubly SOD1G37R;NF-H44-transgenic mice (lane 3), and transgenic mice expressing the human NF-H44 protein (lane 4). (B) Immunodetection of human mutant and mouse SOD1 proteins in spinal cord extracts from normal mice (lane 1), transgenic mice for SOD1G37R(lane 2), SOD1G37R;NF-H44-transgenic mice (lane 3), and transgenic mice expressing the human NF-H44 protein (lane 4).
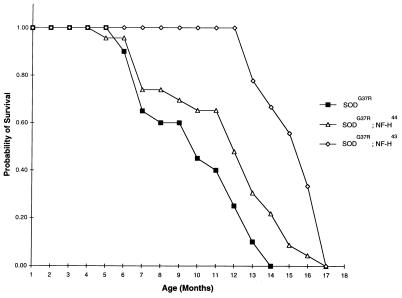
Fig. 4 shows the survival curves of SOD1G37R-transgenic mice. Transgenic mice expressing SOD1G37R alone in a normal neurofilament background had a mean life expectancy of 9.5 ± 2.8 mo (n = 20) and most of them (75%) died before 1 year old. In contrast, 100% of SOD1G37R;NF-H43-transgenic mice were still alive after 1 year for an average lifespan of 15.8 ± 1.5 mo (n = 9). Thus, expression of the human NF-H43 transgene extended the mean longevity of SOD1G37R-expressing mice by ≈6 mo. The protective effect of human NF-H protein also was important in SOD1G37R;NF-H44-transgenic mice, derived from the other NF-H-transgenic line. In this case, the median of life probability of doubly transgenic mice was extended by ≈2 mo (n = 23) (Fig. 4). The onset of paralysis in the doubly SOD1G37R;NF-H-transgenic mice occurred shortly before death with a period of SOD1 disease duration (≈2 wk) comparable with the one observed in singly SOD1G37R-transgenic mice. It should be noted that although the onset of paralysis and death caused by SOD1G37R toxicity were dramatically delayed in SOD1G37R;NF-H-transgenic mice, these doubly transgenics exhibited the mild neurological phenotypes characteristic of the respective parental NF-H-transgenic lines.
Figure 4.
Increased lifespan of SOD1G37R-transgenic mice by NF-H overexpression. Survival curves of transgenic mice expressing SOD1G37R alone or together with NF-H transgenes coding for the NF-H43 or NF-H44 proteins. The survival probability of transgenic mice is plotted as a function of their age in months. It is remarkable that expression of the human NF-H44 transgene increased the mean longevity of SOD1G37R by ≈6 mo.
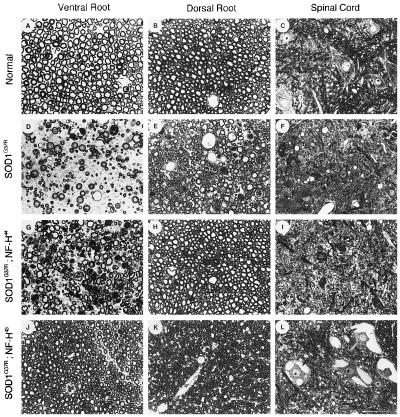
Light microscopy examination of spinal cord and lumbar (L5) spinal root axons from 1-yr-old transgenic mice, carrying the SOD1G37R transgene alone or together with a NF-H transgene further corroborated the protective effect of NF-H against SOD1G37R toxicity (Fig. 5). Although massive axonal loss and cell death had occurred in one-year old SOD1G37R mice (Fig. 5 D–F), the motor and sensory axons were generally spared in SOD1G37R;NF-H43-transgenic mice (Fig. 5 J and K). Note that, like mice expressing the human NF-H43 alone (Fig. 2 G–I), the doubly SOD1G37R;NF-H43-transgenic mice exhibited axonal atrophy (Fig. 5 J and K) and prominent perikaryal neurofilamentous accumulations (Fig. 5L). To a lesser degree, the rescue of motor and sensory axons also was evident in doubly transgenic mice expressing the NF-H44 (Fig. 5 G–I). Remarkably, the different degrees of protection conferred by the two human NF-H transgenes in the SOD1-induced disease correlated with the extent of neurofilament accumulations in motor neuron cell bodies of each NF-H-transgenic line.
Figure 5.
Lessen neurodegeneration in SOD1G37R-transgenic mice coexpressing human NF-H proteins. Light micrographs show the lumbar (L5) ventral root axons, dorsal root axons, and motor neurons from a normal mouse (A, B, and C), a singly SOD1G37R-transgenic mouse (D, E, and F), a doubly SOD1G37R;NF-H44-transgenic mouse (G, H, and I), and a doubly SOD1G37R;NF-H43-transgenic mouse (J, K, and L). Neurofilamentous swellings are indicated by arrows in L. Tissues were embedded in Epon and 1 μm sections were stained with toluidine blue. (Bar = 50 μm.)
DISCUSSION
It is surprising that human NF-H overexpression was able to confer protection against SOD1-mediated toxicity instead of exacerbating disease. Different mechanisms could account for the apparent paradoxical effects of human NF-H overexpression. One mechanism is that the extra neurofilament proteins would act as a sink for toxic oxygen radical species thereby reducing damage to other essential cellular components. To test this possibility, we examined in extracts from the spinal cord and spinal roots of mice the levels of protein-bound nitrotyrosine, using a mAb (Upstate Biotechnology, Lake Placid, NY) and of protein-bound carbonyls as described by Levine et al. (22). Experiments carried out with mice at different ages revealed no detectable changes in the pattern or amount of protein-bound nitrotyrosine or carbonyl modifications in the doubly SOD1G37R; NF-H-transgenic mice when compared with normal, SOD1G37R, and NF-H-transgenic mice (data not shown). These results are in agreement with three recent studies (23–25). After infection of differentiated PC12 cells with adenovirus coding for mutant SOD1 genes (23), the resulting cell death occurred without changes in the content of protein-bound nitrotyrosines, nor was the cell death NO-dependent. The level of protein-bound nitrotyrosine also was reported to remain unchanged in two additional transgenic mouse lines expressing the SOD1G37R mutant (24). In another study, the level of carbonyls was reported not to be significantly increased in brain samples of familial ALS patients bearing SOD1 mutations (25). Therefore, we conclude that targeted oxidative modifications of NF-H protein is unlikely to be responsible for the protection against SOD-1 induced damage.
It is striking that increased synthesis of NF-H43 had more beneficial effects than NF-H44. Although in both cases NF-H levels are increased comparably, the NF-H43 also results in the trapping of many neurofilaments in the cell bodies (Fig. 2I) and a partial depletion of axonal neurofilaments (Fig. 1B). The NF-H44 mice have a more normal axonal neurofilament content than NF-H43 mice (Fig. 1B) and less perikaryal accumulations of neurofilaments (Fig. 2F). Thus, the enhanced beneficial effect of the NF-H43 product and more modest benefits of NF-H44 may derive from (i) protection in proportion to the increased level of perikaryal neurofilament proteins, (ii) protection in proportion to a decreased axonal neurofilament content, (iii) a selective property of the shorter KSP repeat domain of NF-H43, or (iv) a combination of these effects.
Of relevance here are two other mating experiments that have followed SOD1-mediated disease in mice with altered neurofilament content: First, total loss of perikaryal and axonal neurofilaments through disruption of the NF-L gene (26) (with a corresponding elevation of perikaryal NF-M and NF-H protein levels) also leads to ≈2-mo extension of lifespan in SOD1G85R mutant mice (27) demonstrating that SOD1-mediated disease can proceed in the absence of neurofilaments. Second, expressing an NF-H-β-galactosidase fusion protein has been found to eliminate axonal neurofilaments by trapping neurofilaments in perikarya that become grossly distended with masses of neurofilaments. Although this construct confers no net beneficial effect on disease mediated by familial ALS-linked mutation SODG37R (28), it should be emphasized that the NF-H-β-galactosidase mice lose 25% of motor axons during the first year of life anyway (29). Because this loss would strongly be expected to accelerate disease arising from SOD1 mutant killing of motor neurons, the apparent unchanged disease course most probably arises from the altered neurofilament accumulation (an increase in perikarya and elimination in axons) slowing SOD1-mediated neuronal death. Considering all of these together, it seems most likely that the marked beneficial effect in the NF-H43 and NF-H44 mice arises from an increase in perikaryal levels of neurofilament proteins, and a reduction, but not elimination of axonal neurofilaments (most striking in the case of NF-H43).
One explanation for the protective effect of NF-H overexpression is based on previous reports indicating that neurofilament proteins have multiple calcium-binding sites, including high affinity sites, suggesting neurofilament involvement in calcium homeostasis (30, 31). It is conceivable that increasing the NF-H protein levels might confer protection in perikarya against rises in intracellular calcium resulting from oxidative stress and particularly from mitochondrial damage reported in mutant SOD1 transgenic mice (14, 32). This hypothesis also can offer an explanation for the slowing of disease in mice expressing SOD1G85R without neurofilaments as the result of deletion of NF-L. Despite complete absence of neurofilaments and markedly reduced axonal levels of NF-M and NF-H, perikaryal levels of both subunits are elevated in NF-L knockout mice relative to normal mice (27) and thus would confer enhanced protection.
Overexpression of the calcium-binding protein, calbindin D28K, was recently reported to protect against mutant SOD1-mediated death of PC12 cells (23) and of cultured motor neurons expressing mutant SOD1G93A (personal communication from Heather Durham, Montreal Neurological Institute). The hypothesis of calcium involvement in the pathogenesis receives support by the demonstration of selective vulnerability of motor neurons lacking typical calcium-binding proteins, parvalbumin and calbindin, in ALS patients, monkeys (33–36), and a line of transgenic mice expressing mutant SOD1 (15). Indeed, if neurofilament proteins act as calcium chelators, the dramatic declines in neurofilament mRNA levels occurring during aging (37, 38) and to a greater extent in neurodegenerative diseases including ALS (39) may contribute to increase the susceptibility of specific neuronal populations to oxidative stress and calcium-mediated death.
We report here that overexpression of two different alleles of human NF-H extends the lifespan of SOD1G37R-transgenic mice by up to 65%. Other efforts to alleviate SOD1-induced disease in transgenic mice by various therapeutics including vitamin E, riluzole, gabapentin, and d-penicillamine have produced only modest benefits (40, 41). Mating mutant SOD1 mice to mice overexpressing bcl-2 produced transgenic progeny whose longevity was increased by 15% (42). Attempts to increase perikaryal levels of neurofilament proteins could provide the basis for developing new therapeutic approaches for ALS and other neurodegenerative disorders.
Acknowledgments
The technical assistance of Pascale Hince, Gaétan Gagnon, and Daniel Houle is gratefully acknowledged. The work in Julien’s laboratory was supported by the Medical Research Council of Canada, the ALS Association (USA) and the American Health Assistance Foundation (USA). The maintenance of mice also was supported in part by the Canadian Network of Centre of Excellence in NeuroScience. S.C.-D. has a Medical Research Council of Canada studentship and J.-P.J. has a Medical Research Council of Canada Senior Scholarship. Salary support for D.W.C. is provided by the Ludwig Institute for Cancer Research Grant. D.L.P. is supported by National Institutes of Health Grants AG05146 and NS37145. P.W. is supported by the ALS Association and a Cal Ripken/Lou Gehrig Award.
ABBREVIATIONS
- SOD1
superoxide dismutase
- ALS
amyotrophic lateral sclerosis
- NF-H
neurofilament heavy
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Rosen D R, Siddique T, Patterson D, Figlewicz D A, Sapp P, Hentati A, Donaldson D, Goto J, O’Regan J P, Deng H X, et al. Nature (London) 1993;362:59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- 2.Cudkowicz M E, McKenna-Yasek D, Sapp P E, Chin W, Geller B, Hayden D L, Schoenfeld D A, Hosler B A, Horvitz H R, Brown R H. Ann Neurol. 1997;41:210–221. doi: 10.1002/ana.410410212. [DOI] [PubMed] [Google Scholar]
- 3.Fridovich I. Adv Enzymol Relat Areas Mol Biol. 1986;58:61–97. doi: 10.1002/9780470123041.ch2. [DOI] [PubMed] [Google Scholar]
- 4.Tu P H, Gurney M E, Julien J-P, Lee V M-Y, Trojanowski J Q. Lab Invest. 1997;76:441–456. [PubMed] [Google Scholar]
- 5.Cleveland D W, Bruijn L I, Wong P C, Marszalek J R, Vechio J D, Lee M K, Xu X S, Borchelt D R, Sisodia S S, Price D L. Neurology. 1996;47:S54–S61. doi: 10.1212/wnl.47.4_suppl_2.54s. [DOI] [PubMed] [Google Scholar]
- 6.Julien J-P. Trends Cell Biol. 1997;7:243–249. doi: 10.1016/S0962-8924(97)01049-0. [DOI] [PubMed] [Google Scholar]
- 7.Beckman J S, Carson M, Smith C D, Koppenol W H. Nature (London) 1993;364:584. doi: 10.1038/364584a0. [DOI] [PubMed] [Google Scholar]
- 8.Wiedau-Pazos M, Goto J J, Rabizadeh S, Gralla E B, Roe J A, Lee M K, Valentine J S, Bredesen D E. Science. 1996;271:515–518. doi: 10.1126/science.271.5248.515. [DOI] [PubMed] [Google Scholar]
- 9.Yim M B, Kang J-H, Yim H-S, Kwak H-S, Chock P B, Stadtman E R. Proc Natl Acad Sci USA. 1996;93:5709–5714. doi: 10.1073/pnas.93.12.5709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carpenter S. Neurology. 1968;18:841–851. doi: 10.1212/wnl.18.9.841. [DOI] [PubMed] [Google Scholar]
- 11.Rouleau G A, Clark A W, Rooke K, Pramatarova A, Krizus A, Suchowersky O, Julien J-P, Figlewicz D. Ann Neurol. 1996;39:128–131. doi: 10.1002/ana.410390119. [DOI] [PubMed] [Google Scholar]
- 12.Gurney M E, Pu H, Chiu A Y, Canto M C D, Polchow C Y, Alexander D D, Caliendo J, Hentati A, Kwon Y W, Deng H-X, et al. Science. 1994;264:1772–1775. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- 13.Tu P H, Raju P, Robinson K A, Gurney M E, Trojanowski J Q, Lee V M-Y. Proc Natl Acad Sci USA. 1996;93:3155–3160. doi: 10.1073/pnas.93.7.3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wong P C, Pardo C A, Borchelt D R, Lee M K, Copeland N G, Jenkins N A, Sisodia S S, Cleveland D W, Price D L. Neuron. 1995;14:1105–1116. doi: 10.1016/0896-6273(95)90259-7. [DOI] [PubMed] [Google Scholar]
- 15.Morrison B M, Gordon J W, Ripps M E, Morrison J H. J Comp Neurol. 1996;373:619–631. doi: 10.1002/(SICI)1096-9861(19960930)373:4<619::AID-CNE9>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 16.Côté F, Collard J-F, Julien J-P. Cell. 1993;73:35–46. doi: 10.1016/0092-8674(93)90158-m. [DOI] [PubMed] [Google Scholar]
- 17.Xu Z, Cork L C, Griffin J W, Cleveland D W. Cell. 1993;73:23–33. doi: 10.1016/0092-8674(93)90157-l. [DOI] [PubMed] [Google Scholar]
- 18.Lee M K, Marszalek J R, Cleveland D W. Neuron. 1994;13:975–988. doi: 10.1016/0896-6273(94)90263-1. [DOI] [PubMed] [Google Scholar]
- 19.Collard J-F, Côté F, Julien J-P. Nature (London) 1995;375:61–64. doi: 10.1038/375061a0. [DOI] [PubMed] [Google Scholar]
- 20.Figlewicz D A, Krizus A, Martinoli M G, Meininger V, Dib M, Rouleau G A, Julien J-P. Hum Mol Genet. 1994;3:1757–1761. doi: 10.1093/hmg/3.10.1757. [DOI] [PubMed] [Google Scholar]
- 21.Figlewicz D A, Rouleau G A, Krizus A, Julien J-P. Gene. 1993;132:297–300. doi: 10.1016/0378-1119(93)90211-k. [DOI] [PubMed] [Google Scholar]
- 22.Levine R L, Williams J A, Stadtman E R, Shacter E. Methods Enzymol. 1994;233:346–357. doi: 10.1016/s0076-6879(94)33040-9. [DOI] [PubMed] [Google Scholar]
- 23.Ghadge G D, Lee J P, Bindokas V P, Jordan J, Ma L, Miller R J, Roos R P. J Neurosci. 1997;17:8756–5766. doi: 10.1523/JNEUROSCI.17-22-08756.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bruijn L I, Beal M F, Becher M W, Schulz J B, Wong P C, Price D L, Cleveland D W. Proc Natl Acad Sci USA. 1997;94:7606–7611. doi: 10.1073/pnas.94.14.7606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferrante R J, Browne S E, Shinobu L A, Bowling A C, Baik M J, Macgarvey U, Kowall N W, Brown R H, Beal M F. J Neurochem. 1997;69:2064–2074. doi: 10.1046/j.1471-4159.1997.69052064.x. [DOI] [PubMed] [Google Scholar]
- 26.Zhu Q, Couillard-Després S, Julien J-P. Exp Neurol. 1998;148:299–316. doi: 10.1006/exnr.1997.6654. [DOI] [PubMed] [Google Scholar]
- 27.Williamson T L, Bruijn L I, Zhu Q, Anderson K L, Anderson S D, Julien J-P, Cleveland D W. Proc Natl Acad Sci USA. 1998;95:9631–9636. doi: 10.1073/pnas.95.16.9631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eyer J, Cleveland D W, Wong P C, Peterson A C. Nature (London) 1998;391:584–587. doi: 10.1038/35378. [DOI] [PubMed] [Google Scholar]
- 29.Eyer J, Peterson A C. Neuron. 1994;12:389–405. doi: 10.1016/0896-6273(94)90280-1. [DOI] [PubMed] [Google Scholar]
- 30.Lefebvre S, Mushynski W E. Biochem Biophys Res Commun. 1987;145:1006–1011. doi: 10.1016/0006-291x(87)91535-x. [DOI] [PubMed] [Google Scholar]
- 31.Lefebvre S, Mushynski W E. Biochemistry. 1988;27:8503–8508. doi: 10.1021/bi00422a031. [DOI] [PubMed] [Google Scholar]
- 32.Dal Canto M C, Gurney M E. Brain Res. 1995;676:25–40. doi: 10.1016/0006-8993(95)00063-v. [DOI] [PubMed] [Google Scholar]
- 33.Reiner A, Medina L, Figueredo-Cardenas G, Anfinson S. Exp Neurol. 1995;131:239–250. doi: 10.1016/0014-4886(95)90046-2. [DOI] [PubMed] [Google Scholar]
- 34.Elliott J L, Snider W D. NeuroReport. 1995;6:449–452. doi: 10.1097/00001756-199502000-00011. [DOI] [PubMed] [Google Scholar]
- 35.Ince P, Stout N, Shaw P, Slade J, Hunziker W, Heizmann C W, Baimbridge K G. Neuropathol Appl Neurobiol. 1993;19:291–299. doi: 10.1111/j.1365-2990.1993.tb00443.x. [DOI] [PubMed] [Google Scholar]
- 36.Ho B K, Alexianu M E, Colom L V, Mohamed A H, Serrano F, Appel S H. Proc Natl Acad Sci USA. 1996;93:6796–6801. doi: 10.1073/pnas.93.13.6796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parhad I M, Scott J N, Cellars L A, Bains J S, Krekoski C A, Clark A W. J Neurosci Res. 1995;41:355–366. doi: 10.1002/jnr.490410308. [DOI] [PubMed] [Google Scholar]
- 38.Kuchel G A, Poon T, Irshad K, Richard C, Julien J-P, Cowen T. NeuroReport. 1997;8:799–805. doi: 10.1097/00001756-199702100-00044. [DOI] [PubMed] [Google Scholar]
- 39.Bergeron C, Beric-Maskarel K, Muntasser S, Weyer L, Somerville M J, Percy M E. J Neuropathol Exp Neurol. 1994;53:221–230. doi: 10.1097/00005072-199405000-00002. [DOI] [PubMed] [Google Scholar]
- 40.Gurney M E, Cutting F B, Zhai P, Doble A, Taylor C P, Andrus P K, Hall E D. Ann Neurol. 1996;39:147–157. doi: 10.1002/ana.410390203. [DOI] [PubMed] [Google Scholar]
- 41.Hottinger A F, Fine E G, Gurney M E, Zurn A D, Aebischer P. Eur J Neurosci. 1997;9:1548–1551. doi: 10.1111/j.1460-9568.1997.tb01511.x. [DOI] [PubMed] [Google Scholar]
- 42.Kostic V, Jackson-Lewis V, de Bilbao F, Dubois-Dauphin M, Przedborski S. Science. 1997;277:559–562. doi: 10.1126/science.277.5325.559. [DOI] [PubMed] [Google Scholar]