Abstract
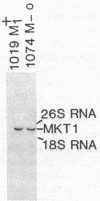
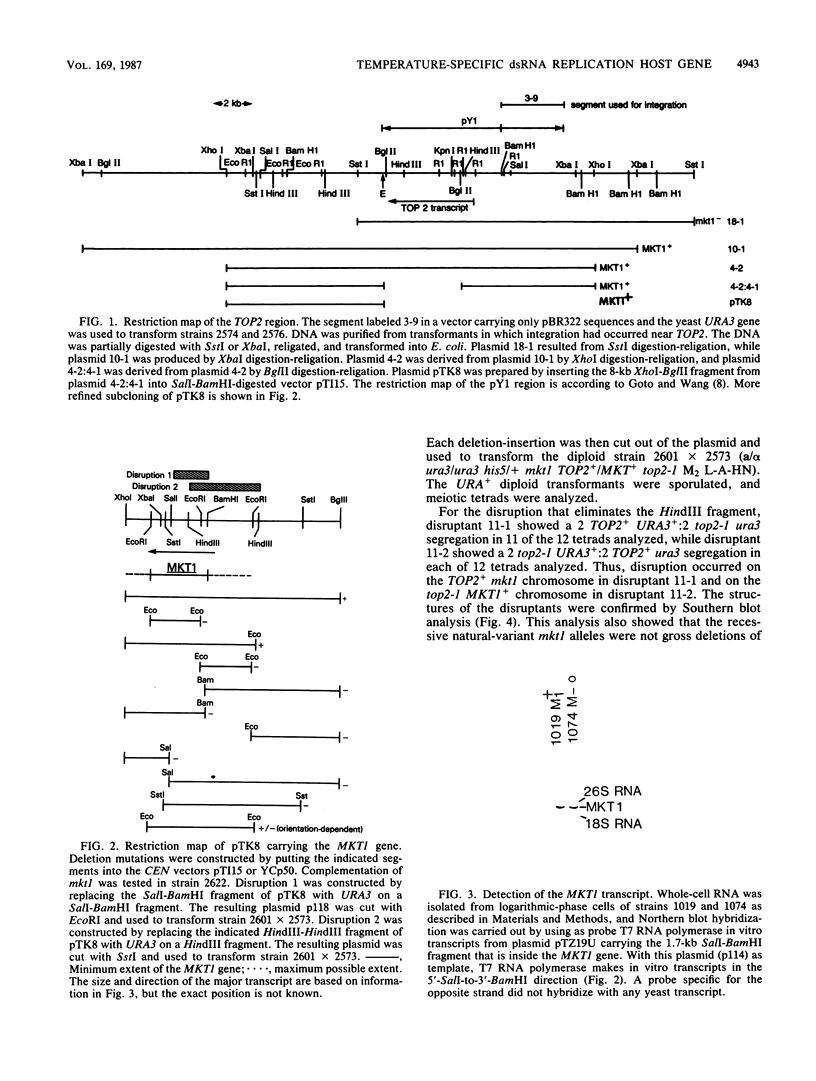
The MKT1 gene was defined by recessive alleles present in many laboratory strains of Saccharomyces cerevisiae that result in loss of M2 double-stranded RNA at temperatures above 30 degrees C if L-A-HN double-stranded RNA is present but not if L-A-H is present. I mapped MKT1 near TOP2 and isolated the gene by chromosome walking from TOP2. The gene location was defined by deletions, and a 2.8-kilobase transcript corresponding to the gene was detected. The recessive natural-variant mutations are not deletions as judged by Southern blots, but deletions of the MKT1 gene constructed in vitro and used to replace the normal gene surprisingly resulted in the same phenotype as that of the mkt1 natural variants, namely, a temperature-dependent maintenance of M2 double-stranded RNA. Thus the MKT1 gene product is only needed for M2 replication or maintenance at temperatures above 30 degrees C and if L-A-HN is present. The temperature dependence does not reflect the thermolability of a mutant gene product, as had previously been thought, nor does L-A double-stranded RNA need MKT1, as previously hypothesized. MKT1 may be involved in the process of packaging M2 double-stranded RNA. MKT1 is dispensable for host cell growth, mating, meiosis, and spore germination.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ball S. G., Tirtiaux C., Wickner R. B. Genetic Control of L-a and L-(Bc) Dsrna Copy Number in Killer Systems of SACCHAROMYCES CEREVISIAE. Genetics. 1984 Jun;107(2):199–217. doi: 10.1093/genetics/107.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostian K. A., Hopper J. E., Rogers D. T., Tipper D. J. Translational analysis of the killer-associated virus-like particle dsRNA genome of S. cerevisiae: M dsRNA encodes toxin. Cell. 1980 Feb;19(2):403–414. doi: 10.1016/0092-8674(80)90514-0. [DOI] [PubMed] [Google Scholar]
- DiNardo S., Voelkel K., Sternglanz R. DNA topoisomerase II mutant of Saccharomyces cerevisiae: topoisomerase II is required for segregation of daughter molecules at the termination of DNA replication. Proc Natl Acad Sci U S A. 1984 May;81(9):2616–2620. doi: 10.1073/pnas.81.9.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban R., Wickner R. B. Three different M1 RNA-containing viruslike particle types in Saccharomyces cerevisiae: in vitro M1 double-stranded RNA synthesis. Mol Cell Biol. 1986 May;6(5):1552–1561. doi: 10.1128/mcb.6.5.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura T., Esteban R., Wickner R. B. In vitro L-A double-stranded RNA synthesis in virus-like particles from Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4433–4437. doi: 10.1073/pnas.83.12.4433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura T., Wickner R. B. L-A double-stranded RNA viruslike particle replication cycle in Saccharomyces cerevisiae: particle maturation in vitro and effects of mak10 and pet18 mutations. Mol Cell Biol. 1987 Jan;7(1):420–426. doi: 10.1128/mcb.7.1.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura T., Wickner R. B. Thermolabile L-A virus-like particles from pet18 mutants of Saccharomyces cerevisiae. Mol Cell Biol. 1986 Feb;6(2):404–410. doi: 10.1128/mcb.6.2.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto T., Wang J. C. Yeast DNA topoisomerase II is encoded by a single-copy, essential gene. Cell. 1984 Apr;36(4):1073–1080. doi: 10.1016/0092-8674(84)90057-6. [DOI] [PubMed] [Google Scholar]
- Hannig E. M., Leibowitz M. J., Wickner R. B. On the mechanism of exclusion of M2 double-stranded RNA by L-A-E double-stranded RNA in Saccharomyces cerevisiae. Yeast. 1985 Sep;1(1):57–65. doi: 10.1002/yea.320010107. [DOI] [PubMed] [Google Scholar]
- Harris M. S. Virus-like particles and double stranded RNA from killer and non-killer strains of Saccharomyces cerevisiae. Microbios. 1978;21(85-86):161–176. [PubMed] [Google Scholar]
- Hopper J. E., Bostian K. A., Rowe L. B., Tipper D. J. Translation of the L-species dsRNA genome of the killer-associated virus-like particles of Saccharomyces cerevisiae. J Biol Chem. 1977 Dec 25;252(24):9010–9017. [PubMed] [Google Scholar]
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983 Jan;153(1):163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibowitz M. J., Wickner R. B. Pet18: a chromosomal gene required for cell growth and for the maintenance of mitochondrial DNA and the killer plasmid of yeast. Mol Gen Genet. 1978 Oct 4;165(2):115–121. doi: 10.1007/BF00269899. [DOI] [PubMed] [Google Scholar]
- Mortimer R. K., Hawthorne D. C. Genetic mapping in yeast. Methods Cell Biol. 1975;11:221–233. doi: 10.1016/s0091-679x(08)60325-8. [DOI] [PubMed] [Google Scholar]
- Newman A. M., Elliott S. G., McLaughlin C. S., Sutherland P. A., Warner R. C. Replication of double-stranded RNA of the virus-like particles in Saccharomyces cerevisiae. J Virol. 1981 Apr;38(1):263–271. doi: 10.1128/jvi.38.1.263-271.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley S. P., Sommer S. S., Wickner R. B. Superkiller mutations in Saccharomyces cerevisiae suppress exclusion of M2 double-stranded RNA by L-A-HN and confer cold sensitivity in the presence of M and L-A-HN. Mol Cell Biol. 1984 Apr;4(4):761–770. doi: 10.1128/mcb.4.4.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein R. J. One-step gene disruption in yeast. Methods Enzymol. 1983;101:202–211. doi: 10.1016/0076-6879(83)01015-0. [DOI] [PubMed] [Google Scholar]
- Sclafani R. A., Fangman W. L. Conservative replication of double-stranded RNA in Saccharomyces cerevisiae by displacement of progeny single strands. Mol Cell Biol. 1984 Aug;4(8):1618–1626. doi: 10.1128/mcb.4.8.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer S. S., Wickner R. B. Gene disruption indicates that the only essential function of the SKI8 chromosomal gene is to protect Saccharomyces cerevisiae from viral cytopathology. Virology. 1987 Mar;157(1):252–256. doi: 10.1016/0042-6822(87)90338-2. [DOI] [PubMed] [Google Scholar]
- Sommer S. S., Wickner R. B. Yeast L dsRNA consists of at least three distinct RNAs; evidence that the non-Mendelian genes [HOK], [NEX] and [EXL] are on one of these dsRNAs. Cell. 1982 Dec;31(2 Pt 1):429–441. doi: 10.1016/0092-8674(82)90136-2. [DOI] [PubMed] [Google Scholar]
- Toh-E A., Guerry P., Wickner R. B. Chromosomal superkiller mutants of Saccharomyces cerevisiae. J Bacteriol. 1978 Dec;136(3):1002–1007. doi: 10.1128/jb.136.3.1002-1007.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toh-e A., Sahashi Y. The PET18 locus of Saccharomyces cerevisiae: a complex locus containing multiple genes. Yeast. 1985 Dec;1(2):159–171. doi: 10.1002/yea.320010204. [DOI] [PubMed] [Google Scholar]
- Wickner R. B. Double-stranded RNA replication in yeast: the killer system. Annu Rev Biochem. 1986;55:373–395. doi: 10.1146/annurev.bi.55.070186.002105. [DOI] [PubMed] [Google Scholar]
- Wickner R. B., Fujimura T., Esteban R. Overview of double-stranded RNA replication in Saccharomyces cerevisiae. Basic Life Sci. 1986;40:149–163. doi: 10.1007/978-1-4684-5251-8_12. [DOI] [PubMed] [Google Scholar]
- Wickner R. B. Killer systems in Saccharomyces cerevisiae: three distinct modes of exclusion of M2 double-stranded RNA by three species of double-stranded RNA, M1, L-A-E, and L-A-HN. Mol Cell Biol. 1983 Apr;3(4):654–661. doi: 10.1128/mcb.3.4.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner R. B., Koh T. J., Crowley J. C., O'Neil J., Kaback D. B. Molecular cloning of chromosome I DNA from Saccharomyces cerevisiae: isolation of the MAK16 gene and analysis of an adjacent gene essential for growth at low temperatures. Yeast. 1987 Mar;3(1):51–57. doi: 10.1002/yea.320030108. [DOI] [PubMed] [Google Scholar]
- Wickner R. B. Mapping chromosomal genes of Saccharomyces cerevisiae using an improved genetic mapping method. Genetics. 1979 Jul;92(3):803–821. doi: 10.1093/genetics/92.3.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner R. B. Plasmids controlled exclusion of the K2 killer double-stranded RNA plasmid of yeast. Cell. 1980 Aug;21(1):217–226. doi: 10.1016/0092-8674(80)90129-4. [DOI] [PubMed] [Google Scholar]
- Wickner R. B., Toh-e A. [HOK], a new yeast non-Mendelian trait, enables a replication-defective killer plasmid to be maintained. Genetics. 1982 Feb;100(2):159–174. doi: 10.1093/genetics/100.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]