Abstract
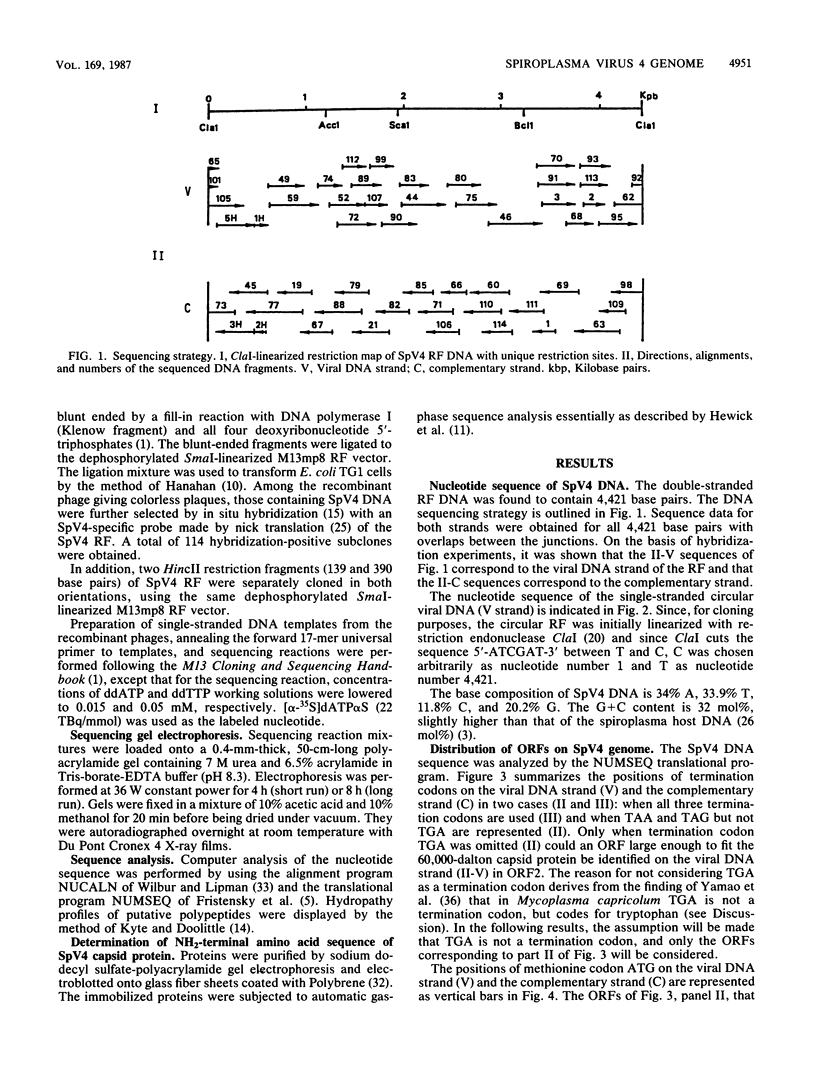
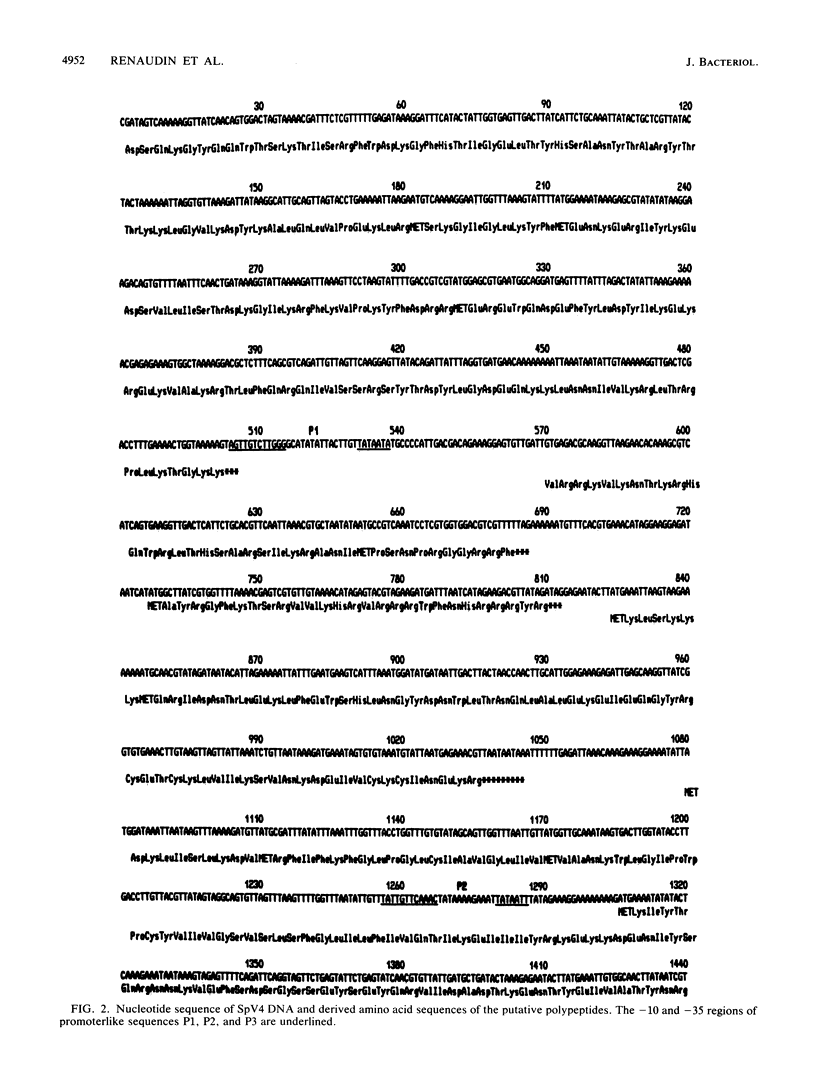
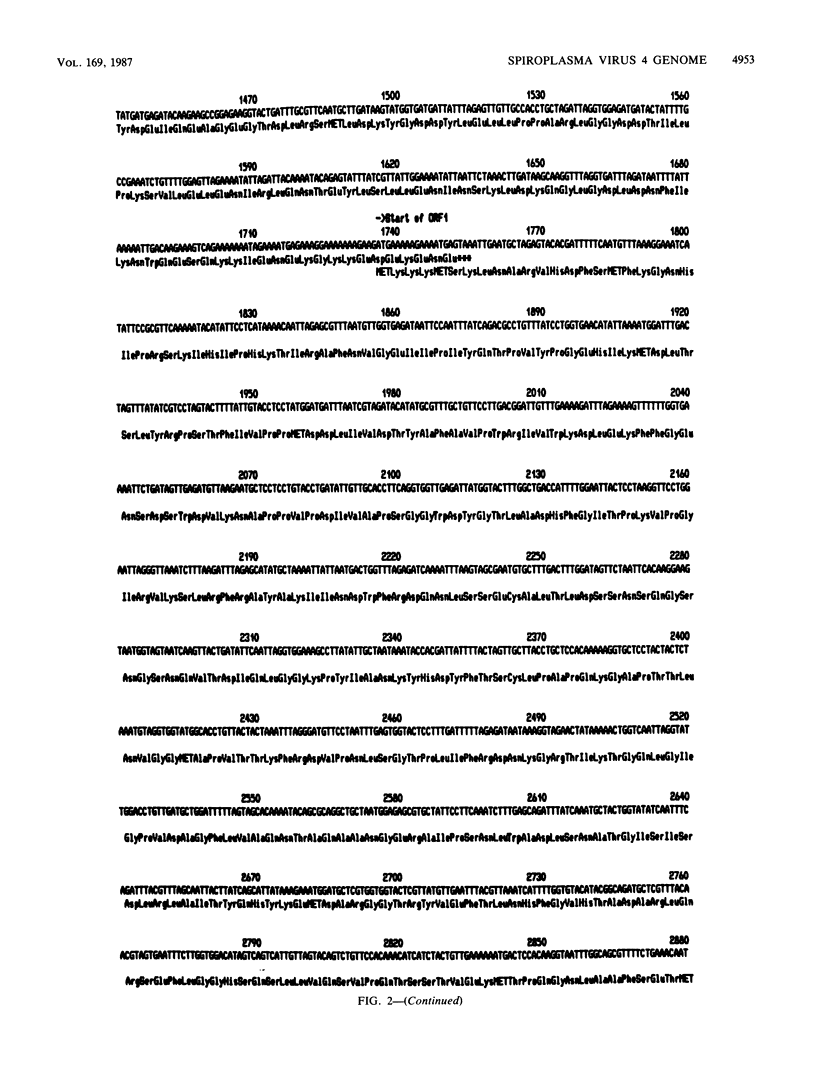
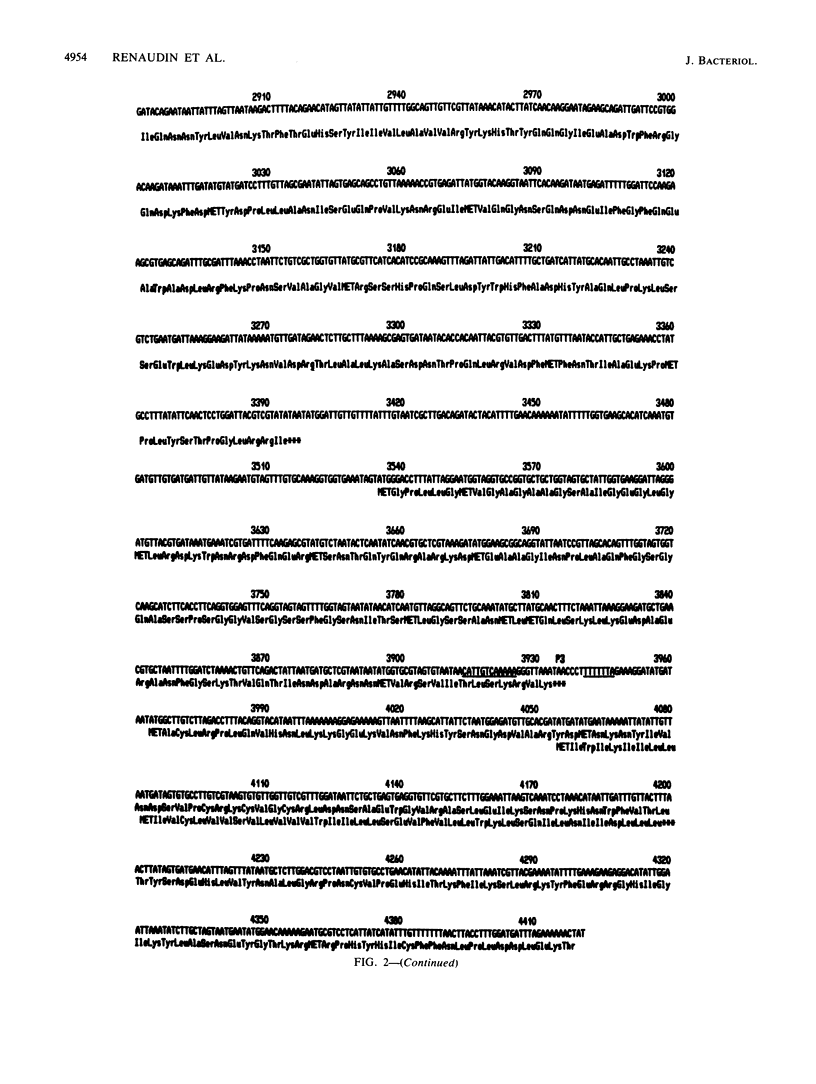
The replicative form (RF) of spiroplasma virus 4 (SpV4) has been cloned in Escherichia coli, and the cloned RF has been shown to be infectious by transfection (M. C. Pascarel-Devilder, J. Renaudin, and J.-M. Bové, Virology 151:390-393, 1986). The cloned SpV4 RF was randomly subcloned and was fully sequenced by the dideoxy chain termination technique, using the M13 cloning and sequencing system. The nucleotide sequence of the SpV4 genome contains 4,421 nucleotides with a G+C content of 32 mol%. The triplet TGA is not a termination codon but, as in Mycoplasma capricolum (F. Yamao, A. Muto, Y. Kawauchi, M. Iwami, S. Iwagani, Y. Azumi, and S. Osawa, Proc. Natl. Acad. Sci. USA 82:2306-2309, 1985), probably codes for tryptophan. With these assumptions, nine open reading frames (ORFs) were identified. All nine are characterized by an ATG or GTG initiation codon, one or several termination codons, and a Shine-Dalgarno sequence upstream of the initiation codon. The nine ORFs are distributed in all three reading frames. One of the ORFs (ORF1) corresponds to the 60,000-dalton capsid protein gene. Analysis of codon usage showed that T- and A-terminated codons are preferably used, reflecting the low G+C content (32 mol%) of the SpV4 genome. The viral DNA contains two G+C-rich inverted repeat sequences. One could be involved in transcription termination and the other in initiation of cDNA strand synthesis. The SpV4 genome was found to contain at least three promoterlike sequences quasi-identical to those of eubacteria. These results fully support the bacterial origin of spiroplasmas.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Deininger P. L. Random subcloning of sonicated DNA: application to shotgun DNA sequence analysis. Anal Biochem. 1983 Feb 15;129(1):216–223. doi: 10.1016/0003-2697(83)90072-6. [DOI] [PubMed] [Google Scholar]
- Fristensky B., Lis J., Wu R. Portable microcomputer software for nucleotide sequence analysis. Nucleic Acids Res. 1982 Oct 25;10(20):6451–6463. doi: 10.1093/nar/10.20.6451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frydenberg J., Christiansen C. The sequence of 16S rRNA from Mycoplasma strain PG50. DNA. 1985 Apr;4(2):127–137. doi: 10.1089/dna.1985.4.127. [DOI] [PubMed] [Google Scholar]
- Gadeau A. P., Mouches C., Bove J. M. Probable insensitivity of mollicutes to rifampin and characterization of spiroplasmal DNA-dependent RNA polymerase. J Bacteriol. 1986 Jun;166(3):824–828. doi: 10.1128/jb.166.3.824-828.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godson G. N., Barrell B. G., Staden R., Fiddes J. C. Nucleotide sequence of bacteriophage G4 DNA. Nature. 1978 Nov 16;276(5685):236–247. doi: 10.1038/276236a0. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Hewick R. M., Hunkapiller M. W., Hood L. E., Dreyer W. J. A gas-liquid solid phase peptide and protein sequenator. J Biol Chem. 1981 Aug 10;256(15):7990–7997. [PubMed] [Google Scholar]
- Iwami M., Muto A., Yamao F., Osawa S. Nucleotide sequence of the rrnB 16S ribosomal RNA gene from Mycoplasma capricolum. Mol Gen Genet. 1984;196(2):317–322. doi: 10.1007/BF00328065. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Maraini G., Ottonello S., Gozzoli F., Merli A. Identification of a membrane protein binding the retinol in retinal pigment epithelium. Nature. 1977 Jan 6;265(5589):68–69. doi: 10.1038/265068a0. [DOI] [PubMed] [Google Scholar]
- McLaughlin J. R., Murray C. L., Rabinowitz J. C. Unique features in the ribosome binding site sequence of the gram-positive Staphylococcus aureus beta-lactamase gene. J Biol Chem. 1981 Nov 10;256(21):11283–11291. [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Moran C. P., Jr, Lang N., LeGrice S. F., Lee G., Stephens M., Sonenshein A. L., Pero J., Losick R. Nucleotide sequences that signal the initiation of transcription and translation in Bacillus subtilis. Mol Gen Genet. 1982;186(3):339–346. doi: 10.1007/BF00729452. [DOI] [PubMed] [Google Scholar]
- Mouchès C., Candresse T., Barroso G., Saillard C., Wroblewski H., Bové J. M. Gene for spiralin, the major membrane protein of the helical mollicute Spiroplasma citri: cloning and expression in Escherichia coli. J Bacteriol. 1985 Dec;164(3):1094–1099. doi: 10.1128/jb.164.3.1094-1099.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascarel-Devilder M. C., Renaudin J., Bove J. M. The spiroplasma virus 4 replicative form cloned in Escherichia coli transfects spiroplasmas. Virology. 1986 Jun;151(2):390–393. doi: 10.1016/0042-6822(86)90060-7. [DOI] [PubMed] [Google Scholar]
- Reznikoff W. S., Siegele D. A., Cowing D. W., Gross C. A. The regulation of transcription initiation in bacteria. Annu Rev Genet. 1985;19:355–387. doi: 10.1146/annurev.ge.19.120185.002035. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rogers M. J., Simmons J., Walker R. T., Weisburg W. G., Woese C. R., Tanner R. S., Robinson I. M., Stahl D. A., Olsen G., Leach R. H. Construction of the mycoplasma evolutionary tree from 5S rRNA sequence data. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1160–1164. doi: 10.1073/pnas.82.4.1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers M. J., Steinmetz A. A., Walker R. T. The nucleotide sequence of a tRNA gene cluster from Spiroplasma meliferum. Nucleic Acids Res. 1986 Apr 11;14(7):3145–3145. doi: 10.1093/nar/14.7.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano T., Hatanaka M. Fate of viral RNA of murine leukemia virus after infection. Proc Natl Acad Sci U S A. 1975 Jan;72(1):343–347. doi: 10.1073/pnas.72.1.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandekerckhove J., Bauw G., Puype M., Van Damme J., Van Montagu M. Protein-blotting on Polybrene-coated glass-fiber sheets. A basis for acid hydrolysis and gas-phase sequencing of picomole quantities of protein previously separated on sodium dodecyl sulfate/polyacrylamide gel. Eur J Biochem. 1985 Oct 1;152(1):9–19. doi: 10.1111/j.1432-1033.1985.tb09157.x. [DOI] [PubMed] [Google Scholar]
- Wilbur W. J., Lipman D. J. Rapid similarity searches of nucleic acid and protein data banks. Proc Natl Acad Sci U S A. 1983 Feb;80(3):726–730. doi: 10.1073/pnas.80.3.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese C. R., Maniloff J., Zablen L. B. Phylogenetic analysis of the mycoplasmas. Proc Natl Acad Sci U S A. 1980 Jan;77(1):494–498. doi: 10.1073/pnas.77.1.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wróblewski H., Robic D., Thomas D., Blanchard A. Comparison of the amino acid compositions and antigenic properties of spiralins purified from the plasma membranes of different spiroplasmas. Ann Microbiol (Paris) 1984 Jan-Feb;135A(1):73–82. doi: 10.1016/s0769-2609(84)80061-7. [DOI] [PubMed] [Google Scholar]
- Yamao F., Muto A., Kawauchi Y., Iwami M., Iwagami S., Azumi Y., Osawa S. UGA is read as tryptophan in Mycoplasma capricolum. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2306–2309. doi: 10.1073/pnas.82.8.2306. [DOI] [PMC free article] [PubMed] [Google Scholar]



