Abstract
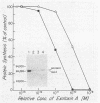
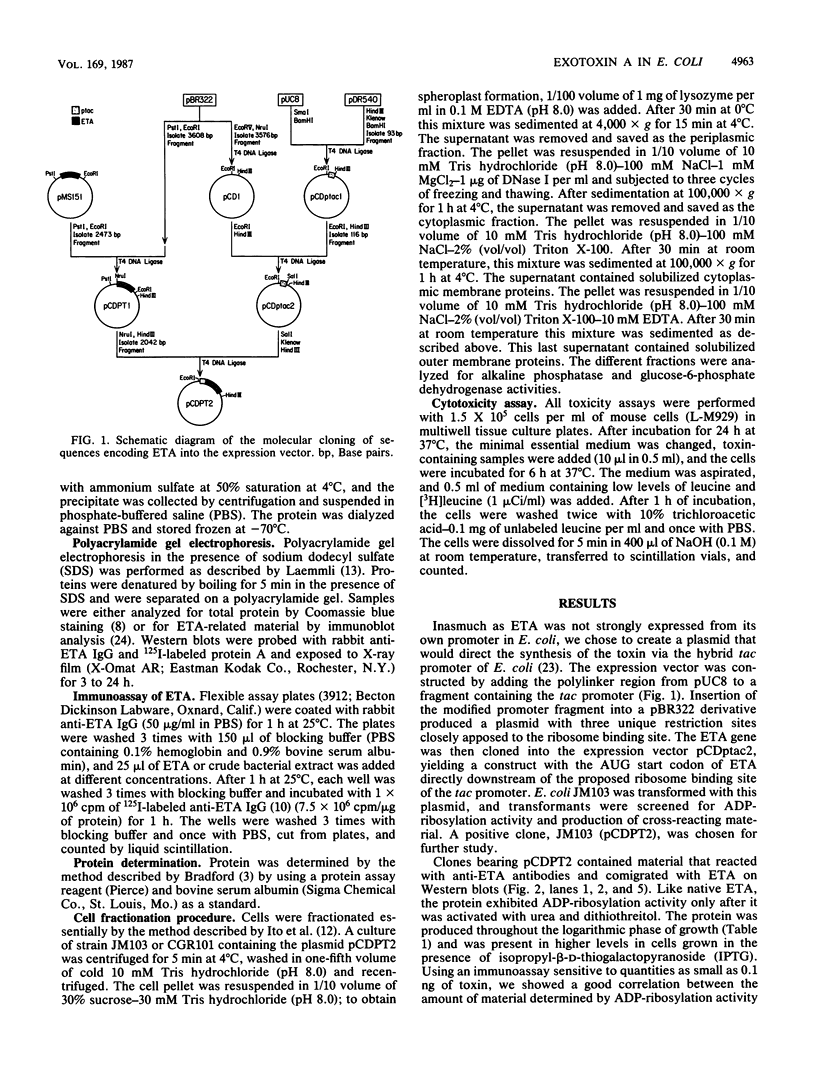
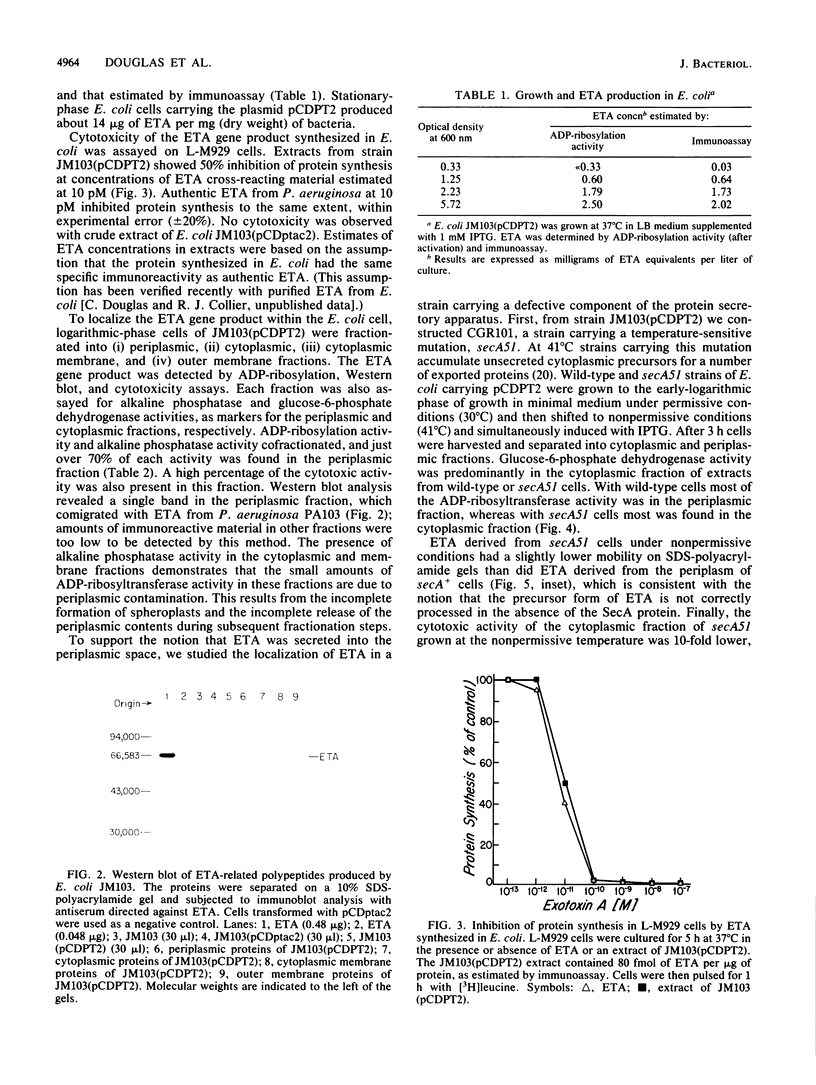
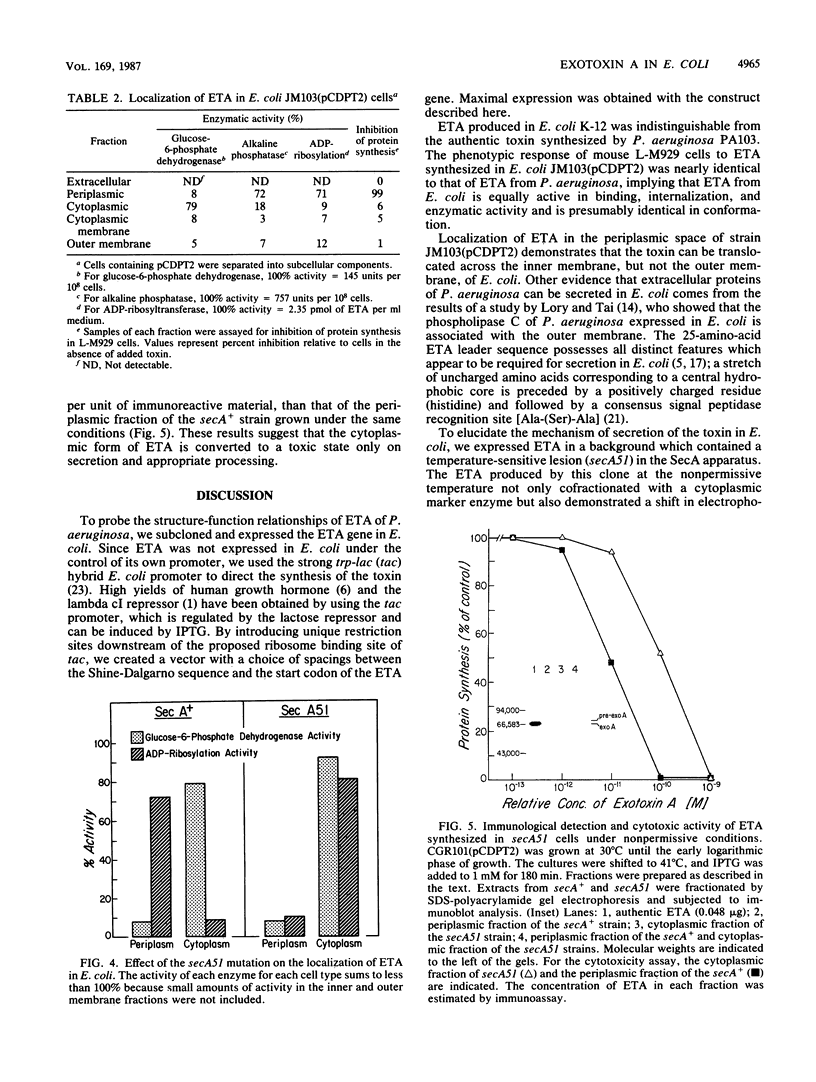
We subcloned the structural gene for exotoxin A (ETA) of Pseudomonas aeruginosa in front of the tac promoter in an Escherichia coli expression vector and studied the intracellular location and properties of the protein product. The E. coli K-12 strain that carried this recombinant plasmid produced an immunoreactive protein that was identical to authentic ETA in size and in cytotoxic and ADP-ribosyl transferase activities per unit of immunoreactive material. The protein was predominantly in the periplasmic fraction; and a mutation in the secA gene blocked secretion, processing, and conversion of the protein to a fully toxic conformation. The results indicate that expression of the ETA gene in E. coli yields native ETA, which is localized within the periplasmic space. This organism may therefore serve as a useful host for studying structure and function in ETA.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amann E., Brosius J., Ptashne M. Vectors bearing a hybrid trp-lac promoter useful for regulated expression of cloned genes in Escherichia coli. Gene. 1983 Nov;25(2-3):167–178. doi: 10.1016/0378-1119(83)90222-6. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chung D. W., Collier R. J. Enzymatically active peptide from the adenosine diphosphate-ribosylating toxin of Pseudomonas aeruginosa. Infect Immun. 1977 Jun;16(3):832–841. doi: 10.1128/iai.16.3.832-841.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis B. D., Tai P. C. The mechanism of protein secretion across membranes. Nature. 1980 Jan 31;283(5746):433–438. doi: 10.1038/283433a0. [DOI] [PubMed] [Google Scholar]
- Douglas C. M., Collier R. J. Exotoxin A of Pseudomonas aeruginosa: substitution of glutamic acid 553 with aspartic acid drastically reduces toxicity and enzymatic activity. J Bacteriol. 1987 Nov;169(11):4967–4971. doi: 10.1128/jb.169.11.4967-4971.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Gray G. L., Smith D. H., Baldridge J. S., Harkins R. N., Vasil M. L., Chen E. Y., Heyneker H. L. Cloning, nucleotide sequence, and expression in Escherichia coli of the exotoxin A structural gene of Pseudomonas aeruginosa. Proc Natl Acad Sci U S A. 1984 May;81(9):2645–2649. doi: 10.1073/pnas.81.9.2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidi-Rontani C., Collier R. J. Exotoxin A of Pseudomonas aeruginosa: evidence that domain I functions in receptor binding. Mol Microbiol. 1987 Jul;1(1):67–72. doi: 10.1111/j.1365-2958.1987.tb00528.x. [DOI] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Iglewski B. H., Kabat D. NAD-dependent inhibition of protein synthesis by Pseudomonas aeruginosa toxin,. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2284–2288. doi: 10.1073/pnas.72.6.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K., Sato T., Yura T. Synthesis and assembly of the membrane proteins in E. coli. Cell. 1977 Jul;11(3):551–559. doi: 10.1016/0092-8674(77)90073-3. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lory S., Tai P. C. Characterization of the phospholipase C gene of Pseudomonas aeruginosa cloned in Escherichia coli. Gene. 1983 Apr;22(1):95–101. doi: 10.1016/0378-1119(83)90068-9. [DOI] [PubMed] [Google Scholar]
- MALAMY M., HORECKER B. L. The localization of alkaline phosphatase in E. coli K12. Biochem Biophys Res Commun. 1961 Jun 2;5:104–108. doi: 10.1016/0006-291x(61)90020-1. [DOI] [PubMed] [Google Scholar]
- Mandel M., Higa A. Calcium-dependent bacteriophage DNA infection. J Mol Biol. 1970 Oct 14;53(1):159–162. doi: 10.1016/0022-2836(70)90051-3. [DOI] [PubMed] [Google Scholar]
- Michaelis S., Beckwith J. Mechanism of incorporation of cell envelope proteins in Escherichia coli. Annu Rev Microbiol. 1982;36:435–465. doi: 10.1146/annurev.mi.36.100182.002251. [DOI] [PubMed] [Google Scholar]
- Middlebrook J. L., Dorland R. B. Bacterial toxins: cellular mechanisms of action. Microbiol Rev. 1984 Sep;48(3):199–221. doi: 10.1128/mr.48.3.199-221.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozola M. A., Wilson R. B., Jordan E. M., Draper R. K., Clowes R. C. Cloning and expression of a gene segment encoding the enzymatic moiety of Pseudomonas aeruginosa exotoxin A. J Bacteriol. 1984 Aug;159(2):683–687. doi: 10.1128/jb.159.2.683-687.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver D. B., Beckwith J. E. coli mutant pleiotropically defective in the export of secreted proteins. Cell. 1981 Sep;25(3):765–772. doi: 10.1016/0092-8674(81)90184-7. [DOI] [PubMed] [Google Scholar]
- Perlman D., Halvorson H. O. A putative signal peptidase recognition site and sequence in eukaryotic and prokaryotic signal peptides. J Mol Biol. 1983 Jun 25;167(2):391–409. doi: 10.1016/s0022-2836(83)80341-6. [DOI] [PubMed] [Google Scholar]
- Pollitt S., Zalkin H. Role of primary structure and disulfide bond formation in beta-lactamase secretion. J Bacteriol. 1983 Jan;153(1):27–32. doi: 10.1128/jb.153.1.27-32.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell D. R., Bennett G. N. Construction and analysis of in vivo activity of E. coli promoter hybrids and promoter mutants that alter the -35 to -10 spacing. Gene. 1982 Dec;20(2):231–243. doi: 10.1016/0378-1119(82)90042-7. [DOI] [PubMed] [Google Scholar]
- Tweten R. K., Collier R. J. Molecular cloning and expression of gene fragments from corynebacteriophage beta encoding enzymatically active peptides of diphtheria toxin. J Bacteriol. 1983 Nov;156(2):680–685. doi: 10.1128/jb.156.2.680-685.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer H. A., Comstock L. J., Vasser M. The tac promoter: a functional hybrid derived from the trp and lac promoters. Proc Natl Acad Sci U S A. 1983 Jan;80(1):21–25. doi: 10.1073/pnas.80.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]