Abstract
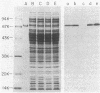
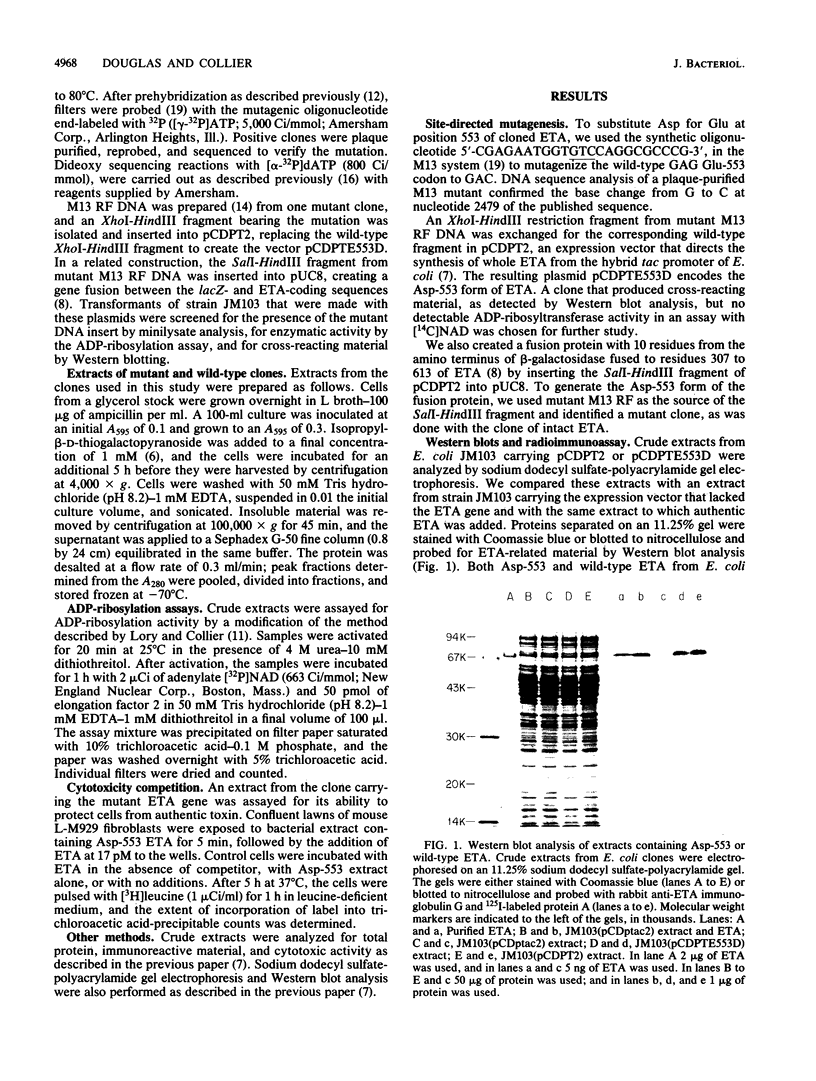
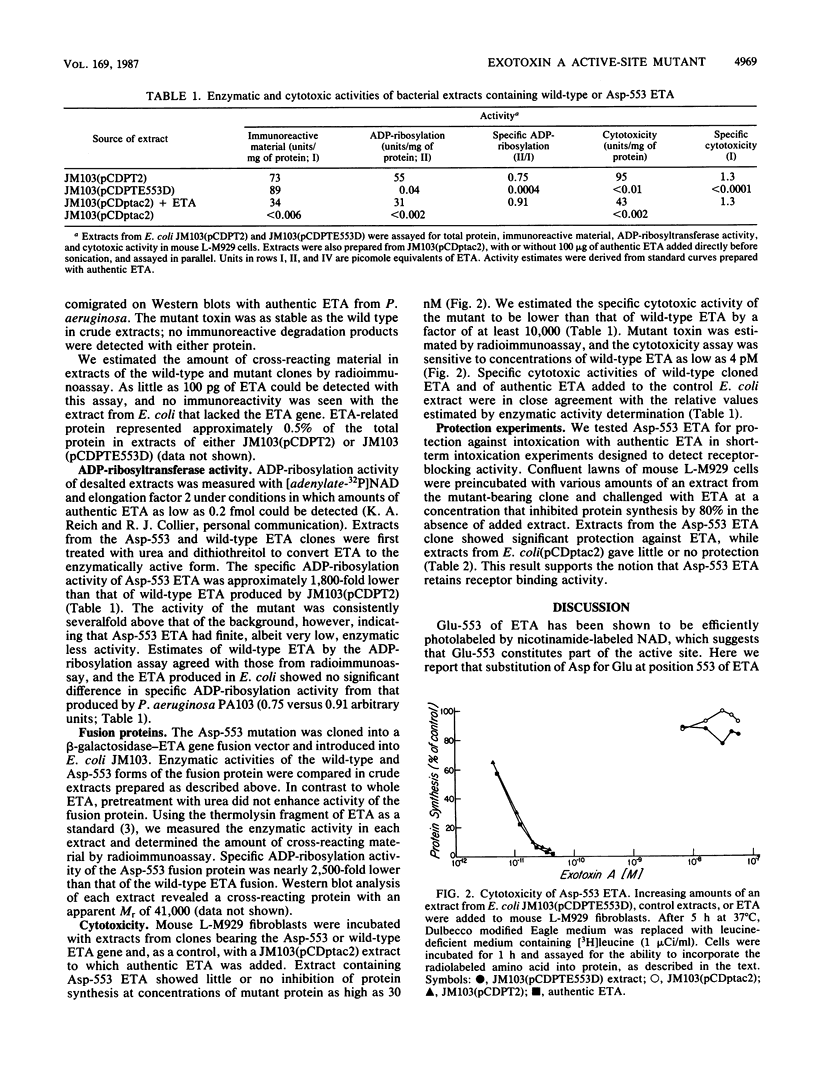
Glutamic acid 553 of Pseudomonas aeruginosa exotoxin A (ETA) has been identified by photoaffinity labeling as a residue within the NAD binding site (S.F. Carroll and R.J. Collier, J. Biol. Chem. 262:8707-8711, 1987). To explore the function of Glu-553 we used oligonucleotide-directed mutagenesis to replace this residue with Asp in cloned ETA and expressed the mutant gene in Escherichia coli K-12. ADP-ribosylation activity of Asp-553 ETA in cell extracts was about 1,800-fold lower and toxicity for mouse L-M929 fibroblasts was at least 10,000-fold lower than that of the wild-type toxin. Extracts containing Asp-553 ETA inhibited the cytotoxicity of authentic ETA on L-M929 fibroblasts, suggesting that the mutant toxin competes for ETA receptors. The results indicate that Glu-553 is crucial for ADP-ribosylation activity and, consequently, cytotoxicity of ETA. Substitution or deletion of this residue may be a route to new ETA vaccines.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Callahan L. T., 3rd, Martinez D., Marburg S., Tolman R. L., Galloway D. R. Toxoids of Pseudomonas aeruginosa exotoxin-A: photoaffinity inactivation of purified toxin and purified toxin derivatives. Infect Immun. 1984 Mar;43(3):1019–1026. doi: 10.1128/iai.43.3.1019-1026.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll S. F., Collier R. J. Active site of Pseudomonas aeruginosa exotoxin A. Glutamic acid 553 is photolabeled by NAD and shows functional homology with glutamic acid 148 of diphtheria toxin. J Biol Chem. 1987 Jun 25;262(18):8707–8711. [PubMed] [Google Scholar]
- Carroll S. F., Collier R. J. NAD binding site of diphtheria toxin: identification of a residue within the nicotinamide subsite by photochemical modification with NAD. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3307–3311. doi: 10.1073/pnas.81.11.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll S. F., McCloskey J. A., Crain P. F., Oppenheimer N. J., Marschner T. M., Collier R. J. Photoaffinity labeling of diphtheria toxin fragment A with NAD: structure of the photoproduct at position 148. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7237–7241. doi: 10.1073/pnas.82.21.7237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas C. M., Guidi-Rontani C., Collier R. J. Exotoxin A of Pseudomonas aeruginosa: active, cloned toxin is secreted into the periplasmic space of Escherichia coli. J Bacteriol. 1987 Nov;169(11):4962–4966. doi: 10.1128/jb.169.11.4962-4966.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray G. L., Smith D. H., Baldridge J. S., Harkins R. N., Vasil M. L., Chen E. Y., Heyneker H. L. Cloning, nucleotide sequence, and expression in Escherichia coli of the exotoxin A structural gene of Pseudomonas aeruginosa. Proc Natl Acad Sci U S A. 1984 May;81(9):2645–2649. doi: 10.1073/pnas.81.9.2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidi-Rontani C., Collier R. J. Exotoxin A of Pseudomonas aeruginosa: evidence that domain I functions in receptor binding. Mol Microbiol. 1987 Jul;1(1):67–72. doi: 10.1111/j.1365-2958.1987.tb00528.x. [DOI] [PubMed] [Google Scholar]
- Hwang J., Fitzgerald D. J., Adhya S., Pastan I. Functional domains of Pseudomonas exotoxin identified by deletion analysis of the gene expressed in E. coli. Cell. 1987 Jan 16;48(1):129–136. doi: 10.1016/0092-8674(87)90363-1. [DOI] [PubMed] [Google Scholar]
- Iglewski B. H., Kabat D. NAD-dependent inhibition of protein synthesis by Pseudomonas aeruginosa toxin,. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2284–2288. doi: 10.1073/pnas.72.6.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lory S., Collier R. J. Expression of enzymic activity by exotoxin A from Pseudomonas aeruginosa. Infect Immun. 1980 May;28(2):494–501. doi: 10.1128/iai.28.2.494-501.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marburg S., Tolman R. L., Callahan L. T., 3rd Pseudomonas exotoxin A: toxoid preparation by photoaffinity inactivation. Proc Natl Acad Sci U S A. 1983 May;80(10):2870–2873. doi: 10.1073/pnas.80.10.2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Middlebrook J. L., Dorland R. B. Bacterial toxins: cellular mechanisms of action. Microbiol Rev. 1984 Sep;48(3):199–221. doi: 10.1128/mr.48.3.199-221.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tweten R. K., Barbieri J. T., Collier R. J. Diphtheria toxin. Effect of substituting aspartic acid for glutamic acid 148 on ADP-ribosyltransferase activity. J Biol Chem. 1985 Sep 5;260(19):10392–10394. [PubMed] [Google Scholar]
- Vasil M. L., Iglewski B. H. Comparative toxicities of diphtherial toxin and Pseudomonas aeruginosa exotoxin A: evidence for different cell receptors. J Gen Microbiol. 1978 Oct;108(2):333–337. doi: 10.1099/00221287-108-2-333. [DOI] [PubMed] [Google Scholar]
- Zoller M. J., Smith M. Oligonucleotide-directed mutagenesis of DNA fragments cloned into M13 vectors. Methods Enzymol. 1983;100:468–500. doi: 10.1016/0076-6879(83)00074-9. [DOI] [PubMed] [Google Scholar]
- de Boer H. A., Comstock L. J., Vasser M. The tac promoter: a functional hybrid derived from the trp and lac promoters. Proc Natl Acad Sci U S A. 1983 Jan;80(1):21–25. doi: 10.1073/pnas.80.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]