Abstract
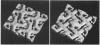
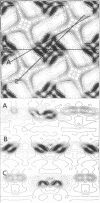
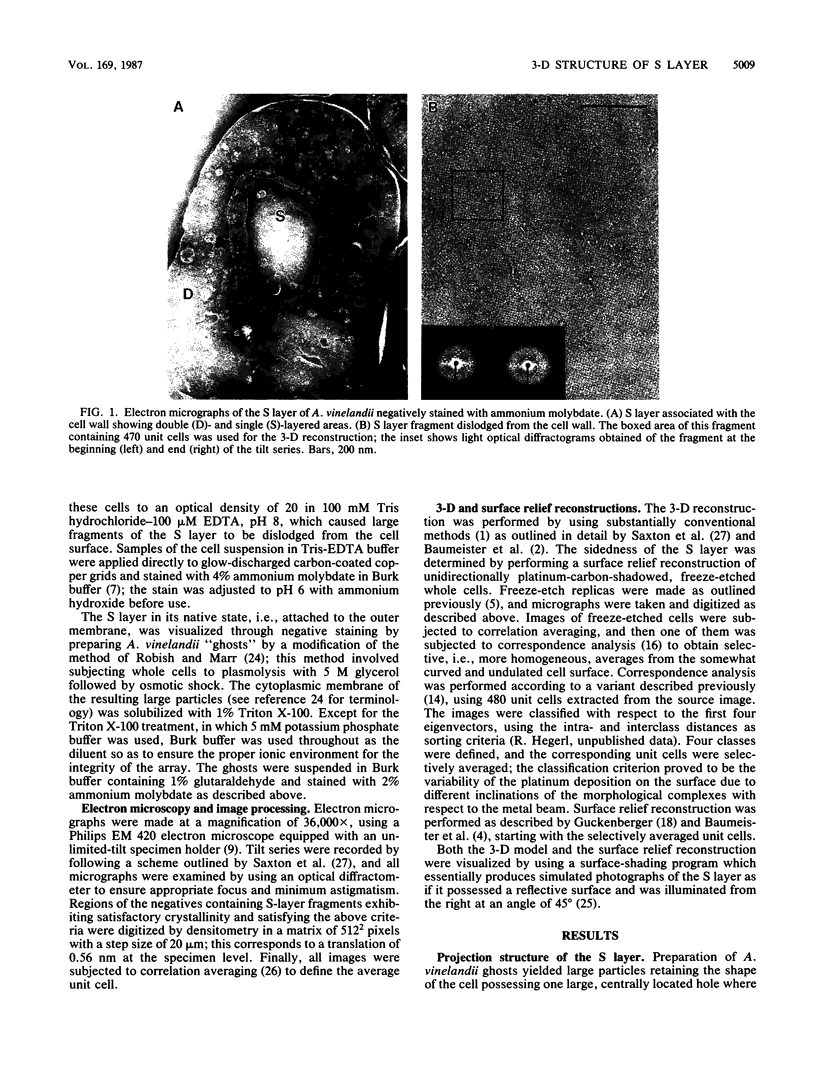
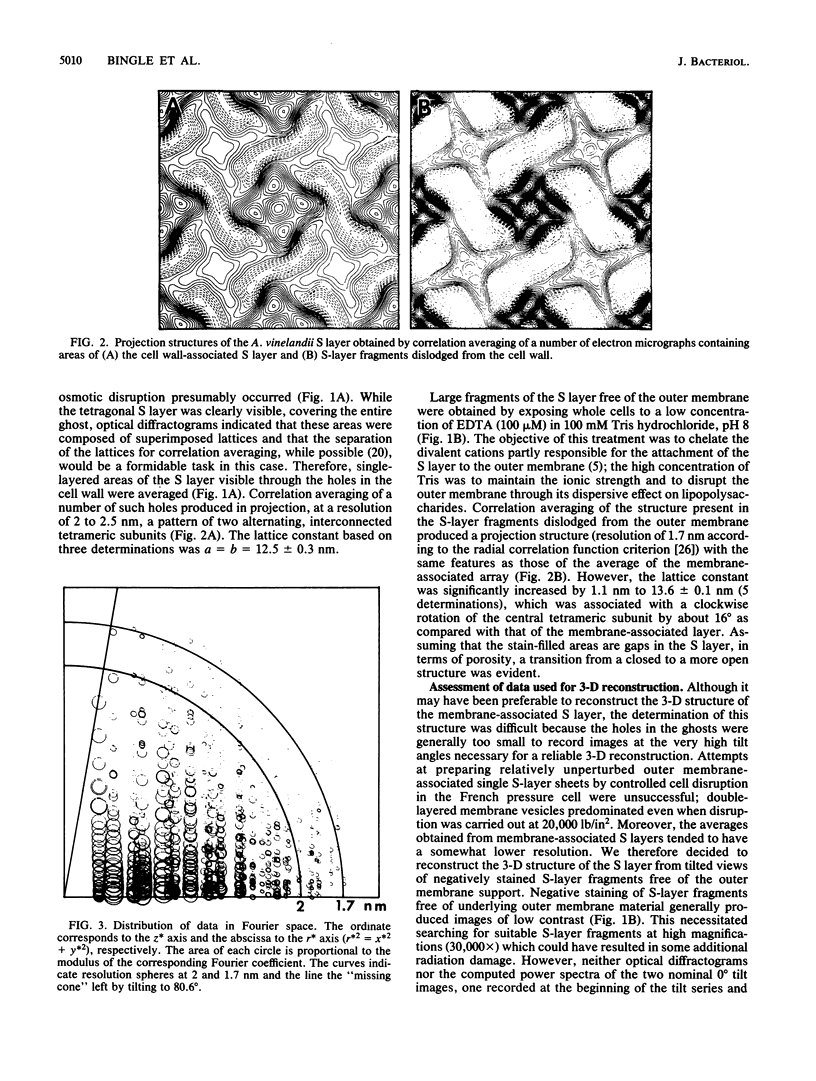
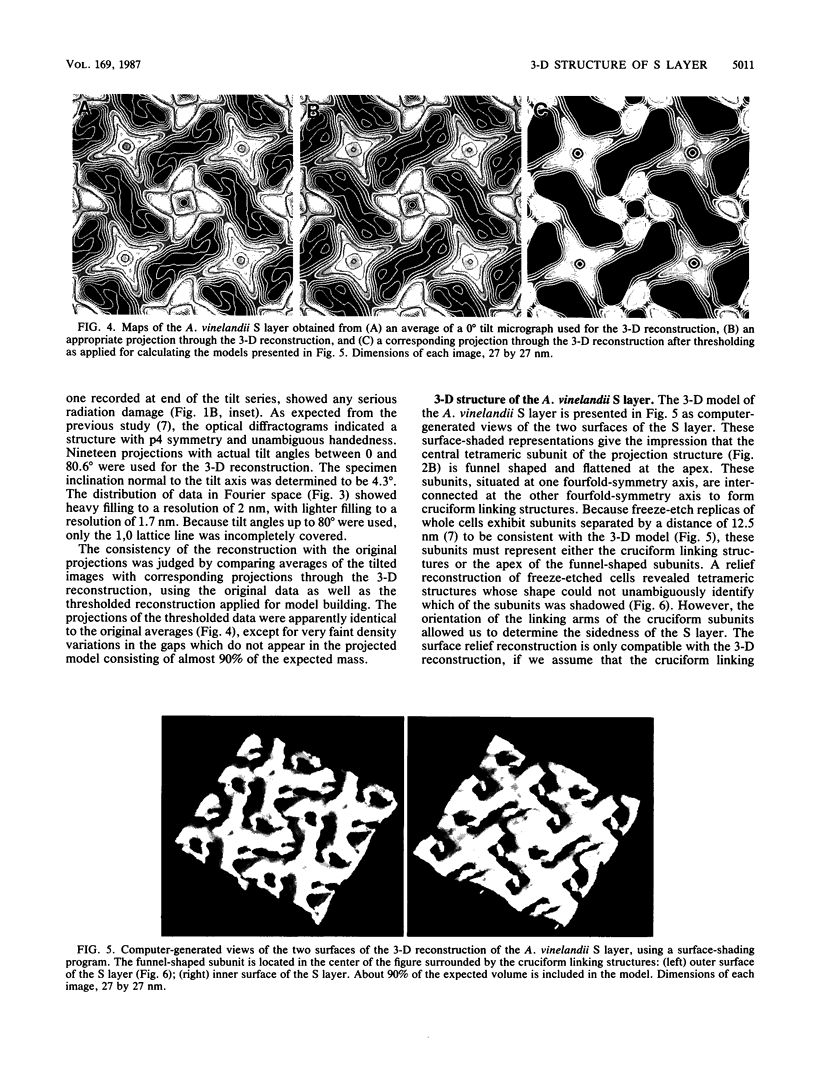
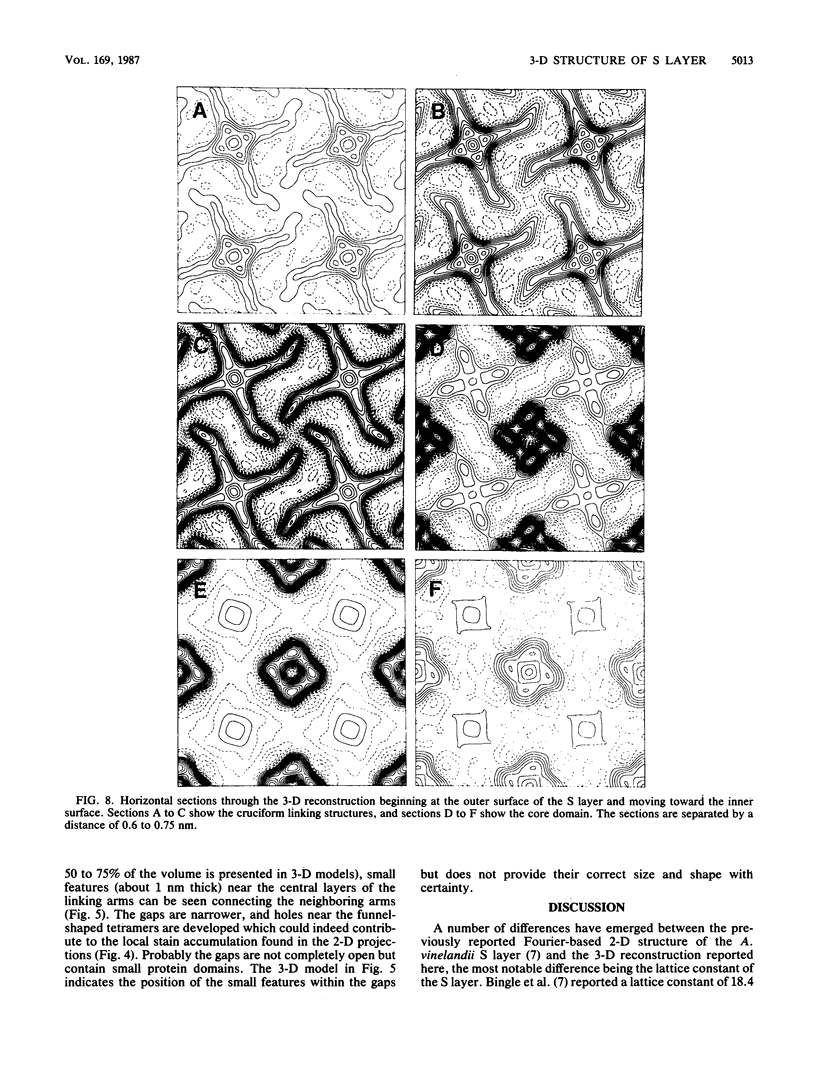
Fragments of the Azotobacter vinelandii tetragonal surface (S) layer, free of outer membrane material, were obtained by treating whole cells with 100 microM EDTA. The three-dimensional structure of the S layer was reconstructed from tilted-view electron micrographs of the S-layer fragments, after computer-assisted image processing by correlation averaging. At a resolution of 1.7 nm, the S layer exhibited funnel-shaped subunits situated at one fourfold-symmetry axis and interconnected at the other fourfold-symmetry axis to form prominent cruciform linking structures. These data, in conjunction with a relief reconstruction of the surface of freeze-etched whole cells, indicated that the apex of the funnel-shaped subunit was associated with the outer membrane, while the funnel "opening" faced the environment; the cruciform linking structures were formed at the outermost surface of the S layer. Electron microscopy and image enhancement were used to compare the structure of the outer membrane-associated S layer with that of fragments of the S layer dislodged from the outer membrane. This analysis revealed an increase in the lattice constant of the S layer from 12.5 to 13.6 nm and an alteration in the position of the cruciform linking structures in the z direction. These conformational changes resulted in a reduction in the thickness of the S layer (minimum estimate, 5 nm) and an apparent increase in the size of the gaps between the subunits. In terms of the porosity of the S layer, this gave the appearance of a transition from a closed to a more open structure.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amos L. A., Henderson R., Unwin P. N. Three-dimensional structure determination by electron microscopy of two-dimensional crystals. Prog Biophys Mol Biol. 1982;39(3):183–231. doi: 10.1016/0079-6107(83)90017-2. [DOI] [PubMed] [Google Scholar]
- Baumeister W., Barth M., Hegerl R., Guckenberger R., Hahn M., Saxton W. O. Three-dimensional structure of the regular surface layer (HPI layer) of Deinococcus radiodurans. J Mol Biol. 1986 Jan 20;187(2):241–250. doi: 10.1016/0022-2836(86)90231-7. [DOI] [PubMed] [Google Scholar]
- Baumeister W., Guckenberger R., Engelhardt H., Woodcock C. L. Metal shadowing and decoration in electron microscopy of biological macromolecules. Ann N Y Acad Sci. 1986;483:57–76. doi: 10.1111/j.1749-6632.1986.tb34497.x. [DOI] [PubMed] [Google Scholar]
- Bingle W. H., Doran J. L., Page W. J. Regular surface layer of Azotobacter vinelandii. J Bacteriol. 1984 Jul;159(1):251–259. doi: 10.1128/jb.159.1.251-259.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingle W. H., Whippey P. W., Doran J. L., Murray R. G., Page W. J. Structure of the Azotobacter vinelandii surface layer. J Bacteriol. 1987 Feb;169(2):802–810. doi: 10.1128/jb.169.2.802-810.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckmire F. L., Murray R. G. Substructure and in vitro assembly of the outer, structured layer of Spirillum serpens. J Bacteriol. 1976 Jan;125(1):290–299. doi: 10.1128/jb.125.1.290-299.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson M. R., Downing K. H., Wu W. H., Glaeser R. M. Three-dimensional structure of the surface layer protein of Aquaspirillum serpens VHA determined by electron crystallography. J Bacteriol. 1986 Sep;167(3):1025–1034. doi: 10.1128/jb.167.3.1025-1034.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhardt H., Saxton W. O., Baumeister W. Three-dimensional structure of the tetragonal surface layer of Sporosarcina ureae. J Bacteriol. 1986 Oct;168(1):309–317. doi: 10.1128/jb.168.1.309-317.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank J., van Heel M. Correspondence analysis of aligned images of biological particles. J Mol Biol. 1982 Oct 15;161(1):134–137. doi: 10.1016/0022-2836(82)90282-0. [DOI] [PubMed] [Google Scholar]
- HOUWINK A. L. A macromolecular mono-layer in the cell wall of Spirillum spec. Biochim Biophys Acta. 1953 Mar;10(3):360–366. doi: 10.1016/0006-3002(53)90266-2. [DOI] [PubMed] [Google Scholar]
- Hastie A. T., Brinton C. C., Jr Specific interaction of the tetragonally arrayed protein layer of Bacillus sphaericus with its peptidoglycan sacculus. J Bacteriol. 1979 Jun;138(3):1010–1021. doi: 10.1128/jb.138.3.1010-1021.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koval S. F., Murray R. The superficial protein arrays on bacteria. Microbiol Sci. 1986 Dec;3(12):357–361. [PubMed] [Google Scholar]
- Lepault J., Martin N., Leonard K. Three-dimensional structure of the T-layer of Bacillus sphaericus P-1. J Bacteriol. 1986 Oct;168(1):303–308. doi: 10.1128/jb.168.1.303-308.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBRISH S. A., MARR A. G. Location of enzymes in Azotobacteragilis. J Bacteriol. 1962 Jan;83:158–168. doi: 10.1128/jb.83.1.158-168.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxton W. O., Baumeister W., Hahn M. Three-dimensional reconstruction of imperfect two-dimensional crystals. Ultramicroscopy. 1984;13(1-2):57–70. doi: 10.1016/0304-3991(84)90057-3. [DOI] [PubMed] [Google Scholar]
- Saxton W. O., Baumeister W. The correlation averaging of a regularly arranged bacterial cell envelope protein. J Microsc. 1982 Aug;127(Pt 2):127–138. doi: 10.1111/j.1365-2818.1982.tb00405.x. [DOI] [PubMed] [Google Scholar]
- Sleytr U. B. Self-assembly of the hexagonally and tetragonally arranged subunits of bacterial surface layers and their reattachment to cell walls. J Ultrastruct Res. 1976 Jun;55(3):360–377. doi: 10.1016/s0022-5320(76)80093-7. [DOI] [PubMed] [Google Scholar]
- Stewart M., Beveridge T. J., Trust T. J. Two patterns in the Aeromonas salmonicida A-layer may reflect a structural transformation that alters permeability. J Bacteriol. 1986 Apr;166(1):120–127. doi: 10.1128/jb.166.1.120-127.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]